INTRODUCTION
With the possible exception of stem cells and tumor cells, ageing is a nearly universal process that, through functional decline, leads to cell death and, eventually, death of the organism. The age-related molecular changes taking place in the human colon have not been exactly determined; moreover, the results of animal tests and human studies are sometimes contradictory.
Researchers have identified a variety of changes in colonic and rectal function associated with ageing. The total number of colonic myenteric neurons decreases with age in rats and in children, particularly during the first 4 years of life[1,2]. While noting an increase in the surface area of myenteric ganglia with age, Hanani et al[3] found that the proportion of ganglia with cavities and other structural abnormalities increases with age. Furthermore, a positive association between age and collagen content within myenteric ganglia has also been identified[2]. These changes in colonic innervation may have an impact on colonic motility[4-9].
As far as colonic epithelium is concerned, its renewal takes 4-5 d in humans[10]. The regulated balance of epithelial proliferation and apoptosis allows normal epithelial regeneration. Any deviation in epithelial cell kinetics may result in a loss of not only structural but also functional integrity. The imbalance of colonic epithelial renewal may lead to either ulcer or carcinoma development of the colonic mucosa.
The effect of ageing on colonic epithelial regeneration is not fully understood. In rat colon, crypt epithelial proliferation and apoptosis were found to be the most active in the 3rd week of life[11], which was thought to be in connection with the development of the gastrointestinal tract. The results of Xiao et al[12] are, however, contradictory; the number of proliferative epithelial cells was higher, while the rate of apoptosis was lower in older rats. The epithelial expression of the anti-apoptotic Bcl protein and of the pro-apoptotic Bak protein was also in accord with ageing: the former was high, while the latter was low in older rats. This phenomenon may explain the survival of genetically defective cells, hence the increasing incidence of colorectal cancer in the elderly. The age-related rise in cell proliferation is thought to be in part the result of enhanced transition from G1 to S phase as well as stimulated progression through the S phase of the cell cycle[13,14]. Inconsistencies in results may be due to different sampling locations and different ages of the animals, together with the effects of errors in sample proceedings, immunohistochemical methods and data evaluation[15].
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease, are one of the major causes of epithelial destruction in the colon. It was recently demonstrated that the perceived differences seen in clinical practice between adults and children with UC are largely due to a decrease in histologic features of colitis in children less than 10 years of age[16]. As children approach adulthood, the degree of inflammation and microscopic architectural distortion seen becomes similar to that found in adults. Interestingly, in rectal biopsies of UC, no differences were found amongst all age groups.
Although the understanding of age-related physiological and pathological processes is of great clinical importance, data about the effect of ageing on the induction of mucosal inflammation and the age-related alterations of mucosal healing are scarce in scientific literature.
THE EFFECT OF AGEING ON INFLAMMATION INDUCTION
Telomere shortening and telomerase activity
Telomeres protect the ends of the chromosomes from end-to-end fusions, degradation and recombination. Telomere length decreases with age in most human tissues, including colon[17], and it has been hypothesized that short telomeres might partially explain the connection between cancer and ageing[18]. Telomeres shorten approximately 100 base pairs in each cell division because of their incomplete replication[19] and also as a consequence of oxidative damage[20]. When telomeres become critically short, and in the absence of efficient DNA repair mechanisms, anaphase bridges are formed during mitosis as a result of end-to-end chromosome fusions, which initiates breakage-fusion-bridge cycles. These cycles facilitate the accumulation of genetic changes and chromosomal instability; hence cells need to acquire mechanisms of telomere maintenance, which usually consist of telomerase reactivation. Telomerase prevents further accumulation of chromosomal instability and confers unlimited replicative potential to the cell[21]. It has been previously demonstrated that in UC, colorectal cancer progression is associated with shorter colonocyte telomeres, chromosomal instability and anaphase bridges[22]. It has also been observed that age-related telomere shortening is accelerated in UC[23]. It seems that there is a minimal viable length of colonocyte telomeres, consistent with data from both human cell lines[24] and a telomerase-deficient mouse model[25]. After reaching this critical length, colonocytes defective for DNA damage checkpoints can continue to proliferate but with increased chromosomal instability. Together with alterations in p53 and p16, which are frequent in the non-neoplastic epithelium of UC[10,26,27], this could be a common pathway of tumor progression in this chronic inflammatory disease.
Interestingly, decreased telomerase activity was observed not just in the non-neoplastic epithelium of severely active UC, but also in the non-affected normal mucosa[28,29]. This result suggests that telomerase deficiency may contribute to the pathogenesis of the disease. Moreover, the elevated epithelial telomerase expression found in mildly active UC[30] may help survival and immortalization of the genetically defective epithelial cells in long-standing, chronic inflammation, and thus create the basis for subsequent pathological cell proliferation.
Alterations of immune response
Ageing is associated with a progressive dysregulation of immune response. During ageing, adaptive immunity significantly declines, a phenomenon called immunosenescence, whereas innate immunity seems to be activated, which induces a characteristic pro-inflammatory profile. The latter is called inflamm-ageing[31,32].
Recently, a new subset of CD4+ T cells has been identified. The Th17 cells are distinct from the Th1 and Th2 cells, and secrete interleukin 17 (IL-17) and IL-22[33,34]. IL-17 has been shown to be a primary mediator in several autoimmune and inflammatory diseases, including IBD[35]. Ouyang et al[36] demonstrated that the induction of Th17 cytokines is significantly elevated in both aged humans and mice. In addition, they found that memory T cells are an important cell type for the induction of IL-17, and that the transfer of CD4+CD45Rbhi cells from aged mice induced more severe colitis in recombination-activating gene 1-deficient mice compared to cells from young mice. Their results suggest that ageing promotes an intrinsic predisposition towards the pathological Th17 immune response, which may explain the second peak (between 50-80 years of age) in the incidence of IBD which occurs in humans.
Ageing also results in alterations in the function of Toll-like receptors (TLRs), which have an important role in the pathogenesis of IBD[37]. Recent studies have begun to elucidate the consequences of ageing on TLR function in human cohorts and add to existing findings established in animal models. In general, these studies show that human TLR function is impaired in the context of ageing, and in addition there is evidence for inappropriate persistence of TLR activation in specific systems[38-41].
It has been shown in a mouse model that ageing and TLR2 deficiency have significant effects on the levels of pro-inflammatory cytokines, such as IL-10 and IFN-γ, which could potentially provide a microenvironment that favors the development of more severe colitis following epithelial damage[42]. It has also been reported that the level of trefoil factor 3 (TFF3), an important colonic protective and repair factor, decreases over time in mice, and is negatively regulated by TLR2 signaling[42]. Cytokines such as TNF and IL-1β negatively regulate TFF3 expression in an epithelial cell line by activation of NF-κB, which has been demonstrated to negatively regulate the transcription of TFF3[43,44]. These interactions among ageing-associated changes, TLR deficiency and TFF3 regulation may be of particular relevance in understanding the development of chronic intestinal inflammation and mucosal injury in the elderly.
Several TLR polymorphisms are also involved in the course of IBD[45-47], although it seems likely that large-scale population studies will be needed to clarify the role of key TLR polymorphisms in ageing-related alterations of IBD pathogenesis.
THE EFFECT OF AGEING ON COLONIC MUCOSAL HEALING
Stem cells
The luminal border of the colonic wall is lined by an epithelial monolayer which has several functions, such as water and electrolyte absorption, and it is also a barrier against luminal pathogens[48,49]. Due to the high turnover of shedding epithelial cells, their continuous replacement from the local stem cell pool is required even in healthy colon. Stem cells are located at the basal part of crypts; their progeny migrate towards the luminal surface and undergo terminal differentiation to secretory (Paneth, enteroendocrine and goblet cells) and absorptive (epithelial) cells[48-50]. In the case of tissue injury, such as in IBD or graft-versus-host disease (GVHD), the capacity of intestinal stem cells is not sufficient for the perfect tissue repair, hence the homing of bone marrow-derived multipotent cells is also essential for mucosal regeneration[48,51,52]. It is well known that circulating hematopoietic stem cells (HSCs) play an important role not just in hematopoietic homeostasis, but in the regeneration of solid-organ tissue, which has been certified by several studies[53]. Stem cells are long-lived cells; therefore, they can sustain several genetic and epigenetic changes during cellular senescence. There is evidence both for and against stem cell ageing, and publications are not in agreement with regard to quality and quantity alterations of stem cells in the course of ageing[54].
HSCs in older mice have decreased per-cell repopulating activity, self-renewal and homing abilities, myeloid skewing of differentiation, and increased apoptosis related to stress[55]. It was recently reported that the cyclin-dependent kinase inhibitor p16INK4a, the level of which was previously noted to increase in other cell types with age, accumulates and modulates specific age-associated HSC functions[56]. Notably, in the absence of p16INK4a, HSC repopulating defects and apoptosis were mitigated, improving the stress tolerance of cells and the survival of animals in successive transplants, in a stem-cell-autonomous tissue regeneration model. As p16INK4a is involved in colorectal carcinogenesis[57], it may be supposed to have a specific role in the regulation of the behavior of migrating stem cells of the human colon as well.
Stem cells are in close connection with their niche by means of mechanical and/or chemical processes. Based on the results of bone marrow transplantation studies, one can assume that stem cell function and life span depend on the recipient’s age, since increased post-transplant autoimmunity has been observed in the case of older recipients[54]. The age-related decrease of regenerative stem cell capacity, however, needs to be further studied.
The balance of cell proliferation and death ensures adequate epithelial regeneration. Although colonic epithelial stem cells can be distinguished from other epithelial cells only by morphology, several cellular markers may help the identification of both normal and cancer stem cells of the intestinal tract[58-60].
The effect of ageing on colonic epithelial regeneration and crypt-base stem cell function has come to the frontline of discovery recently. In a mouse model, a few apoptotic cells were seen around the stem cell position and this frequency did not alter with age. However, the apoptotic index within crypts was nearly twice as high in older mice after low dose gamma-irradiation and the number of surviving crypts decreased significantly faster after increasing the dose of irradiation; moreover, in the post-irradiation period the crypt regeneration was much slower in older animals[61]. It was shown that clonogenic cells are more radiosensitive in old mice, and that the growth of surviving crypts after injury was delayed in old mice even though the number of resident clonogenic cells were higher in older colonic crypts[62].
Methylation
In non-cancerous colonic mucosa, repeated injuries are likely to induce adaptive methylation changes that enable efficient wound healing and act against cancer development. The methylation-variable sites that are located in promoter or noncoding neutral regions have demonstrated gradual methylation changes associated with the ageing or long-term adaptation process[63]. The presence of transitional-CpG sites between the unmethylated promoters and nearby densely methylated retroelements has been proposed, in order to describe the complexity of variable methylation in gene control regions[64,65]. The transitional-CpG sites at the margin of the CpG islands and at the non-island CpG sites around the transcription start sites have been found to be either under- or over-methylated in a tissue-specific manner as well as to be methylated to various degrees in the same tissue type[65]. This result suggests that the methylation-variable sites nearest to the transcription start sites may serve as epigenetic markers for adaptive DNA methylation. The methylation-variable site of a strongly expressed tissue-specific gene can influence the expression of the nearby gene as well as its related genes. Therefore, the methylation-variable sites of the key colon-specific genes are expected to participate in both the discrete mucosal adaptation and the interactive changes of methylation patterns that lead to mucosal alterations. In the stomach, recent evidence suggests that mucosal injury induces adaptive changes in DNA methylation[66]. The ulcer-healing genes, such as trefoil factor 1 and 2, (TFF1, -2), cadherin 1 (CDH1), and peroxisome proliferator-activated receptor gamma (PPARG), were found to be concurrently methylated with other genes depending on the presence or absence of CpG islands in the normal mucosa of healthy individuals, while both the TFF2 and PPARG genes were frequently undermethylated in gastric ulcer patients.
Age-related methylation loci, such as estrogen receptor 1 (ESR1) and myogenic differentiation 1 (MYOD1), have also been highlighted[67-69]. These loci showed age-dependent methylation in normal colon mucosa, and this type of methylation is considered to serve as a functional link between ageing and cancer, possibly by deregulating the growth and differentiation of normal colonic epithelial cells and predisposing them to tumorous transformation. In addition, methylation of ESR1 in colon epithelium occurs more frequently in patients with UC who have neoplasia than in UC patients without neoplasia[70].
Differences in methylation levels of age-related methylation loci between the proximal and distal colon have also been described[71]. This phenomenon may have an impact not just in colorectal carcinogenesis, but in the understanding of the pathogenesis of UC.
Growth factor receptors
Gastrointestinal mucosal cell proliferation is known to be under the regulation of a number of nutritional and hormonal factors. It was reported that an age-related rise in gastric and colonic mucosal cell proliferation is accompanied by a marked rise in expression and activation of several tyrosine kinases, including epithelial growth factor receptor (EGFR)[72-74]. The age-related rise in EGFR activation in the mucosa of the gastrointestinal tract is not fully understood, but some recent data suggest this could be partly the result of loss of EGFR-related peptide (ERRP), a “negative regulator”[75]. It was recently reported that in Fischer-344 rats, ageing is associated with increased activation of EGFR in the colonic mucosa, as evidenced by a 30%-35% increase in the levels of tyrosine phosphorylated EGFR in the proximal and distal colon of aged (20-22 mo old) compared to young (4-6 mo old) rats. In contrast, the levels of ERRP in both regions of the colon of aged rats were decreased by 50%-60%, compared to their younger counterparts[76]. Expression of ERRP was also found to be high in benign human colon, stomach and pancreas, but low in the respective invasive adenocarcinomas[77,78].
Age-related decrease of EGFR signaling in cells of ectodermal origin has additionally been described[79]. This may cause delayed mucosal healing in the case of older individuals.
Hepatocyte-derived growth factor (HGF) is mainly produced by mesenchymal cells and acts on cells of epithelial origin which express the HGF receptor C-met (HGFR). The HGF-HGFR system is important in gut homeostasis, and has a crucial role in gastrointestinal wound healing[30]. Furthermore, this system has morphogenetic effects, and it also regulates the formation of epithelial tubular and gland structures[30,80]. It was demonstrated that the production of HGF by fibroblasts increased sharply after about 70% completion of their lifespan in culture, which is regulated at the transcriptional level[80]. The expression of HGFR may decrease with ageing as well[81], which may have consequences on tissue repair.
Revealing age-related alterations in the expression of growth factor receptors involved in colonic mucosal repair, and the better understanding of alterations in receptor signaling, may result in new therapeutic targets of colonic mucosal damage.
CONCLUSION
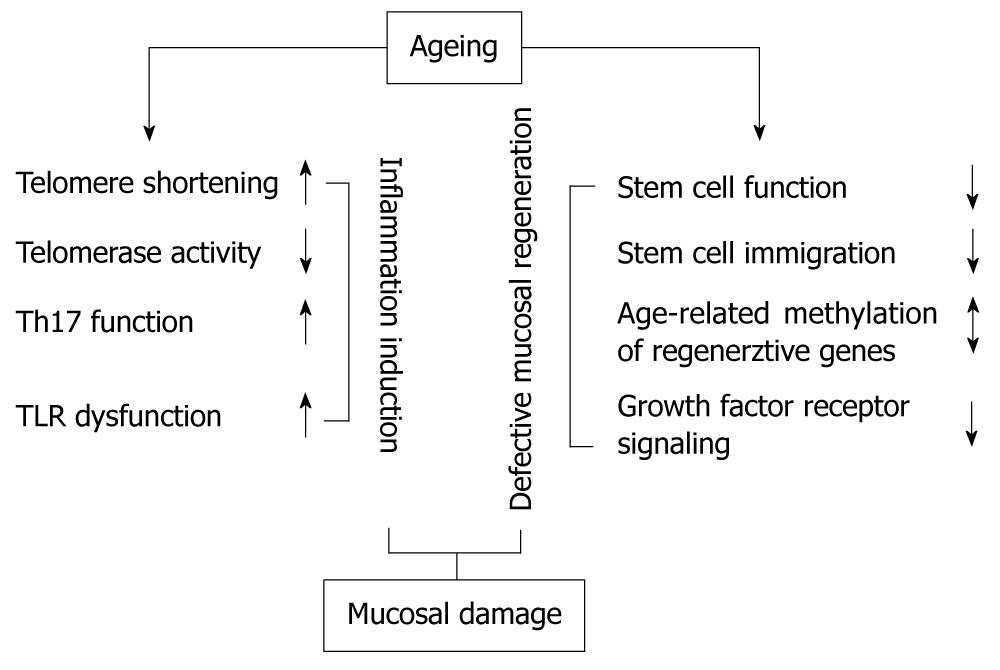
There are numerous signs of ageing in the human gastrointestinal tract, including the colon (Figure 1). Beyond macro- and microscopic alterations, some of these can be detected at the genetic, gene expression and/or epigenetic level. The connection between ageing and colonic mucosal regeneration has been reported by several studies, and their results may provide an insight into physiologic and pathologic mucosal healing in the context of ageing. An understanding of the influence of these age-related mechanisms may help to develop new therapeutic strategies for chronic inflammatory bowel diseases accompanied by mucosal damage.
Figure 1 The effect of ageing on factors resulting in colonic mucosal damage.