Published online Jun 21, 2011. doi: 10.3748/wjg.v17.i23.2791
Revised: March 8, 2011
Accepted: March 15, 2011
Published online: June 21, 2011
Irritable bowel syndrome (IBS) is defined by the Rome III criteria as symptoms of recurrent abdominal pain or discomfort with the onset of a marked change in bowel habits with no evidence of an inflammatory, anatomic, metabolic, or neoplastic process. As such, many clinicians regard IBS as a central nervous system problem of altered pain perception. Here, we review the recent literature and discuss the evidence that supports an organic based model, which views IBS as a complex, heterogeneous, inter-dependent, and multi-variable inflammatory process along the neuronal-gut axis. We delineate the organic pathophysiology of IBS, demonstrate the role of inflammation in IBS, review the possible differences between adult and pediatric IBS, discuss the merits of a comprehensive treatment model as taught by the Institute of Functional Medicine, and describe the potential for future research for this syndrome.
- Citation: Katiraei P, Bultron G. Need for a comprehensive medical approach to the neuro-immuno-gastroenterology of irritable bowel syndrome. World J Gastroenterol 2011; 17(23): 2791-2800
- URL: https://www.wjgnet.com/1007-9327/full/v17/i23/2791.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i23.2791
Functional abdominal pain (FAP) and irritable bowel syndrome (IBS) are debilitating and common conditions. IBS is defined by the Rome III criteria as, “symptoms of recurrent abdominal pain or discomfort and a marked change in bowel habit for at least six months, with symptoms experienced on at least three days of at least three months, with two of the three following findings: (1) Pain is relieved by a bowel movement; (2) Onset of pain is related to a change in frequency of stool; and (3) Onset of pain is related to a change in the appearance of stool”[1].
FAP occurs in 10%-15% of school-aged children, of which 17%-24% have pain significant enough to disrupt their activity, and 13%-15% awaken from their sleep due to the pain[2,3]. Up to 53% of children with abdominal pain continue to have abdominal pain as adults, and 18% are ultimately diagnosed with IBS[4].
Chronic abdominal pain is associated with significant morbidity, including depression[2], decreased quality of life measures[5], and disability leading to inability to work[6]. Adults with abdominal pain have higher rates of potentially unnecessary surgeries[7-10]. Patients with IBS and FAP are costly to the medical system[11,12]. Both children and adults with IBS frequently visit the offices of primary care physicians and gastroenterologists[13]. Adults with IBS have significantly more hospitalizations, outpatient visits, diagnostic testing, and overall medication use than well patients[14]. A large percentage of the medical costs associated with IBS are related to hospitalizations and inpatient diagnostic testing, such as endoscopies[15]. Antidepressants and other neuropharmacological agents help the symptoms of IBS[16], but these treatments have their own limitations and potential adverse effects.
IBS is thought to be just a functional problem that is “without demonstrable evidence of a pathological condition such as an anatomic, metabolic, infectious, inflammatory, or neoplastic disorder”[17]. IBS is seen as a non-organic syndrome, primarily involving altered perception and processing of pain. As a result, the majority of current therapies for IBS revolve around stress reduction, alteration of pain pathways, and alleviation of symptoms[16].
In this literature review, we delineate the gastrointestinal-neuro-immune pathophysiology of IBS and discuss the link between inflammation and pain. We believe that more effective treatment models are possible through a patient-centered approach that simultaneously treats the multiple variables that lead to IBS, as addressed in this review. The integration of this IBS treatment model may improve patient outcomes while reducing the medical cost burden of IBS. This paper will also discuss the possible differences between adult and pediatric IBS and present potential areas of future research.
Stress in various forms predisposes individuals to developing IBS[18-20] and increases IBS symptoms in children[21]. Abuse or other significant stressors change the neurobiology of stress and alters the levels of corticotropin-releasing factor (CRF) or hormone (CRH)[22], a hypothalamic stress hormone. CRF activates the pituitary-adrenal axis and mediates behavioral, autonomic, immune, and visceral responses to stress[23]. Patients with IBS have enhanced stress responses and release higher amounts of CRF in response to stress[24].
Stress changes the physiology of the gastrointestinal tract. Maternal separation of rat pups causes CRF-mediated mucosal barrier dysfunction with macromolecular permeability and increased bacterial adherence/penetration of the gastrointestinal mucosa with translocation to the spleen[25]. These animals also have mitochondrial swelling of the gut epithelial cells, immune cell infiltration, mucus depletion, and mast cell degranulation[23,26-29]. Stressed human beings show similar findings[30].
Stress compromises the integrity of the gut and induces inflammation through numerous pathways, as demonstrated by several published papers[22,28,31]. CRF released from the hypothalamus can directly influence human colonic mast cells[32,33], which then induce intestinal epithelial pathophysiology and mucosal barrier defects[34-37]. Substance P (SP) and calcitonin gene-related peptide (CGRP)-containing gastrointestinal efferent neurons can also influence mast cells[38-41] and result in degranulation[42] and release of TNF-α[43]. These compounds, in turn, result in gut inflammation and intestinal permeability[44].
These stress-induced changes in the gastrointestinal tract “persist after the stressor is removed from the animal”[37]. This is likely to be due to the ability of mast cells to influence their environment. In rats, inflammation results in increased mast cell-neuronal contacts and mucosal nerve cell density that last well beyond the initial insult[43,45]. Gastrointestinal inflammation in humans also results in neuron proliferation[46-48]. Stress and inflammation modulate nerve growth factor (NGF), which then affects mucosal nerve remodeling[49,50], sprouting, and synaptogenesis[51]. Mast cells, in close contact with neurons, synthesize and release NGF, and thus, can alter neuronal density and synaptogenesis[49,52].
Furthermore, inflammation preceding a psychological stress can alter the epithelial response to stress signals and make the gut more susceptible to stress[53]. In addition, inflammation can change the morphology of mast cells and their intracellular contents, further changing the susceptibility of the gut to various future stressors[54-57].
Inflammation can play an important part in the manifestation of IBS symptoms[58]. Once the inflammatory cascade is activated, this immune response can create a vicious cycle of self-perpetuating inflammation. Activated mast cells can directly release CRF[59]. Patients with inflammatory bowel disease (IBD) and IBS have CRF-immunoreactive macrophages, enterochromaffin cells, lymphocytes, neutrophils, and eosinophils, which are present in higher concentrations than in healthy controls[60-63]. CRF induces lymphocyte proliferation[64] and macrophage release of pro-inflammatory cytokines (TNF-α, IL-1, and IL-6)[65]. These activated immune cells, in turn, locally release CRF and other immune peptides[61,66], which then activate mast cells[22]. Mast cell-derived tryptase is another compound that recruits lymphocytes, eosinophils, and macrophages[67], and can further perpetuate inflammation.
Patients with IBS have central processing abnormalities associated with the perception of pain[68-71]. Colonic irritation can lead to visceral hypersensitivity[72]. Patients with IBS have inflammatory changes in their gut mucosa, which can only be identified by quantitative histopathology, immunohistochemistry, and electron microscopy[73]. These patients have increased numbers of mast cells in the mucosa of the colon[30,74,75]. Mast cell concentrations and their distance from mucosal nerve cells are positively associated with various IBS symptoms[75]. The tryptase released from these mast cells can directly activate gastrointestinal neurons in animals and humans, and can cause visceral hypersensitivity[76-78]. Tryptase cleaves and activates transmembrane proteins called proteinase-activated receptor-2 (PAR2), which are found on the primary afferent neurons of the gastrointestinal tract[79]. Activation of PAR2 receptors leads to neuronal activation, which then creates the experience of chronic pain.
In addition to central nervous system activation, patients with IBS also have sensitization and upregulation of the dorsal horn[70,71,80], which explains the cutaneous hyperalgesia found in the lower extremities, rather than upper extremities, due to viscerosomatic convergence of nociceptive afferent neurons from the colon/rectum and lower extremities[81]. Seybold et al[82] review the mechanisms by which gastrointestinal inflammation leads to gastrointestinal primary afferent neuronal activation and spinal cord activation/sensitization and inflammation.
If true, the perpetual mild mast-cell mediated inflammation can trigger the “excessive or prolonged stimulation of extrinsic afferents (that) may also result in the development of neuronal sensitization, at peripheral, spinal, or higher CNS levels, such that perception of sensations from the bowel is heightened, resulting in symptoms of urgency, bloating, and pain”[83]. This subclinical inflammation may also influence gastrointestinal serotonin pathways.
Serotonin (5-HT) can influence the motor function and sensitivity of the gastrointestinal tract[84-89]. Serotonin exerts a range of effects via its seven receptor subtypes (5-HT1 to 5HT-7). Serotonin receptor 5HT5, 5HT6, 5HT7 are found in the brain, whereas 5HT1, 5HT2, 5HT3, 5HT4, and 5HT7 are the gastrointestinal serotonin receptors[90]. A large majority of the body’s serotonin is stored in gastrointestinal enterochromaffin cells (EC)[85]. Patients with diarrhea predominant IBS have increased EC cells[91-93], which are activated by inflammation to release serotonin and may result in the elevated serotonin levels found in patients with IBS[94,95]. Tegaserod, a partial 5HT4 agonist has been used for constipation dominant IBS and Alosetron, a 5HT3 antagonist, in diarrhea dominant IBS.
Serotonin reuptake transporters (SERT) in the gut epithelial cells terminate the effects of serotonin[96,97] and influence serotonin concentrations and symptoms of IBS[85]. Patients with IBS have genetic polymorphisms that lead to lower expression of transport proteins and less serotonin reuptake[83,98,99]. The noted inflammation may also alter SERT expression and decrease its function in patients with IBS[100]. Further studies on the modulation of the gastrointestinal tract serotonin pathways may help further define and treat IBS.
The presence and activity of mast cells, along with other inflammatory cells, alone are not likely result in chronic inflammation. Other intestinal antigens, such as food, bacteria, and fungi, are likely to be needed to perpetuate the inflammation in the presence of an impaired gastrointestinal epithelial barrier.
Healthy individuals have tight junctions that help to form the gastrointestinal epithelial barrier along with mucous, SIgA, and other peptides. This epithelial barrier controls the interaction between luminal bacteria and antigens and the mucosal immune system[22,101]. It also allows immune tolerance of food antigens and bacteria. Activation of PAR2 not only leads to neuronal activation, but also to epithelial barrier defects in patients with IBS[102,103].
Low level PAR2 activation of the myosin light chain kinase (MLCK), causes phosphorylation of the myosin light chain, which then leads to contraction of the actin-myosin ring. Tight junction protein zona occludens-1 (ZO-1) relocalizes into the cytoplasm and disrupts the tight junctions, which increases paracellular permeability. High level PAR2 activation in the rat colon results in localized inflammation and increased production of TNF-α and IFN-γ. INF-γ decreases ZO-1 expression and alters the actin cytoskeleton organization[104]. TNF-α activates MLCK and results in tight junction protein relocation[105,106]. A more detailed discussion of these pathways can be found in articles by Gareau et al[23] and Cenac et al[103].
Children and adults with IBS have increased intestinal permeability[107,108]. Increased intestinal permeability results in “mucosal barrier defects (that) allow the passage of an increased load of luminal antigens of dietary and bacterial origin which, in turn, elicit the activation of mucosal immune responses”[109].
Various triggers can activate mast cells. Bacteria are powerful antigens for the gastrointestinal immune system[110-115]. Stress can result in increased bacterial adherence and penetration into the gastrointestinal mucosa[23,25-27], which may increase the interaction between the luminal bacteria and local immune response. This may explain why patients with IBS have higher antibody titers to specific bacterial flagella than healthy controls[116]. The DNA of these bacteria can interact with toll-like receptors[117], which then influence the immune system through regulation of tumor necrosis factor alpha and interferon gamma[118].
Escherichia coli, Campylobacter, and other bacteria can negatively influence the GI immune system and result in gastrointestinal inflammation and intestinal permeability[46,91,119-121]. Conversely, commercially available beneficial bacteria, in the form of probiotics, can reduce gastrointestinal inflammation[122-125], reverse or prevent intestinal permeability[120], and stop bacterial adhesion[126] and translocation[27,127]. Probiotics can also reverse visceral hypersensitivity from various causes[128,129], including stress[130]. Probiotics attenuate the upregulation of pain pathways at the spinal and supraspinal levels[131], and induce epithelial cells to express micro-opiate receptor 1 (MOR1) and cannabinoid 2 (CB2) opioid receptors[132]. Probiotics can reduce the symptoms of IBS[133,134].
Adults with IBS have gastrointestinal microflora that are significantly different than those of healthy populations[135]. Children with IBS are also likely to have significant alterations in their gastrointestinal microflora. We speculate that there may be a subset of children who are predisposed to developing IBS through repeated or prolonged exposure to antibiotics for various reasons (recurrent otitis media, sepsis, meningitis, osteomyelitis, vesicoureteral reflux, acne, etc). Various antibiotics, including Augmentin, the macrolides, and amoxicillin significantly alter the composition of the bacteria in the GI tract[136-138]. Antibiotic use has been related to increased rates of IBS and functional abdominal pain[139,140].
Gastrointestinal bacteria are also influenced by the diet. Dietary soluble fiber encourages the growth of beneficial species like lactobacilli and bifidobacteria[141-143]. In mice, a white bread diet significantly prolonged antibiotic induced bacterial perturbations[136]. It is common knowledge that the standard American diet lacks fiber, and thus may predispose human beings to have prolonged antibiotic induced bacterial perturbations.
Prebiotics are short chain carbohydrates that help some of the beneficial bacteria or probiotics in the intestines to grow more effectively[142,143]. Prebiotics may decrease IBS symptoms[144-146]. Prebiotics are fermented by probiotics and metabolized into short chain fatty acids (SCFA). SCFAs can decrease inflammation and are used in maintaining the intestinal epithelial lining[147]. While breast milk naturally contains prebiotics[148], up until a few years ago, most infant formulas did not contain prebiotics. Thus, there may be a population of children who were formula fed and required several courses of antibiotics that now have perturbed gastrointestinal flora, as well as intestinal epithelial barriers. We believe that these children may be at risk of developing IBS.
Food proteins are other significant antigens for the gut immune system. Food antigens induce mast cell activation[149] and degranulation, which can lead to visceral hypersensitivity. In children, certain foods may exacerbate intestinal permeability and the elimination of the foods help resolve the IBS symptoms[150]. Elimination of certain foods may decrease immune activation by removing the allergic antigenic load to the local immune system. In patients with IBS, sodium cromoglycate can eliminate IBS symptoms[151-153] by preventing the degranulation of mast cells and inhibiting the release of inflammatory mediators, following contact with an allergen[154].
Over 60% of patients believe that certain foods worsen their IBS symptoms and that elimination of these foods can reduce their symptoms[155-157]. Some believe that these food reactions are psychological in origin[158-160]. Blinded food challenges have raised many questions about the validity of elimination diets for IBS treatment[161-163]. There is also a growing body of evidence to support the use of elimination diets as part of a treatment protocol for IBS[164-167]. Milk, wheat, and eggs are the most commonly identified food triggers[163].
Another potential antigen for the gastrointestinal immune system is Candida albicans. Adult studies have shown that Candida does not play a significant role in patients with IBS[168,169]. To our knowledge, the role of candida in pediatric IBS has not been determined. Some children who have received numerous courses of antibiotics, such as amoxicillin, can have disruption of the bacterial balance and have overgrowth of the commensal Candida[137,170-173]. Candida induces inflammation. It produces alcohols and glycoproteins that stimulate mast cells to produce histamine and prostaglandins[174,175]. Candida also produces inflammatory prostaglandins that affect mammalian cells[176], as well as proteases that degrade the gastrointestinal IgA and, thus, allow candida to overcome the local immune defense mechanisms[177]. Candidal proteases can induce a B-cell response and result in increased inflammation[174]. In animals and humans, Candida perpetuates intestinal inflammation[169].
Another possible contributing factor to IBS signs and symptoms is small intestinal bacterial overgrowth (SIBO), defined as bacterial counts greater than 105 cf/mL from small intestinal aspirates[178]. Controversy exists over the ideal method of assessing SIBO[178-181]. A significant number of patients with IBS complain of bloating and pain. SIBO may explain this bloating and pain, as well as other IBS-like-symptoms[182-184]. Several studies have shown antibiotics to be helpful in reducing the symptoms of IBS[185-188].
Patients with IBS who have delayed gastric emptying have a higher risk of developing SIBO[189-192]. Stress is one cause of delayed gastric emptying[193-196]. Once SIBO is present, it can trigger an inflammatory response. SIBO, through abnormal gastrointestinal flora fermentation, may be another cause of IBS symptoms and must be considered in the evaluation of the patient. Furthermore, proton pump inhibitors can also increase the risk of SIBO by decreasing gastric acidity and further perturbations of the gastrointestinal flora species[170,197-200]. We speculate that SIBO may play a larger role in adults with IBS than in children. Further studies are required to elucidate the various differences between adult and pediatric IBS.
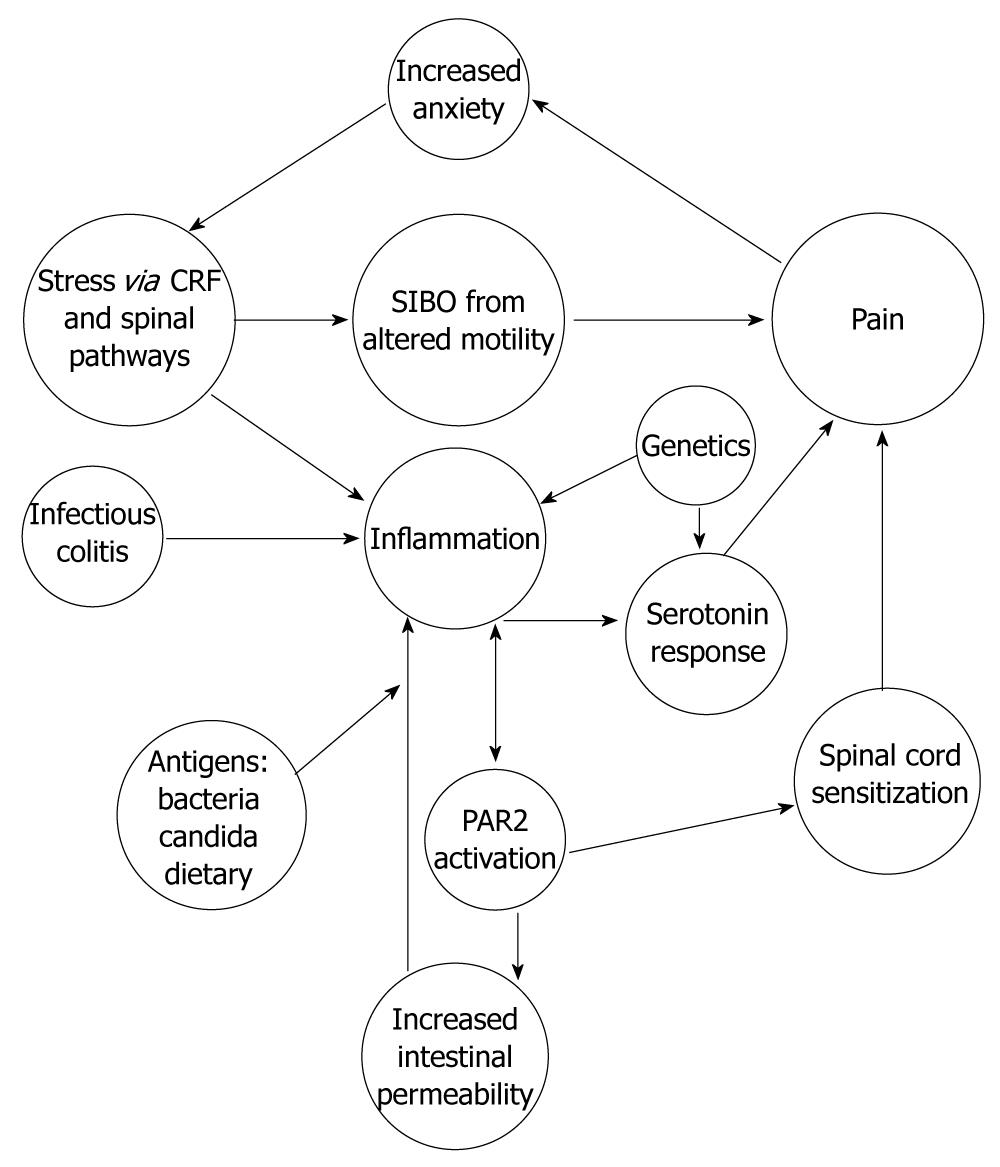
The evidence presented in our review suggests that IBS is an organic disease with a complex pathophysiology (Figure 1) that is difficult to identify by standard diagnostic tools. The pathophysiology of IBS varies from person to person and from children to adults. The underlying mast cell mediated inflammation of IBS, along with serotonin signaling, can drive the chronic nociceptive input from the periphery to dynamically maintain the altered central processing defects and perception of pain[70,80,201].
In addition to the pathophysiology, clinicians must focus more attention on the well known and less well characterized risk factors that may predispose individuals to developing IBS (Table 1). It is our belief that clinicians should further use the field of neurogastroenterology to better understand the effects of stress on the gastrointestinal tract. Clinicians and researchers must work to develop and adopt models to help us better predict and prevent this condition in susceptible individuals. For children, these models will require additional studies to evaluate the impact of recurrent antibiotic use and resultant overgrowth of candida on the development of IBS.
| Genetics/family history |
| Stress/high academic performance/parental psychiatric disorders |
| Recurrent or chronic antibiotic use |
| Bacterial or viral enteritis |
| Unrecognized food sensitivities |
| Low fiber diet/diet high in simple carbohydrates |
| Formula feeding |
| Chronic acid suppression |
Effective treatment models for IBS must reflect the complex physiology of IBS and simultaneously address multiple pathophysiological factors to break the vicious cycle of inflammation and ultimately allow for cessation of symptoms. The Institute of Functional Medicine (IFM)[202] has created such a model of care for IBS. The IFM model has the potential to provide significant improvement in patient care, while reducing healthcare costs and deserves further consideration and evaluation. Please refer to the IFM website and various publications for a more detailed discussion on treatment options.
| 1. | Available from: http://www.romecriteria.org. |
| 2. | Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr. 1996;129:220-226. |
| 3. | Hyams JS, Treem WR, Justinich CJ, Davis P, Shoup M, Burke G. Characterization of symptoms in children with recurrent abdominal pain: resemblance to irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 1995;20:209-214. |
| 4. | Christensen MF, Mortensen O. Long-term prognosis in children with recurrent abdominal pain. Arch Dis Child. 1975;50:110-114. |
| 5. | Frank L, Kleinman L, Rentz A, Ciesla G, Kim JJ, Zacker C. Health-related quality of life associated with irritable bowel syndrome: comparison with other chronic diseases. Clin Ther. 2002;24:675-689; discussion 674. |
| 6. | Creed F, Ratcliffe J, Fernandez L, Tomenson B, Palmer S, Rigby C, Guthrie E, Read N, Thompson D. Health-related quality of life and health care costs in severe, refractory irritable bowel syndrome. Ann Intern Med. 2001;134:860-868. |
| 7. | Longstreth GF, Yao JF. Irritable bowel syndrome and surgery: a multivariable analysis. Gastroenterology. 2004;126:1665-1673. |
| 8. | Hasler WL, Schoenfeld P. Systematic review: Abdominal and pelvic surgery in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:997-1005. |
| 9. | Talley NJ. Unnecessary abdominal and back surgery in irritable bowel syndrome: time to stem the flood now? Gastroenterology. 2004;126:1899-1903. |
| 11. | Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109:1736-1741. |
| 12. | Leong SA, Barghout V, Birnbaum HG, Thibeault CE, Ben-Hamadi R, Frech F, Ofman JJ. The economic consequences of irritable bowel syndrome: a US employer perspective. Arch Intern Med. 2003;163:929-935. |
| 13. | Lane MM, Weidler EM, Czyzewski DI, Shulman RJ. Pain symptoms and stooling patterns do not drive diagnostic costs for children with functional abdominal pain and irritable bowel syndrome in primary or tertiary care. Pediatrics. 2009;123:758-764. |
| 14. | Longstreth GF, Wilson A, Knight K, Wong J, Chiou CF, Barghout V, Frech F, Ofman JJ. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol. 2003;98:600-607. |
| 15. | Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. 2006;24:21-37. |
| 16. | Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367-378. |
| 17. | Chronic abdominal pain in children. Pediatrics. 2005;115:812-815. |
| 18. | White DL, Savas LS, Daci K, Elserag R, Graham DP, Fitzgerald SJ, Smith SL, Tan G, El-Serag HB. Trauma history and risk of the irritable bowel syndrome in women veterans. Aliment Pharmacol Ther. 2010;32:551-561. |
| 19. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-1988. |
| 20. | Alonso C, Guilarte M, Vicario M, Ramos L, Ramadan Z, Antoln M, Martnez C, Rezzi S, Saperas E, Kochhar S. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163-172.e1. |
| 21. | Walker LS, Garber J, Smith CA, Van Slyke DA, Claar RL. The relation of daily stressors to somatic and emotional symptoms in children with and without recurrent abdominal pain. J Consult Clin Psychol. 2001;69:85-91. |
| 22. | Kiank C, Tach Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: role of corticotropin-releasing factor receptors. Brain Behav Immun. 2010;24:41-48. |
| 23. | Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008;8:274-281. |
| 24. | Posserud I, Agerforz P, Ekman R, Bjrnsson ES, Abrahamsson H, Simrn M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102-1108. |
| 25. | Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res. 2006;59:83-88. |
| 26. | Mazzon E, Sturniolo GC, Puzzolo D, Frisina N, Fries W. Effect of stress on the paracellular barrier in the rat ileum. Gut. 2002;51:507-513. |
| 27. | Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553-1560. |
| 28. | Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Bueno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580:347-356. |
| 29. | Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007;293:G198-G203. |
| 30. | Piche T, Saint-Paul MC, Dainese R, Marine-Barjoan E, Iannelli A, Montoya ML, Peyron JF, Czerucka D, Cherikh F, Filippi J. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57:468-473. |
| 31. | Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy: implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116:207-235. |
| 32. | Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: stress, intestinal hyperpermeability and inflammation. World J Gastroenterol. 2007;13:3027-3030. |
| 33. | Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, Perdue MH, Sderholm JD. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50-58. |
| 34. | Santos J, Saunders PR, Hanssen NP, Yang PC, Yates D, Groot JA, Perdue MH. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol. 1999;277:G391-G399. |
| 35. | Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci. 2002;47:208-215. |
| 36. | Pothoulakis C, Castagliuolo I, Leeman SE. Neuroimmune mechanisms of intestinal responses to stress. Role of corticotropin-releasing factor and neurotensin. Ann N Y Acad Sci. 1998;840:635-648. |
| 37. | Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol. 1996;271:G884-G892. |
| 38. | Miceli PC, Jacobson K. Cholinergic pathways modulate experimental dinitrobenzene sulfonic acid colitis in rats. Auton Neurosci. 2003;105:16-24. |
| 39. | Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci USA. 1987;84:2975-2979. |
| 40. | Santos J, Guilarte M, Alonso C, Malagelada JR. Pathogenesis of irritable bowel syndrome: the mast cell connection. Scand J Gastroenterol. 2005;40:129-140. |
| 41. | De Jonge F, De Laet A, Van Nassauw L, Brown JK, Miller HR, van Bogaert PP, Timmermans JP, Kroese AB. In vitro activation of murine DRG neurons by CGRP-mediated mucosal mast cell degranulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G178-G191. |
| 42. | Janiszewski J, Bienenstock J, Blennerhassett MG. Picomolar doses of substance P trigger electrical responses in mast cells without degranulation. Am J Physiol. 1994;267:C138-C145. |
| 43. | Stead RH, Colley EC, Wang B, Partosoedarso E, Lin J, Stanisz A, Hillsley K. Vagal influences over mast cells. Auton Neurosci. 2006;125:53-61. |
| 44. | Wang L, Stanisz AM, Wershil BK, Galli SJ, Perdue MH. Substance P induces ion secretion in mouse small intestine through effects on enteric nerves and mast cells. Am J Physiol. 1995;269:G85-G92. |
| 45. | Stead RH, Kosecka-Janiszewska U, Oestreicher AB, Dixon MF, Bienenstock J. Remodeling of B-50 (GAP-43)- and NSE-immunoreactive mucosal nerves in the intestines of rats infected with Nippostrongylus brasiliensis. J Neurosci. 1991;11:3809-3821. |
| 46. | Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096-1101. |
| 47. | Di Sebastiano P, Fink T, Weihe E, Friess H, Beger HG, Bchler M. Changes of protein gene product 9.5 (PGP 9.5) immunoreactive nerves in inflamed appendix. Dig Dis Sci. 1995;40:366-372. |
| 48. | Leonard N, Hourihane DO, Whelan A. Neuroproliferation in the mucosa is a feature of coeliac disease and Crohn's disease. Gut. 1995;37:763-765. |
| 49. | Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582-590. |
| 50. | Stead RH. Nerve remodelling during intestinal inflammation. Ann N Y Acad Sci. 1992;664:443-455. |
| 51. | Burgos I, Cuello AC, Liberini P, Pioro E, Masliah E. NGF-mediated synaptic sprouting in the cerebral cortex of lesioned primate brain. Brain Res. 1995;692:154-160. |
| 52. | Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA. 1994;91:3739-3743. |
| 53. | Saunders PR, Miceli P, Vallance BA, Wang L, Pinto S, Tougas G, Kamath M, Jacobson K. Noradrenergic and cholinergic neural pathways mediate stress-induced reactivation of colitis in the rat. Auton Neurosci. 2006;124:56-68. |
| 54. | Lantz CS, Huff TF. Differential responsiveness of purified mouse c-kit mast cells and their progenitors to IL-3 and stem cell factor. J Immunol. 1995;155:4024-4029. |
| 55. | Rennick D, Hunte B, Holland G, Thompson-Snipes L. Cofactors are essential for stem cell factor-dependent growth and maturation of mast cell progenitors: comparative effects of interleukin-3 (IL-3), IL-4, IL-10, and fibroblasts. Blood. 1995;85:57-65. |
| 56. | Nakahata T, Toru H. Cytokines regulate development of human mast cells from hematopoietic progenitors. Int J Hematol. 2002;75:350-356. |
| 57. | Blennerhassett MG, Bienenstock J. Sympathetic nerve contact causes maturation of mast cells in vitro. J Neurobiol. 1998;35:173-182. |
| 58. | Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51 Suppl 1:i41-i44. |
| 59. | Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563-568. |
| 60. | Saito-Nakaya K, Hasegawa R, Nagura Y, Ito H, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol Motil. 2008;20:1147-1156. |
| 61. | Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918-932. |
| 62. | Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-1783. |
| 63. | Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392-400. |
| 64. | Singh VK. Stimulatory effect of corticotropin-releasing neurohormone on human lymphocyte proliferation and interleukin-2 receptor expression. J Neuroimmunol. 1989;23:257-262. |
| 65. | Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect Immun. 2002;70:6068-6074. |
| 66. | Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622-653. |
| 67. | He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159:6216-6225. |
| 68. | Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99-110. |
| 69. | Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64-72. |
| 70. | Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002;4:322-328. |
| 71. | Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529-535. |
| 72. | Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276-1285. |
| 73. | De Giorgio R, Barbara G. Is irritable bowel syndrome an inflammatory disorder? Curr Gastroenterol Rep. 2008;10:385-390. |
| 74. | Park JH, Rhee PL, Kim HS, Lee JH, Kim YH, Kim JJ, Rhee JC. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:71-78. |
| 75. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. |
| 76. | Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37. |
| 77. | Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137:1425-1434. |
| 78. | Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636-647. |
| 79. | Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579-621. |
| 80. | Moshiree B, Zhou Q, Price DD, Verne GN. Central sensitisation in visceral pain disorders. Gut. 2006;55:905-908. |
| 81. | Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7-14. |
| 82. | Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;451-491. |
| 83. | Yeo A, Boyd P, Lumsden S, Saunders T, Handley A, Stubbins M, Knaggs A, Asquith S, Taylor I, Bahari B. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452-1458. |
| 84. | Spiller R. Serotonin, inflammation, and IBS: fitting the jigsaw together? J Pediatr Gastroenterol Nutr. 2007;45 Suppl 2:S115-S119. |
| 85. | Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698-2709. |
| 86. | Lesurtel M, Soll C, Graf R, Clavien PA. Role of serotonin in the hepato-gastroIntestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 2008;65:940-952. |
| 87. | Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13 Suppl 2:15-30. |
| 88. | Crowell MD. The role of serotonin in the pathophysiology of irritable bowel syndrome. Am J Manag Care. 2001;7:S252-S260. |
| 89. | Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285-1293. |
| 90. | Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47-55. |
| 91. | Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662-1671. |
| 92. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. |
| 93. | Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651-1659. |
| 94. | Wang H, Steeds J, Motomura Y, Deng Y, Verma-Gandhu M, El-Sharkawy RT, McLaughlin JT, Grencis RK, Khan WI. CD4T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949-957. |
| 95. | Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34-43. |
| 96. | Chen JX, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol. 1998;275:G433-G448. |
| 97. | Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352-2364. |
| 98. | Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-1664. |
| 99. | Kohen R, Jarrett ME, Cain KC, Jun SE, Navaja GP, Symonds S, Heitkemper MM. The serotonin transporter polymorphism rs25531 is associated with irritable bowel syndrome. Dig Dis Sci. 2009;54:2663-2670. |
| 100. | Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067-1076. |
| 101. | Kraehenbuhl JP, Corbett M. Immunology. Keeping the gut microflora at bay. Science. 2004;303:1624-1625. |
| 102. | Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bhm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci USA. 1997;94:8884-8889. |
| 103. | Cenac N, Chin AC, Garcia-Villar R, Salvador-Cartier C, Ferrier L, Vergnolle N, Buret AG, Fioramonti J, Bueno L. PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways. J Physiol. 2004;558:913-925. |
| 104. | Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795-804. |
| 105. | Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496-G504. |
| 106. | Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422-G430. |
| 107. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. |
| 108. | Barau E, Dupont C. Modifications of intestinal permeability during food provocation procedures in pediatric irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 1990;11:72-77. |
| 109. | Barbara G. Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am J Gastroenterol. 2006;101:1295-1298. |
| 110. | Rautava S, Walker WA. Commensal bacteria and epithelial cross talk in the developing intestine. Curr Gastroenterol Rep. 2007;9:385-392. |
| 111. | Tlaskalova-Hogenova H, Tuckova L, Mestecky J, Kolinska J, Rossmann P, Stepankova R, Kozakova H, Hudcovic T, Hrncir T, Frolova L. Interaction of mucosal microbiota with the innate immune system. Scand J Immunol. 2005;62 Suppl 1:106-113. |
| 112. | Kelly D, Conway S. Bacterial modulation of mucosal innate immunity. Mol Immunol. 2005;42:895-901. |
| 113. | Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernndez-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359-2364. |
| 114. | Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224-5231. |
| 115. | Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945-953. |
| 116. | Schoepfer AM, Schaffer T, Seibold-Schmid B, Mller S, Seibold F. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol Motil. 2008;20:1110-1118. |
| 117. | Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520-528. |
| 118. | Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, De Simone C, Madsen K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358-1373. |
| 119. | Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873-1882. |
| 120. | Mangell P, Nejdfors P, Wang M, AhrnS , Westrm B, Thorlacius H, Jeppsson B. Lactobacillus plantarum 299v inhibits Escherichia coli-induced intestinal permeability. Dig Dis Sci. 2002;47:511-516. |
| 121. | Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374-G379. |
| 122. | McCarthy J, O'Mahony L, O'Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O'Sullivan GC, Kiely B, Collins JK. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975-980. |
| 123. | Zoumpopoulou G, Foligne B, Christodoulou K, Grangette C, Pot B, Tsakalidou E. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int J Food Microbiol. 2008;121:18-26. |
| 124. | Peran L, Sierra S, Comalada M, Lara-Villoslada F, Bailn E, Nieto A, Concha A, Olivares M, Zarzuelo A, Xaus J. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007;97:96-103. |
| 125. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. |
| 126. | Gopal PK, Prasad J, Smart J, Gill HS. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int J Food Microbiol. 2001;67:207-216. |
| 127. | Mangell P, Lennerns P, Wang M, Olsson C, Ahrn S, Molin G, Thorlacius H, Jeppsson B. Adhesive capability of Lactobacillus plantarum 299v is important for preventing bacterial translocation in endotoxemic rats. APMIS. 2006;114:611-618. |
| 128. | Verd EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55:182-190. |
| 129. | Liebregts T, Adam B, Bertel A, Jones S, Schulze J, Enders C, Sonnenborn U, Lackner K, Holtmann G. Effect of E. coli Nissle 1917 on post-inflammatory visceral sensory function in a rat model. Neurogastroenterol Motil. 2005;17:410-414. |
| 130. | Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthsy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901-1907. |
| 131. | Ait-Belgnaoui A, Eutamene H, Houdeau E, Bueno L, Fioramonti J, Theodorou V. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterol Motil. 2009;21:567-573, e18-e19. |
| 132. | Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35-37. |
| 133. | Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. |
| 134. | Barbara G, Stanghellini V, Cremon C, De Giorgio R, Gargano L, Cogliandro R, Pallotti F, Corinaldesi R. Probiotics and irritable bowel syndrome: rationale and clinical evidence for their use. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2:S214-S217. |
| 135. | Kassinen A, Krogius-Kurikka L, Mkivuokko H, Rinttil T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24-33. |
| 136. | Dubos R, Schaedler RW, Stephens M. The effect of antibacterial drugs on the fecal flora of mice. J Exp Med. 1963;117:231-243. |
| 137. | Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001;1:101-114. |
| 138. | Edlund C, Nord CE. Effect on the human normal microflora of oral antibiotics for treatment of urinary tract infections. J Antimicrob Chemother. 2000;46 Suppl 1:41-48; discussion 63-65. |
| 139. | Mendall MA, Kumar D. Antibiotic use, childhood affluence and irritable bowel syndrome (IBS). Eur J Gastroenterol Hepatol. 1998;10:59-62. |
| 140. | Maxwell PR, Rink E, Kumar D, Mendall MA. Antibiotics increase functional abdominal symptoms. Am J Gastroenterol. 2002;97:104-108. |
| 141. | Blaut M. Relationship of prebiotics and food to intestinal microflora. Eur J Nutr. 2002;41 Suppl 1:I11-I16. |
| 142. | Delzenne NM. Oligosaccharides: state of the art. Proc Nutr Soc. 2003;62:177-182. |
| 143. | Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J Nutr. 1999;129:1438S-1441S. |
| 144. | Giannini EG, Mansi C, Dulbecco P, Savarino V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition. 2006;22:334-342. |
| 145. | Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508-518. |
| 146. | Paineau D, Payen F, Panserieu S, Coulombier G, Sobaszek A, Lartigau I, Brabet M, Galmiche JP, Tripodi D, Sacher-Huvelin S. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br J Nutr. 2008;99:311-318. |
| 147. | Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006;24:701-714. |
| 148. | Newburg DS. Oligosaccharides in human milk and bacterial colonization. J Pediatr Gastroenterol Nutr. 2000;30 Suppl 2:S8-S17. |
| 149. | Marshall JS. Repeated antigen challenge in rats induces a mucosal mast cell hyperplasia. Gastroenterology. 1993;105:391-398. |
| 150. | Barau E, Dupont C. Modifications of intestinal permeability during food provocation procedures in pediatric irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 1990;11:72-77. |
| 151. | Grazioli I, Melzi G, Balsamo V, Castellucci G, Castro M, Catassi C, Rtsch JM, Scotta S. [Food intolerance and irritable bowel syndrome of childhood: clinical efficacy of oral sodium cromoglycate and elimination diet]. Minerva Pediatr. 1993;45:253-258. |
| 152. | Lunardi C, Bambara LM, Biasi D, Cortina P, Peroli P, Nicolis F, Favari F, Pacor ML. Double-blind cross-over trial of oral sodium cromoglycate in patients with irritable bowel syndrome due to food intolerance. Clin Exp Allergy. 1991;21:569-572. |
| 153. | Stefanini GF, Prati E, Albini MC, Piccinini G, Capelli S, Castelli E, Mazzetti M, Gasbarrini G. Oral disodium cromoglycate treatment on irritable bowel syndrome: an open study on 101 subjects with diarrheic type. Am J Gastroenterol. 1992;87:55-57. |
| 154. | Zar S, Kumar D, Benson MJ. Food hypersensitivity and irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15:439-449. |
| 155. | Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60:667-672. |
| 156. | Dainese R, Galliani EA, De Lazzari F, Di Leo V, Naccarato R. Discrepancies between reported food intolerance and sensitization test findings in irritable bowel syndrome patients. Am J Gastroenterol. 1999;94:1892-1897. |
| 157. | Nanda R, James R, Smith H, Dudley CR, Jewell DP. Food intolerance and the irritable bowel syndrome. Gut. 1989;30:1099-1104. |
| 158. | Teufel M, Biedermann T, Rapps N, Hausteiner C, Henningsen P, Enck P, Zipfel S. Psychological burden of food allergy. World J Gastroenterol. 2007;13:3456-3465. |
| 159. | Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109:1204-1214. |
| 160. | Rief W, Barsky AJ. Psychobiological perspectives on somatoform disorders. Psychoneuroendocrinology. 2005;30:996-1002. |
| 161. | Farah DA, Calder I, Benson L, MacKenzie JF. Specific food intolerance: its place as a cause of gastrointestinal symptoms. Gut. 1985;26:164-168. |
| 162. | Bernstein M, Day JH, Welsh A. Double-blind food challenge in the diagnosis of food sensitivity in the adult. J Allergy Clin Immunol. 1982;70:205-210. |
| 163. | Niec AM, Frankum B, Talley NJ. Are adverse food reactions linked to irritable bowel syndrome? Am J Gastroenterol. 1998;93:2184-2190. |
| 164. | Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464. |
| 165. | Zar S, Mincher L, Benson MJ, Kumar D. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand J Gastroenterol. 2005;40:800-807. |
| 166. | Stefanini GF, Saggioro A, Alvisi V, Angelini G, Capurso L, di Lorenzo G, Dobrilla G, Dodero M, Galimberti M, Gasbarrini G. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand J Gastroenterol. 1995;30:535-541. |
| 167. | Jones VA, McLaughlan P, Shorthouse M, Workman E, Hunter JO. Food intolerance: a major factor in the pathogenesis of irritable bowel syndrome. Lancet. 1982;2:1115-1117. |
| 168. | Middleton SJ, Coley A, Hunter JO. The role of faecal Candida albicans in the pathogenesis of food-intolerant irritable bowel syndrome. Postgrad Med J. 1992;68:453-454. |
| 169. | Zwolinska-Wcislo M, Brzozowski T, Budak A, Kwiecien S, Sliwowski Z, Drozdowicz D, Trojanowska D, Rudnicka-Sosin L, Mach T, Konturek SJ. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J Physiol Pharmacol. 2009;60:107-118. |
| 170. | O'May GA, Reynolds N, Smith AR, Kennedy A, Macfarlane GT. Effect of pH and antibiotics on microbial overgrowth in the stomachs and duodena of patients undergoing percutaneous endoscopic gastrostomy feeding. J Clin Microbiol. 2005;43:3059-3065. |
| 171. | Loy CE. Antibiotic-associated diarrhoea: an overlooked aetiology? Br J Biomed Sci. 2005;62:166-169. |
| 172. | Song HJ, Shim KN, Jung SA, Choi HJ, Lee MA, Ryu KH, Kim SE, Yoo K. Antibiotic-associated diarrhea: candidate organisms other than Clostridium difficile. Korean J Intern Med. 2008;23:9-15. |
| 173. | Maraki S, Mouzas IA, Kontoyiannis DP, Chatzinikolaou I, Tselentis Y, Samonis G. Prospective evaluation of the impact of amoxicillin, clarithromycin and their combination on human gastrointestinal colonization by Candida species. Chemotherapy. 2001;47:215-218. |
| 174. | Santelmann H, Howard JM. Yeast metabolic products, yeast antigens and yeasts as possible triggers for irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2005;17:21-26. |
| 175. | Nosál R, Novotný J, Sikl D. The effect of glycoprotein from Candida albicans on isolated rat mast cells. Toxicon. 1974;12:103-108. |
| 176. | Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69:2957-2963. |
| 177. | Reinholdt J, Krogh P, Holmstrup P. Degradation of IgA1, IgA2, and S-IgA by Candida and Torulopsis species. Acta Pathol Microbiol Immunol Scand C. 1987;95:265-274. |
| 179. | Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci. 2008;53:1443-1454. |
| 180. | Corazza GR, Menozzi MG, Strocchi A, Rasciti L, Vaira D, Lecchini R, Avanzini P, Chezzi C, Gasbarrini G. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302-309. |
| 181. | Ghoshal UC, Ghoshal U, Das K, Misra A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J Gastroenterol. 2006;25:6-10. |
| 182. | Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852-858. |
| 183. | Parodi A, Dulbecco P, Savarino E, Giannini EG, Bodini G, Corbo M, Isola L, De Conca S, Marabotto E, Savarino V. Positive glucose breath testing is more prevalent in patients with IBS-like symptoms compared with controls of similar age and gender distribution. J Clin Gastroenterol. 2009;43:962-966. |
| 184. | Lin HC, Pimentel M. Bacterial concepts in irritable bowel syndrome. Rev Gastroenterol Disord. 2005;5 Suppl 3:S3-S9. |
| 185. | Majewski M, Reddymasu SC, Sostarich S, Foran P, McCallum RW. Efficacy of rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci. 2007;333:266-270. |
| 186. | Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139-142. |
| 187. | Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503-3506. |
| 188. | Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412-419. |
| 189. | Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47:2639-2643. |
| 190. | Husebye E. The pathogenesis of gastrointestinal bacterial overgrowth. Chemotherapy. 2005;51 Suppl 1:1-22. |
| 191. | Nieuwenhuijs VB, Verheem A, van Duijvenbode-Beumer H, Visser MR, Verhoef J, Gooszen HG, Akkermans LM. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg. 1998;228:188-193. |
| 192. | Reddymasu SC, McCallum RW. Small intestinal bacterial overgrowth in gastroparesis: are there any predictors? J Clin Gastroenterol. 2010;44:e8-e13. |
| 193. | Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1037-G1044. |
| 194. | Martnez V, Wang L, Rivier JE, Vale W, Tach Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611-617. |
| 195. | Martinez V, Wang L, Million M, Rivier J, Tach Y. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733-1744. |
| 196. | Million M, Maillot C, Saunders P, Rivier J, Vale W, Tach Y. Human urocortin II, a new CRF-related peptide, displays selective CRF(2)-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G34-G40. |
| 198. | Thorens J, Froehlich F, Schwizer W, Saraga E, Bille J, Gyr K, Duroux P, Nicolet M, Pignatelli B, Blum AL. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54-59. |
| 199. | Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557-561. |
| 200. | Spiegel BM, Chey WD, Chang L. Bacterial overgrowth and irritable bowel syndrome: unifying hypothesis or a spurious consequence of proton pump inhibitors? Am J Gastroenterol. 2008;103:2972-2976. |
| 201. | Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175-194. |
| 202. |
|
Peer reviewer: Dr. Wei-Dong Tong, MD, PhD, Associate Professor, Daping Hospital, Third Military Medical University, Chongqing 400042, China
S- Editor Tian L L- Editor Stewart GJ E- Editor Ma WH