Published online Jun 28, 2011. doi: 10.3748/wjg.v17.i24.2924
Revised: November 15, 2010
Accepted: November 22, 2010
Published online: June 28, 2011
AIM: To characterize a culture model of rat CCA cells, which were derived from a transplantable TTA-induced CCA and designated as Chang Gung CCA (CGCCA).
METHODS: The CGCCA cells were cultured at in vitro passage 12 times on a culture dish in DMEM medium. To measure the doubling time, 103 cells were plated in a 96-well plate containing the growth medium. The cells were harvested 4 to 10 d after seeding, and a standard MTT assay was used to measure the growth. The phenotype of CACCA cell and xenograft was determined by immunohistochemical study. We also determine the chromosomal alterations of CGCCA, G-banding and spectral karyotyping studies were performed. The CGCCA cell line was transplanted into the nude mice for examining its tumorigenicity. 2-Deoxy-2-(18F)fluoro-D-glucose (FDG) autoradiography was also performed to evaluate the FDG uptake of the tumor xenograft.
RESULTS: The doubling time for the CGCCA cell line was 32 h. After transplantation into nude mice, FDG autoradiography showed that the tumors formed at the cell transplantation site had a latency period of 4-6 wk with high FDG uptake excluding necrosis tissue. Moreover, immunohistochemical staining revealed prominent cytoplasmic expression of c-erb-B2, CK19, c-Met, COX-II, EGFR, MUC4, and a negative expression of K-ras. All data confirmed the phenotypic features of the CGCCA cell line coincide with the xenograft mice tumors, indicating cells containing the tumorigenicity of CCA originated from CCA. In addition, karyotypic banding analysis showed that the diploid (2n) cell status combines with ring and giant rod marker chromosomes in these clones; either both types simultaneously appeared or only one type of marker chromosome in a pair appeared in a cell. The major materials contained in the marker chromosome were primarily identified from chromosome 4.
CONCLUSION: The current CGCCA cell line may be used as a non-K-ras effect CCA model and to obtain information and reveal novel pathways for CCA. Further applications regarding tumor markers or therapeutic targeting of CCA should be addressed accordingly.
- Citation: Yeh CN, Lin KJ, Chen TW, Wu RC, Tsao LC, Chen YT, Weng WH, Chen MF. Characterization of a novel rat cholangiocarcinoma cell culture model-CGCCA. World J Gastroenterol 2011; 17(24): 2924-2932
- URL: https://www.wjgnet.com/1007-9327/full/v17/i24/2924.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i24.2924
Cholangiocarcinoma is a malignant neoplasm derived from bile duct epithelium (i.e. cholangiocytes). It is characterized by a great diversity of symptoms commonly occurring in the late course of the disease, and therefore making treatment puzzling. The biological behavior of the tumor and early intrahepatic and/or extrahepatic spread limit the efficacy of surgical management to perform a curative resection; although liver transplantation may provide an alternative option for CCA treatment, high rates of recurrence still limit liver transplantation for most CCA patients[1] and usually lead to a poor prognosis. Three- to five-year survival rates, even with resection, remain dismal[2-7]; in addition, neither radiation therapy nor chemotherapy significantly improves long-term survival rates. This cancer is related to a wide range of risk factors, such as infestation with liver flukes, primary sclerosing cholangitis, and hepatolithiasis, that cause the incidence rates of CCA to vary greatly among different areas of the world[8]. However, many data have shown that the incidence and mortality rates of CCA have been rising worldwide over the past several decades, particularly the intrahepatic CCA[9-11].
Therefore, our goal is to identify potential possible diagnostic biomarkers as the investigation of the molecular pathophysiology associated with this disease becomes more and more important and necessary. In our previous study, a thioacetamide (TAA)-induced CCA rat model was successfully established, and serves as a powerful pre-clinical platform for therapeutic and chemoprevention strategies for human CCA[12]. Herein, we further developed the rat CCA tumor cells as a cell line designated as Chang Gung CCA (CGCCA), and then transplanted the cells into a xenograft of nude mice to further confirm the characteristics of this cell line. A series of IHC studies, including CK19, c-Met, COX-II, and MUC4, were performed to determine the phenotype of the cell line. The genotype was examined by cytogenetic studies, and the 2-Deoxy-2-(18F)fluoro-D-glucose (FDG)-avid character of the CGCCA xenograft of the nude mice was demonstrated by animal PET. All of the evidence proved that the CGCCA cell line rat was derived from the original primary tumor formed by the TAA carcinogen. Our current work supports the view that the systematic cell cultures may provide a relevant CCA model to study the complex mechanisms involved in CCA by revealing the potential pathogenesis of this disease. In addition, we may be able to determine the possible diagnostic markers for the early detection and diagnosis of this disease.
This study was approved by the experimental animal ethics committee at the Chang Gung Memorial Hospital, Taiwan. The investigation conformed to the US National Institute of Health (NIH) guidelines for the care and use of laboratory animals (Publication No. 85-23, revised 1996). Male Sprague-Dawley (SD) rats weighing 319 ± 14 g were used in the experiments. The rats were housed in an animal room with a 12:12 hour light-dark cycle (light from 08:00 AM to 08:00 PM) at an ambient temperature of 22 ± 1°C. Food and water were available ad libitum.
The cell isolation procedure as described by Lai et al[13] was applied with minor modifications in this study. In brief, hyperplastic bile ductular epithelial cells were obtained from the liver of the male Sprague-Dawley (SD) rats treated with TAA 300 mg/L daily after 25 wk of exposure. The isolation of the CGCCA cell line was established from the nest of cholangiocarcinoma by employing differential cell harvesting with 0.05% trypsin and 0.53 mmol/L EDTA (Life Technologies), and then combined with subsequent serial propagation suspended in a cell culture medium composed of DMEM with 100 U/mL penicillin and 100 U/mL streptomycin (basal medium) plus 10% fetal bovine serum[13].
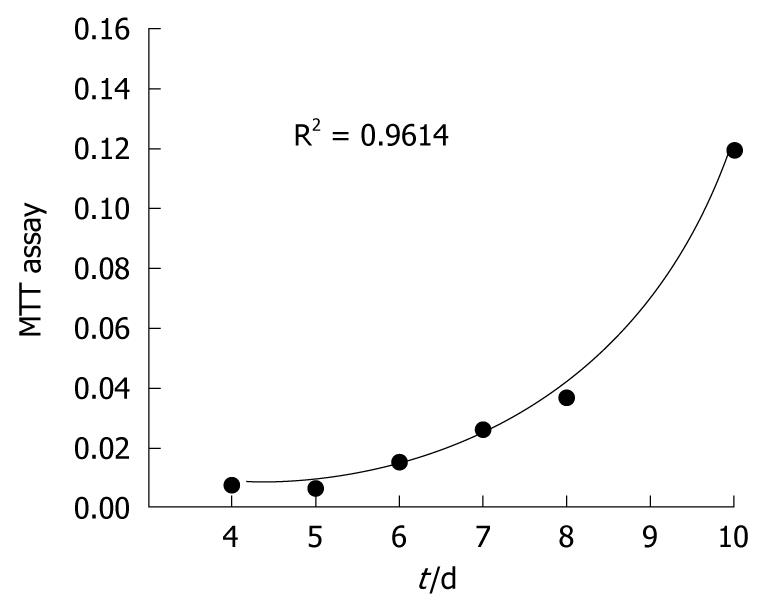
The CGCCA cells were cultured at in vitro passage 12 times on a culture dish in DMEM medium containing 10% fetal bovine serum. To measure the doubling time, 103 cells were plated in a 96-well plate containing the growth medium as described above. The cells were harvested 4 to 10 d after seeding, and a standard MTT assay was used to measure the growth according to the instruction manual (MTT Cell Growth Assay Kit; #CT01; Chemicon). The doubling time of the cell population was estimated based on the slope angle of the linear regression model for the four time points.
CGCCA rat cells were grown on a miniature cell culture vessel chamber, which permits cells to be grown, fixed, stained, and analyzed all on the same slide (#154461 Lab-Tek II Chamber Slide System; Nalge Nunc International, USA). A total of 2000 cells per well were grown over night, rinsed with PBS twice, fixed with 4% PFA for 1 min, and perforated with 1% Tween 20 for 1 min. In addition, we further compared the cells with the xenograft mice tumors. The mice tissues were obtained for the immunoreactivities study once the mice tumors reached a diameter of 1.0 cm. The slides were then stained according to the routine IHC staining as described previously[14]. In brief, the primary antibodies CK19 (MAB-1675; Millipore; Temecula, CA), K-ras (clone sc-30; Santa Cruz Biotechnology Vision Corporation; Fremont, CA), c-erb-B2, COX-II, EGFR, MET, and MUC4 were diluted at 1:200 and 1:400, respectively (clone sc-284; Santa Cruz Biotechnologies; Santa Cruz, CA; M-3563; Dako Cytomation; RB-9072-9; Lab Vision Corporation; Fremont, CA; clone sc-161; Santa Cruz Biotechnology Vision Corporation; Fremont, CA; and 35-4900; Zymed S; San Francisco, CA) and incubated overnight at 4°C. The slides were then washed three times with TBST, mounted, and analyzed under microscope by authors blindly before visualization with the DAKO LSAB2 System (Peroxidase; DAKO A/S; No. K0675). Control slides were incubated with the secondary antibody only.
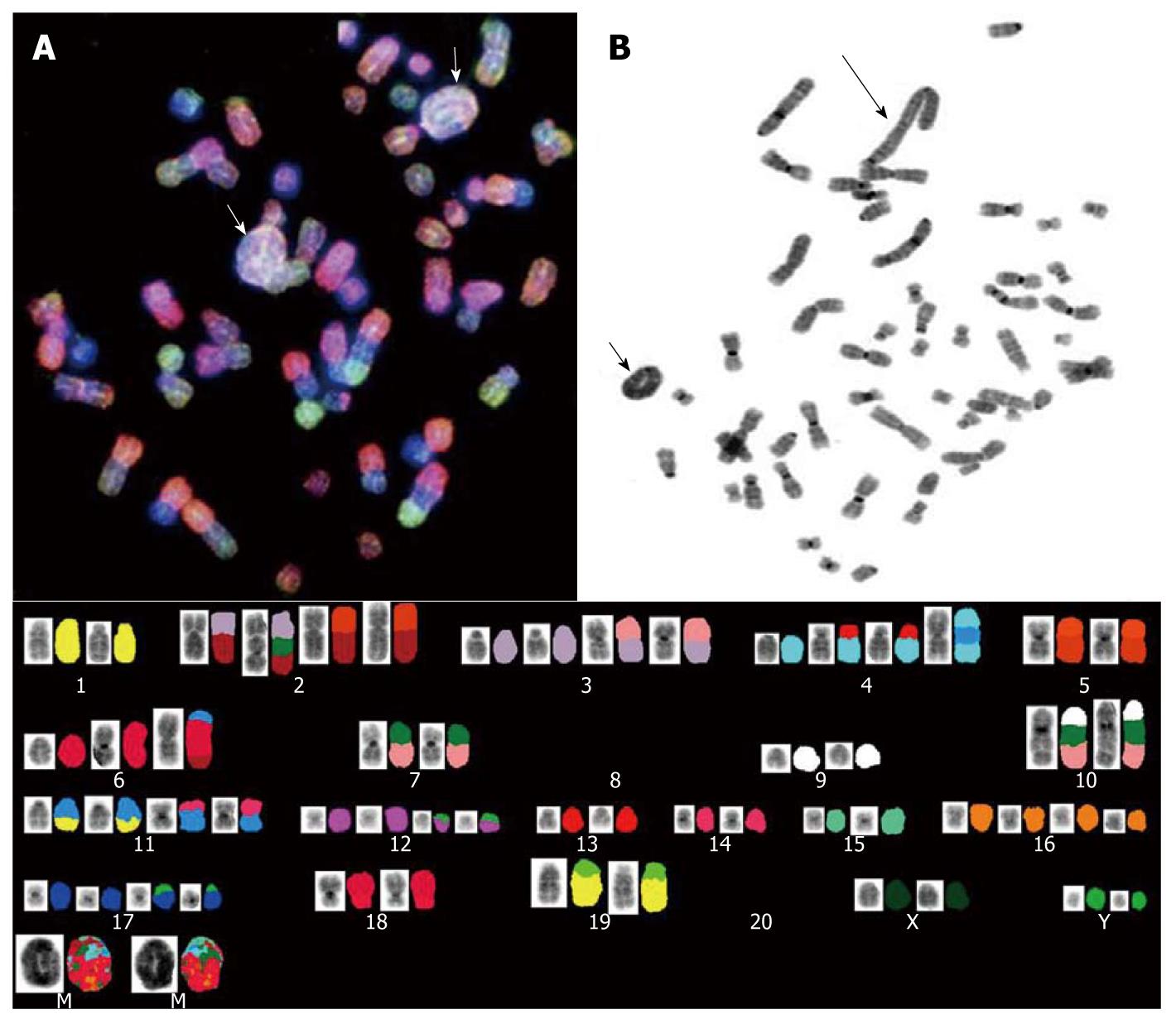
To determine the chromosomal alterations of CGCCA, G-banding and spectral karyotyping (SKY) studies were performed. The CGCCA cells were grown under the conditions as described above. After the cells were harvested, a metaphase chromosome spread was prepared for G-banding and SKY analysis[15]. At least ten metaphases were analyzed after G-banding (data not shown). For SKY analysis, 22 differentially labeled chromosome-specific painting probes and Cot-1 DNA were denatured and hybridized to the tumor metaphase chromosomes according to the protocol recommended by the manufacturer (Applied Spectral Imaging; Migdal Haemek, Israel) with some modifications as previously described[15]. Image acquisitions were performed using a SD200 Spectracube system (Applied Spectral Imaging) mounted on a Leica DM2500 microscope with a custom-designed optical filter (SKY-1; Chroma Technology; Brattleboro, VT). The clonality criteria and the karyotype description followed the recommendations of the International System for Human Cytogenetic Nomenclature (ISCN 2005)[16].
Seven male nude mice (BALB/cA Jcl nu/nu) (6 to 8 wk old; 20 to 22 g body weight) were purchased from Clea Japan (Ohita, Japan). The CGCCA cell line was transplanted into the nude mice for examining its tumorigenicity. Each of the 7 mice received a 50 μL subcutaneous inoculation that contained ten million CGCCA cells suspended in the thigh area. Tumor size was measured with a digimatic caliper, and tumor volume was calculated according to the subcutaneous tissue on the flank of each mouse using the formula V = 1/6πabc (a, b, and c indicate the diameter in each axis). The xenograft developed after implantation of CGCCA; 4 to 6 wk static later, all animals were sacrificed by CO2 asphyxia. The xenograft was dissected, and the histopathology of the xenograft was evaluated as described[12]. The xenografted tumors were compared with the original cell line for morphology changes.
FDG positron emission tomography (PET) is an important imaging technique for the evaluation CCA in humans[17]. To evaluate the different levels of fluorodeoxyglucose uptake of the CGCCA cell line xenograft of the nude mice, FDG autoradiography was performed 4-6 wk after tumor implantation. Animals were food-deprived for 8 h, and 37 MBq of FDG was given intravenously. The animals were sacrificed by decapitation after deep anesthesia with isoflurane 45 min after radiotracer injection. The details of the quantitative autoradiography procedures have been described by the authors[18]. In brief, the target tissues, including liver, tumor, and thigh muscle, were quickly removed and placed on dry ice for fixation. The frozen samples were cut to a 10-μm thickness and mounted on glass slides. The slides were exposed to a phosphor image plate (IP) for 2 h and digitized using FLA5100 scanner (Fuji; Japan, Tokyo). The radioactivities of the adjacent tissue sections (assumed to possess the same radiotracer distribution) were measured by γ counter and decay corrected to the time of injection. The correlations between the radioactivities and IP signal intensities were established. All IP signal intensities were converted to radioactivities by using the following calibration curve. Regions of interest (ROI) were manually drawn around the edge of the tumor xenograft activity by visual inspection. The mean activities were recorded from the entire ROI. The percentage injected dose per gram (%ID/g) was calculated as follows: %ID/g = activity in a gram of tissue (Ct)/injected dose × 100%. The slices were stained with hematoxylin-eosin (HE) for histologic examination.
All data are presented as mean ± SD. Group comparisons of FDG uptake among tissues were determined using analysis of variance (ANOVA). All statistical analysis was performed using SPSS computer software (Chicago, IL) and a P value of < 0.05 was considered statistically significant.
To establish a TAA-induced rat cell line, 25 wk of orally administered TAA rat liver tissues were harvested for culturing. CGCCA rat cells showed a typical growth curve that included lag, logarithmic, and stationary phases (as shown in Figure 1). The doubling time of the CGCCA cell line was calculated to compare the growth of 103 cells. Under the series surveillance, a cell population doubling time of 32 h was determined (6 points at day 4, 5, 6, 7, 8, and 10). When the CGCCA cell line underwent the 26th in vitro passage, we harvested cells to perform the cytogenetic or IHC experiments in the current study. The cell line has been maintained in culture for approximately a year so far. Therefore, viable CGCCA cells have been cryopreserved at almost every in vitro passage time, from which new cultures may be established.
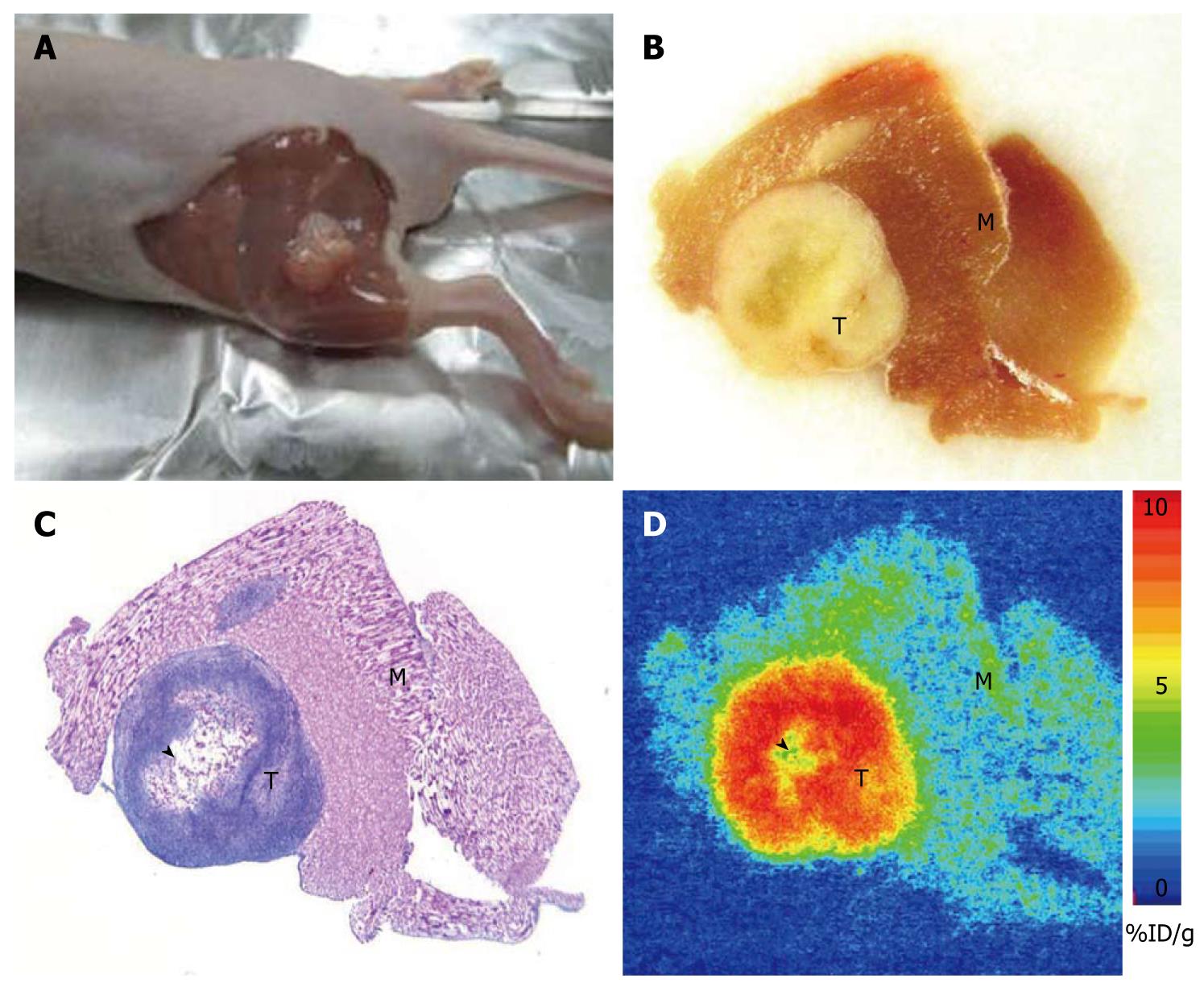
To examine whether the CGCCA cell line contained the tumorigenicity of CCA, the cells were transplanted into the thigh area of seven recipient nude mice and given a 100% incidence of CCA. In addition, their morphological features were essentially identical to those of the parental tumor from which the tumorigenic CCA cells were originally isolated. Tumors formed at the cell transplantation site had a latency period of 4-6 wk, as represented by the photomicrographs shown in Figure 2.
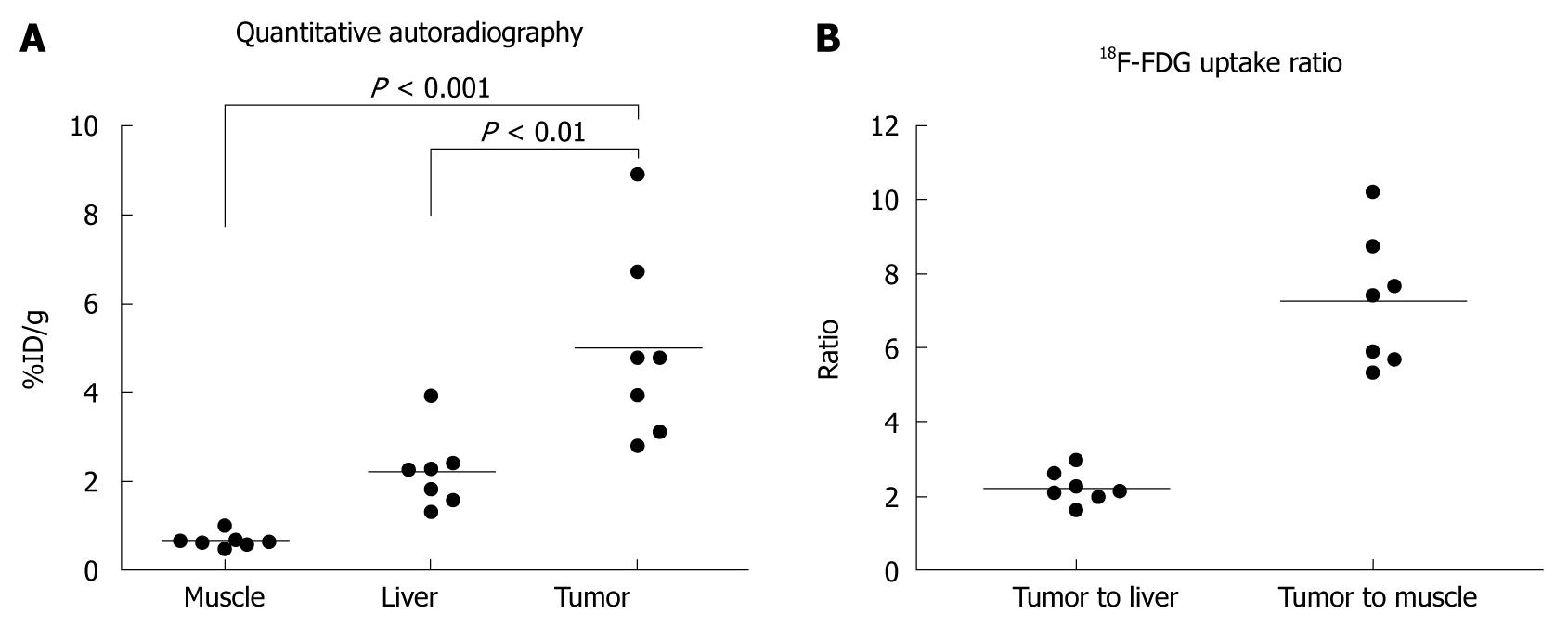
All seven mice developed a large tumor 4-6 wk after tumor cell implantation. Necropsy and histology confirmed the presence of TAA-induced CCA in 7 mice (Figure 2). Central necrosis could be observed in all large tumors suggesting the fast growth of this malignant tumor. Of note, all tumor cells, excluding necrosis tissue, possessed high FDG uptake. The quantitative uptake value of fluorodeoxyglucose in muscle, liver, and tumor were 0.67 ± 0.17, 2.23 ± 0.85, and 5.00 ± 2.15 %ID/g, respectively (Figure 3A). The tumor to liver and tumor to muscle ratios of FDG uptake were 2.25 ± 0.43 and 7.48 ± 1.78, respectively (Figure 3B). These data are highly consistent with our previous in vivo findings, indicating that FDG metabolic activity is significantly higher in the CGCCA xenograft in the nude mice.
The rat CGCCA cell line and heterotransplantation of nude mice tissues were evaluated by immunohistochemistry (IHC) stainings.
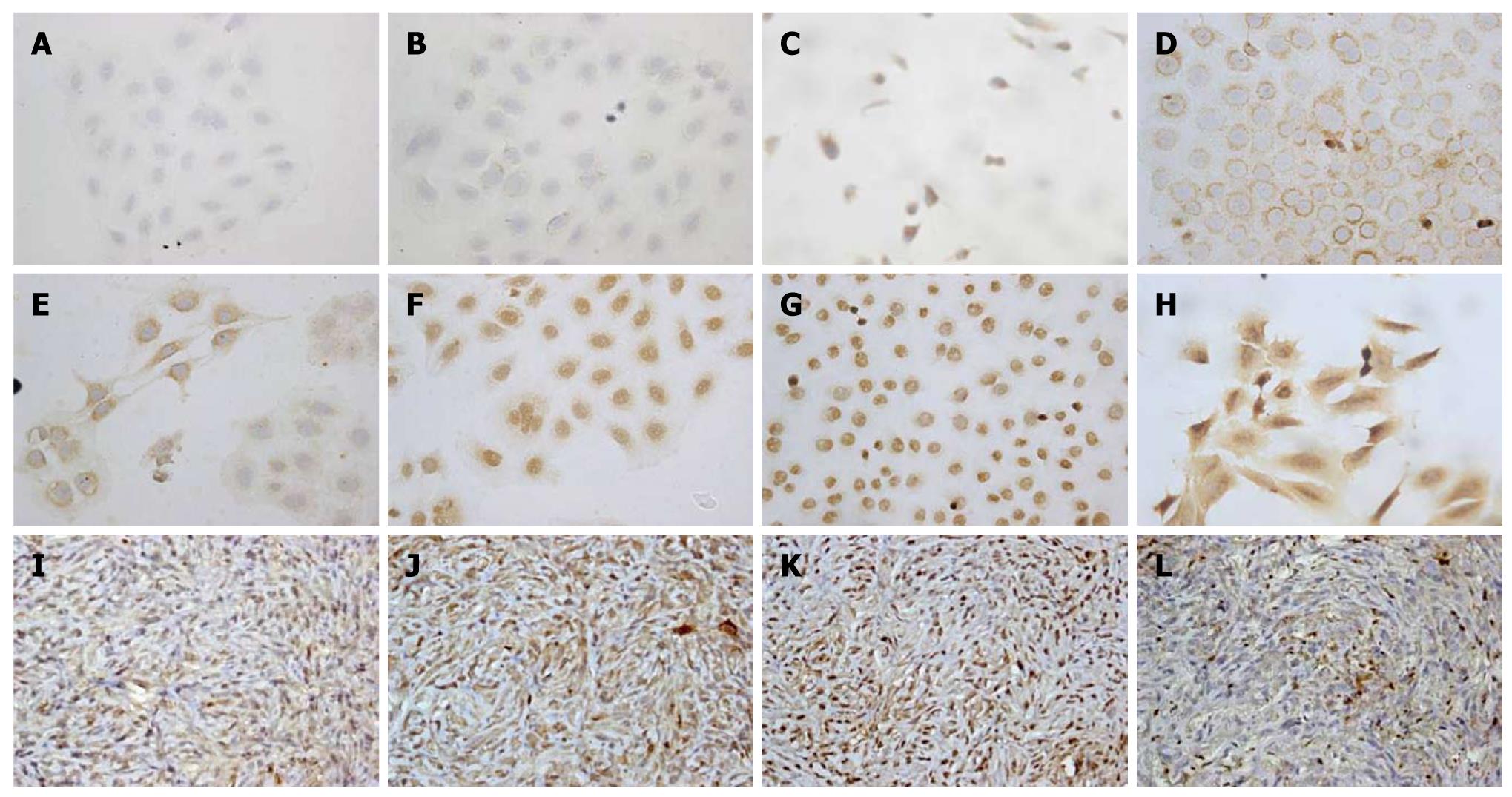
To demonstrate the phenotype characteristics and histopathology of CGCCA cells, IHC staining was performed on either the cell line or mouse xenograft tissues. The CGCCA cells revealed prominent cytoplasmic expression of CK19 (biliary cytokeratin 19), as well as c-erb-B2, c-Met, COX-II, EGFR, and MUC4 (Figure 4C-H). However, the CGCCA cells revealed a negative expression of K-ras (Figure 4A and B). All of the data from the neoplastic glandular epithelia of the TAA-induced rat CGCCA cell line were highly consistent with previous in vivo findings, indicating that these proto-oncogenes are concordantly overexpressed. The tumor tissues from the heterotransplanted nude mice retained the characteristic traits of their in vitro cell counterparts. Phenotypically, xenograft highly expressed cytoplasmic immunostaining of CK19, mucin-producing tubular adenocarcinomas closely resembling in their histological and phenotypic features those of the parent tumor; besides, COX-II, MET, and MUC4 revealed strong and diffuse cytoplasmic immunostaining as well (Figure 4I-L).
Spectral karyotyping (SKY) and G-banded analyses were performed to determine the genetic alterations in the CGCCA cell line using the 26th week’s TAA-induced rat CGCCA cell line. The cytogenetics study revealed a number of chromosomes ranging between 50 to 56 diploidy (2n) karyotype with complicated genetic abnormalities of marker chromosomes. Obviously, at least two clones were identified in this cell line. In the clones, the loss of whole chromosome 8 and 20 was observed in all analyzed cells; multiple translocated chromosomes formed at the site of chromosome 2q10, such as t(2;3), t(2;5), t(2;3;10); similarly, at the site of chromosome 4q10, commonly formed t(4;13) and t(4;11;4) fusion chromosomes were observed as well. Further, i(6)(q10) was frequently observed in most of the clones. Notably, two major similar contents of ring and giant rod marker chromosomes were involved in these clones; they either simultaneously appeared in a cell or only one type of marker chromosome appeared as a pair to be observed (Figure 5).
CCA is the second most common primary hepatic tumor after hepatocellular carcinoma, which is also known as a slowly progressing bile-duct cancer. CCA usually originates in the liver or the intrahepatic bile ducts, and it is characterized by a poor prognosis. Incidence rates account for between 5% and 30% of primary liver cancers and vary geographically[19]. For instance, peripheral CCA represented 3.58% of all primary liver cancers reported by the Japan Liver Cancer Society[1]. According to the report of Bartlett[20], the mortality of intrahepatic CCA has increased 15-fold, and it is currently a more common cause of mortality than hepatocellular carcinoma. New insights into the pathogenesis of this disease, either by using a panel of systematic in vitro cell lines for revealing the obscure mechanisms of CCA, or animal models for development of novel therapeutic strategies, are urgently required for clinical-therapeutic purposes.
To our knowledge, there are two published human CCA cell lines available for the purpose of study; however, both have limited availability due to their long-term passage in vitro, and they have been shown to be markedly aneuploid. With respect to experimental animal CCA culture models, two published reports have described the process of establishment; one was developed from liver fluke-associated CCA induced in a hamster model[21]; the other was developed from furan-fed rat CCA culture model[22]. We have been working for several years and have succeeded in establishing a TAA-induced rat intestinal-type CCA model. This model provides a unique and useful study tool for CCA investigation. The important aspects of the cellular and molecular pathogenesis of CCA that are potentially relevant to the human disease have been addressed in previous reports as well[12,23]. In the current study, we further aim to establish a CCA cell line from a TAA rat CCA model and report its identical karyotype as well as immunohistochemical characteristics. This systematic in vitro cell line panel will be used as a valuable study tool for revealing the obscure mechanisms of CCA, and searching for the potential biomarkers for CCA tumorigenesis.
Regarding the growth kinetics of the CGCCA cell, CGCCA cells exhibit a 32-h doubling time. This is longer than C611B cells derived from a furan rat model, which exhibited a cell doubling time of approximately 24 h and were aneuploid[13]; but comparable with the hamster liver fluke-associated CCA cells[21]. While skeptics question whether human population doubling times can be slower than animal cell lines, after comparing the population doubling times with some human CCA cell lines cultured under similar conditions, the human cell lines presented range from 50 to 180 h[24-27].
Regarding phenotype, the strong and diffuse expression of biliary cytokeratin (CK19) confirms the bile ductular ontogeny of the CGCCA cells and xenograft. The molecular alterations involved in CGCCA cells are similar to TAA rat models previously described[12,22,28,29]; the receptor tyrosine kinases c-Met, c-erb-B2 (also known as HER-2/neu), and EGFR and their interact elements, COX-II, MUC4, were over-expressed in the current neoplastic cells, either in CGCCA cells or xenograft tissues. It is well known that receptor tyrosine kinases have become important therapeutic targets for anti-tumor molecularly targeted therapies. There is increasing evidence to suggest that, when the HGF/SF interacts with the receptor tyrosine kinases c-Met, the activation could lead to a plethora of biological and biochemical effects in the cell[30] and may play an important role in the development and/or progression of human CCA[31-34]. Other molecular alterations include the overexpression of COX-2[5,35,36] and MUC4, which acts as a ligand for c-erb-B2 and was also confirmed to be overexpressed in the CGCCA cells in our current study. Furthermore, we recently demonstrated that MUC4 could be an independent risk factor of poor prognosis in clinical patients with the mass-forming type of intrahepatic CCA who underwent hepatectomy[37].
Notably, negative K-ras expression was observed in the current CGCCA cells. It is known that very high frequency multiple K-ras gene mutations at codon 12 are commonly detected in CCA (15/15 showed one mutation, and 9/15 showed more than two mutations)[38]. Evidence has further indicated that the mutation status of the K-ras gene affects the response of cetuximab, an epidermal growth factor receptor (EGFR) inhibitor[39]. Therefore, K-ras is considered to be one of the important factors involved in the stepwise progression of neoplastic cells to full malignancy. It is also proven to be related to the higher incidence of bile duct cancers that arise distally in the common bile duct[40,41]. Therefore, the current CGCCA cell line could offer a valuable suitable model that could avoid the influence from K-ras expression.
Nevertheless, evidence of the overexpression of these typical oncoproteins in the CGCCA cell line provides strong resemblances with the human CCA disease, as well as an avenue for future pathogenetic or pharmacologic studies.
In addition, there is evidence that FDG PET imaging may be useful in the diagnosis and management of both hilar and peripheral cholangiocarcinomas in humans[17]. We have previously shown that the FDG uptake pattern in TAA-induced rat CCA was similar to that observed in other human studies[18]. In the present study, our CGCCA xenograft closely mimicked the TAA-induced rat CCA with regard to fluorodeoxyglucose uptake as evaluated by FDG autoradiography. The xenograft had a high tumor to liver and tumor to muscle FDG uptake ratio, which makes in vivo tumor detection possible by FDG microPET. The data of the animal PET with regard to the TAA mice models were highly consistent with our previously published in vivo findings, indicating that FDG metabolic activity is significantly higher in the CGCCA xenograft in the nude mice[18].
The results of cytogenetic analysis with regard to CGCCA cells revealed complicated chromosomal alterations, and the hyperdiploid karyotype has been characterized in the CGCCA cell line. Basically, the hyperdiploid karyotype is thought to arise from the maintenance of heterozygosity. According to the marker chromosomes observed in the CGCCA cell line, at least three clones could be identified in current cell line, suggesting that the hyperdiploidy does not arise from a near haploid precursor. In addition, although hyperdiploid clones have been identified, all the clones tend to show a pattern of chromosome loss with all copies of chromosomes 8 and 20; further, the gains are more often tetrasomic than trisomic for the chromosomes 2, 3, 4, 11, 12, 16, and 17 (Figure 5). Notably, the marker rings and/or giant rod chromosomes could be observed in every cell as well; the materials contained in the marker chromosomes of the ring or rod shapes varied from cell to cell; however, the major materials mostly came from chromosome 4 as was demonstrated by SKY (Figure 5). To our knowledge, rings are rare in benign tumors, whereas they are common in certain invasive tumors. In addition, the ring chromosomes are even common in certain tumors, especially in subgroups of sarcomas where they may be used as diagnostic indicators for these lesions as described by Gisselsson et al[42]. The other additional anomalies, such as translocations and other structural chromosome abnormalities, were present in approximately half of the cells; apparently, the presence of non-random alterations in every clone is most likely the primary change event; in addition, the duplication of chromosome 4 fragments are the most common additional change; deletion of chromosomes 8 and 20, and other random structural abnormalities, such as der(2)t(2;3)(q10;q2), der(2)t(3;10;2), der(2)t(2;5)(q10;q1), der(4)t(4;13)(q10;q?) × 2, der(4)t(4;11;4), are probably a secondary event (Figure 5).
However, although the presence of non-random translocations, such as der(3)t(3;7)(q10;q13), der(3)t(3;7)(q10;q13), der(5)t(5;8)(q10;q1), der(7)t(7;10)(q10;q1), der(11)t(11;1)(q10;?), der(11)t(11;14)(q10;?), der(11)t(11;14)(q10;?), der(17)t(17;Y), der(18)t(18;6)(p10;q1), der(19)t(19;1)(q21;qter), indicates the occurrence of translocation and hyperdiploidy, none of them are known have clinical prognostic implications so far. Therefore, to improve the classification of the disease according to translocation rather than diploidy group in order to assign the correct prognostic implications, further cytogenetic studies to compare the disease stages combined with a series of clinical data for the evaluation of cases are necessary.
Accordingly, our findings suggest that the genes involved in the ring marker chromosomes could play a role in the early stage of tumor development. The correlation between the conjecture of the cytogenetic changes and tumor progression was also consistent with our previous PET findings. We detected that a 100% initial visual yield of invasive CCA was observed by the 22nd week in rats; however, a 50% yield rate of invasive CCA was observed by the 16th week, and the occurrence of biliary dysplasia and invasive CCA precedes the development of hepatic fibrosis by 4 wk[12].
In conclusion, the current CGCCA cell line was well established and characterized in order to obtain information regarding diagnostically useful tumor markers, which could shed light on a dark area of CCA tumorigenesis for a future understanding of human clinical therapeutics.
Cholangiocarcinoma is characterized by a great diversity of symptoms commonly occurring in the late course of the disease, and therefore making treatment puzzling. In addition, neither radiation therapy nor chemotherapy significantly improves long-term survival rates. However, many data have shown that the incidence and mortality rates of CCA have been rising worldwide over the past several decades, particularly the intrahepatic CCA. Therefore, the goal is to identify potential possible diagnostic biomarkers as the investigation of the molecular pathophysiology associated with this disease becomes more and more important and necessary. Herein, the authors developed the rat CCA tumor cells as a cell line designated as Chang Gung CCA (CGCCA).
Positive immunostaining of CK19, c-Met, COX-II, and MUC4 determined the phenotype of the cell line. The genotype was examined by cytogenetic studies, and the 2-Deoxy-2-(18F)fluoro-D-glucose-avid character of the CGCCA xenograft of the nude mice was demonstrated by animal PET. All of the evidence proved that the CGCCA cell line rat was derived from the original primary tumor formed by the TAA carcinogen.
The current work supports the view that the systematic cell cultures may provide a relevant CCA model to study the complex mechanisms involved in CCA by revealing the potential pathogenesis of this disease. In addition, the authors may be able to determine the possible diagnostic markers for the early detection and diagnosis of this disease.
The current CGCCA cell line was well established and characterized in order to obtain information regarding diagnostically useful tumor markers, which could shed light on a dark area of CCA tumorigenesis for a future understanding of human clinical therapeutics.
The authors describe the characterization of a new cholangiocarcinoma cell line model and report its successful implantation into nude mice. The paper is well-written, clear and concise.
| 1. | Liver Cancer Study Group of Japan. Classification of primary liver cancer. 1st English ed. Tokyo: Kanehara Shuppan 1997; . |
| 2. | Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384-391. |
| 3. | Uenishi T, Hirohashi K, Kubo S, Yamamoto T, Hamba H, Tanaka H, Kinoshita H. Histologic factors affecting prognosis following hepatectomy for intrahepatic cholangiocarcinoma. World J Surg. 2001;25:865-869. |
| 4. | Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-517; discussion 517-519. |
| 5. | de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368-1378. |
| 6. | Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357. |
| 7. | Shirabe K, Shimada M, Harimoto N, Sugimachi K, Yamashita Y, Tsujita E, Aishima S. Intrahepatic cholangiocarcinoma: its mode of spreading and therapeutic modalities. Surgery. 2002;131:S159-S164. |
| 8. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. |
| 9. | Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472-477. |
| 10. | Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. |
| 11. | Chang KY, Chang JY, Yen Y. Increasing incidence of intrahepatic cholangiocarcinoma and its relationship to chronic viral hepatitis. J Natl Compr Canc Netw. 2009;7:423-427. |
| 12. | Yeh CN, Maitra A, Lee KF, Jan YY, Chen MF. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25:631-636. |
| 13. | Lai GH, Sirica AE. Establishment of a novel rat cholangiocarcinoma cell culture model. Carcinogenesis. 1999;20:2335-2340. |
| 14. | Yeh CN, Pang ST, Chen TW, Wu RC, Weng WH, Chen MF. Expression of ezrin is associated with invasion and dedifferentiation of hepatitis B related hepatocellular carcinoma. BMC Cancer. 2009;9:233. |
| 15. | Weng WH, Wejde J, Ahlén J, Pang ST, Lui WO, Larsson C. Characterization of large chromosome markers in a malignant fibrous histiocytoma by spectral karyotyping, comparative genomic hybridization (CGH), and array CGH. Cancer Genet Cytogenet. 2004;150:27-32. |
| 16. | Shaffer LG, Tommerup N. An international system for human cytogenetic nomenclature. Basel: S. Karger 2005; . |
| 17. | Fritscher-Ravens A, Bohuslavizki KH, Broering DC, Jenicke L, Schäfer H, Buchert R, Rogiers X, Clausen M. FDG PET in the diagnosis of hilar cholangiocarcinoma. Nucl Med Commun. 2001;22:1277-1285. |
| 18. | Yeh CN, Lin KJ, Hsiao IT, Yen TC, Chen TW, Jan YY, Chung YH, Lin CF, Chen MF. Animal PET for thioacetamide-induced rat cholangiocarcinoma: a novel and reliable platform. Mol Imaging Biol. 2008;10:209-216. |
| 19. | Chen MF. Peripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatment. J Gastroenterol Hepatol. 1999;14:1144-1149. |
| 21. | Tengchaisri T, Prempracha N, Thamavit W, Boonpucknavig S, Sriurairatana S, Sirisinha S. Establishment and characterization of cell lines from liver fluke-associated cholangiocarcinoma induced in a hamster model. Southeast Asian J Trop Med Public Health. 1995;26:231-239. |
| 22. | Sirica AE. Biliary proliferation and adaptation in furan-induced rat liver injury and carcinogenesis. Toxicol Pathol. 1996;24:90-99. |
| 23. | Jan YY, Yeh TS, Yeh JN, Yang HR, Chen MF. Expression of epidermal growth factor receptor, apomucins, matrix metalloproteinases, and p53 in rat and human cholangiocarcinoma: appraisal of an animal model of cholangiocarcinoma. Ann Surg. 2004;240:89-94. |
| 24. | Yamaguchi N, Morioka H, Ohkura H, Hirohashi S, Kawai K. Establishment and characterization of the human cholangiocarcinoma cell line HChol-Y1 in a serum-free, chemically defined medium. J Natl Cancer Inst. 1985;75:29-35. |
| 25. | Miyagiwa M, Ichida T, Tokiwa T, Sato J, Sasaki H. A new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In Vitro Cell Dev Biol. 1989;25:503-510. |
| 26. | Sirisinha S, Tengchaisri T, Boonpucknavig S, Prempracha N, Ratanarapee S, Pausawasdi A. Establishment and characterization of a cholangiocarcinoma cell line from a Thai patient with intrahepatic bile duct cancer. Asian Pac J Allergy Immunol. 1991;9:153-157. |
| 27. | Shimizu Y, Demetris AJ, Gollin SM, Storto PD, Bedford HM, Altarac S, Iwatsuki S, Herberman RB, Whiteside TL. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252-260. |
| 28. | Elmore LW, Sirica AE. “Intestinal-type” of adenocarcinoma preferentially induced in right/caudate liver lobes of rats treated with furan. Cancer Res. 1993;53:254-259. |
| 29. | Radaeva S, Ferreira-Gonzalez A, Sirica AE. Overexpression of C-NEU and C-MET during rat liver cholangiocarcinogenesis: A link between biliary intestinal metaplasia and mucin-producing cholangiocarcinoma. Hepatology. 1999;29:1453-1462. |
| 30. | Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41-59. |
| 31. | Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180. |
| 32. | Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439-450. |
| 33. | Hansel DE, Rahman A, Hidalgo M, Thuluvath PJ, Lillemoe KD, Shulick R, Ku JL, Park JG, Miyazaki K, Ashfaq R. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am J Pathol. 2003;163:217-229. |
| 34. | Sirica AE, Lai GH, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol. 2001;16:363-372. |
| 35. | Muller D, Zimmerman SI, Schiller F. Drug metabolism in rat liver injured by thioacetamide. Arch Toxicol. 1982;5:368-371. |
| 36. | Dashti HM, Mathew TC, Jadaon MM, Ashkanani E. Zinc and liver cirrhosis: biochemical and histopathologic assessment. Nutrition. 1997;13:206-212. |
| 37. | Yeh CN, Pang ST, Wu RC, Chen TW, Jan YY, Chen MF. Prognostic value of MUC4 for mass-forming intrahepatic cholangiocarcinoma after hepatectomy. Oncol Rep. 2009;21:49-56. |
| 38. | Levi S, Urbano-Ispizua A, Gill R, Thomas DM, Gilbertson J, Foster C, Marshall CJ. Multiple K-ras codon 12 mutations in cholangiocarcinomas demonstrated with a sensitive polymerase chain reaction technique. Cancer Res. 1991;51:3497-3502. |
| 39. | Xu L, Hausmann M, Dietmaier W, Kellermeier S, Pesch T, Stieber-Gunckel M, Lippert E, Klebl F, Rogler G. Expression of growth factor receptors and targeting of EGFR in cholangiocarcinoma cell lines. BMC Cancer. 2010;10:302. |
| 40. | Ohashi K, Tstsumi M, Nakajima Y, Nakano H, Konishi Y. Ki-ras point mutations and proliferation activity in biliary tract carcinomas. Br J Cancer. 1996;74:930-935. |
| 41. | Hidaka E, Yanagisawa A, Seki M, Takano K, Setoguchi T, Kato Y. High frequency of K-ras mutations in biliary duct carcinomas of cases with a long common channel in the papilla of Vater. Cancer Res. 2000;60:522-524. |
Peer reviewer: Giuseppe Garcea, 13 Kinchley Close, Bradgate Heights, Leicester, LE3 9SE, United Kingdom
S- Editor Tian L L- Editor Rutherford A E- Editor Zheng XM