Published online Jun 7, 2011. doi: 10.3748/wjg.v17.i21.2632
Revised: March 23, 2011
Accepted: March 30, 2011
Published online: June 7, 2011
AIM: To investigate the effect of keratinocyte growth factor (KGF) gene therapy in acetic acid-induced ulcerative colitis in rat model.
METHODS: The colitis of Sprague-Dawley rats was induced by intrarectal infusion of 1 mL 5% (v/v) acetic acid. Twenty-four hours after exposed to acetic acid, rats were divided into three experimental groups: control group, attenuated Salmonella typhimurium Ty21a strain (SP) group and SP strain carrying human KGF gene (SPK) group, and they were separately administered orally with 10% NaHCO3, SP or SPK. Animals were sacrificed and colonic tissues were harvested respectively on day 3, 5, 7 and 10 after administration. Weights of rats, colonic weight/length ratio and stool score were evaluated. Histological changes of colonic tissues were examined by hematoxylin and eosin (HE) staining method. The expression of KGF, KGF receptor (KGFR) and TNF-α were measured either by enzyme-linked immunosorbent assay or Western blotting. Immunohistochemistry was used to detect the cellular localization of KGFR and Ki67. In addition, superoxide dismutase (SOD) activity and malondialdehyde (MDA) contents in the homogenate were measured.
RESULTS: Body weight and colonic weight/length ratio were declined in SPK group compared with SP and control groups (body weight: 272.78 ± 17.92 g vs 243.72 ± 14.02 g and 240.68 ± 12.63 g, P < 0.01; colonic weight/length ratio: 115.76 ± 7.47 vs 150.32 ± 5.99 and 153.67 ± 5.50 mg/cm, P < 0.01). Moreover, pathological changes of damaged colon were improved in SPK group as well. After administration of SPK strain, KGF expression increased markedly from the 3rd d, and remained at a high level till the 10th d. Furthermore, KGFR expression and Ki67 expression elevated, whereas TNF-α expression was inhibited in SPK group. In the group administered with SPK, SOD activity increased significantly (d 5: 26.18 ± 5.84 vs 18.12 ± 3.30 and 18.79 ± 4.74 U/mg, P < 0.01; d 7: 35.48 ± 3.35 vs 22.57 ± 3.44 and 21.69 ± 3.94 U/mg, P < 0.01; d 10: 46.10 ± 6.23 vs 25.35 ± 4.76 and 27.82 ± 6.42 U/mg, P < 0.01) and MDA contents decreased accordingly (d 7: 7.40 ± 0.88 vs 9.81 ± 1.21 and 10.45 ± 1.40 nmol/mg, P < 0.01; d 10: 4.36 ± 0.62 vs 8.41 ± 0.92 and 8.71 ± 1.27 nmol/mg, P < 0.01), compared with SP and control groups.
CONCLUSION: KGF gene therapy mediated by attenuated Salmonella ameliorates ulcerative colitis induced by acetic acids, and it may be a safe and effective treatment for ulcerative colitis.
- Citation: Liu CJ, Jin JD, Lv TD, Wu ZZ, Ha XQ. Keratinocyte growth factor gene therapy ameliorates ulcerative colitis in rats. World J Gastroenterol 2011; 17(21): 2632-2640
- URL: https://www.wjgnet.com/1007-9327/full/v17/i21/2632.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i21.2632
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder in gastrointestinal tract mainly involving ulcerative colitis (UC) and Crohn’s disease (CD). It is characterized by tissue edema, inflammatory response and increased gut epithelial permeability[1,2]. Although many studies of IBD in vivo and vitro have been reported, the pathogenesis of IBD is still not completely understood. As it is reported, IBD may be related to a complex interaction of immune system, genetic and environmental factors[3,4]. Conventional drugs, such as corticosteroids and 5-aminosalicylate preparations, are effective in the treatment of IBD, however, long-term medication would induce severe side effects that have harmful impact on life quality of patients[5-7]. Hence, it is necessary to develop new approaches with fewer side effects for treatment of IBD.
Keratinocyte growth factor (KGF), a member of the fibroblast growth factor family (FGFs), could specifically stimulate proliferation and differentiation of various epithelial cells of different organs by a paracrine fashion including skin, lung, intestine and bladder[8]. In patients with IBD, which was mainly located in the lamina propria of gastrointestinal tract, endogenous KGF expression was increased markedly[9,10]. Previous studies indicated that KGF could promote proliferation and differentiation of intestinal epithelial cells both in vivo and in vitro, and protect the intestine[11,12]. Therefore, KGF may be a potential cytokine to ameliorate IBD. However, the recombinant protein of cytokines is limited in clinical application because of their instability, high dosage and repeated administration which increased the incidence of side effects[13]. We attempted to develop an oral gene therapy of KGF mediated by attenuated Salmonella typhimurium to improve the rat acetic acid-induced colitis, which has similar characteristics to human ulcerative colitis[14].
Attenuated Salmonella typhimurium Ty21a strain (SP) and SP strain carrying human KGF gene (SPK) were constructed by electrotransformation with empty pCDNA3 plasmid (Invitrogen, Carlsbad, CA, USA) or recombinant pCDNA3 plasmid carrying human KGF gene.
Female Sprague-Dawley rats (aged 8-12 wk, n = 80, weighing 250-300 g), were purchased from the Animal Experimental Center of Lanzhou University (Lanzhou, China). They were housed in wire-bottom cages in a temperature-controlled room with a 12 h light-dark cycle. Rats were acclimatized to the environment for 7 d before experiment. All animal experiments were conducted following the institutional guidelines and approved by the Ethical Committee for Animal Care and Use, the General Hospital of Lanzhou Military Command.
Six rats were randomly assigned into normal group and 74 rats into experimental groups. Animals of experimental groups were anesthetized by intraperitoneal injection with pentobarbital sodium (30 mg/kg). A plastic catheter was inserted into the colon at 8 cm proximal to the anus, and 1 mL of 5% (v/v) acetic acid was infused into the rat colon. After 30 s, 2 mL saline was infused to remove the residual acid. Twenty-four hours after exposure to acetic acid, the rats were randomly divided into three groups: control group (n = 24), SP group (n = 25) and SPK group (n = 25). They were treated respectively with 1 mL solvent of 10% NaHCO3, 1.0 × 108 colony forming unit (CFU) of SP strains, or SPK strains by gavage once every other day. Six rats of each group were sacrificed separately on day 3, 5, 7 and 10 after the first administration. The colons were quickly removed, freeze-clamped and dropped in liquid N2 for various assays
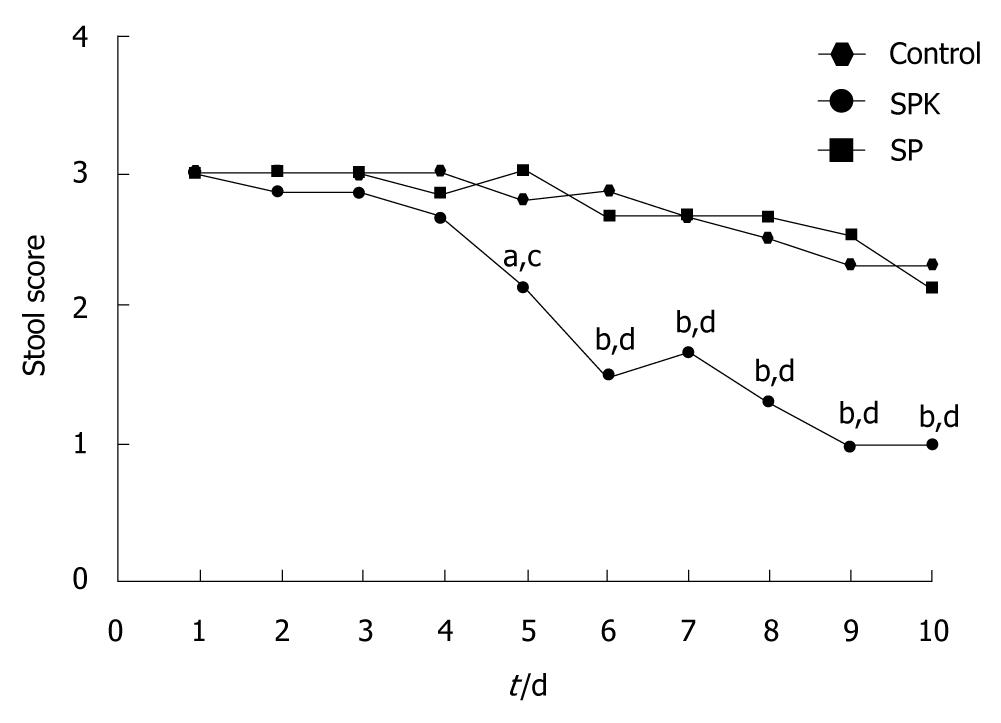
The stool examination of each group was scored daily from day 1 to day 10 after drug administration. The score of stool was defined as 1 for normal, 2 for soft stool and 3 for watery diarrhea.
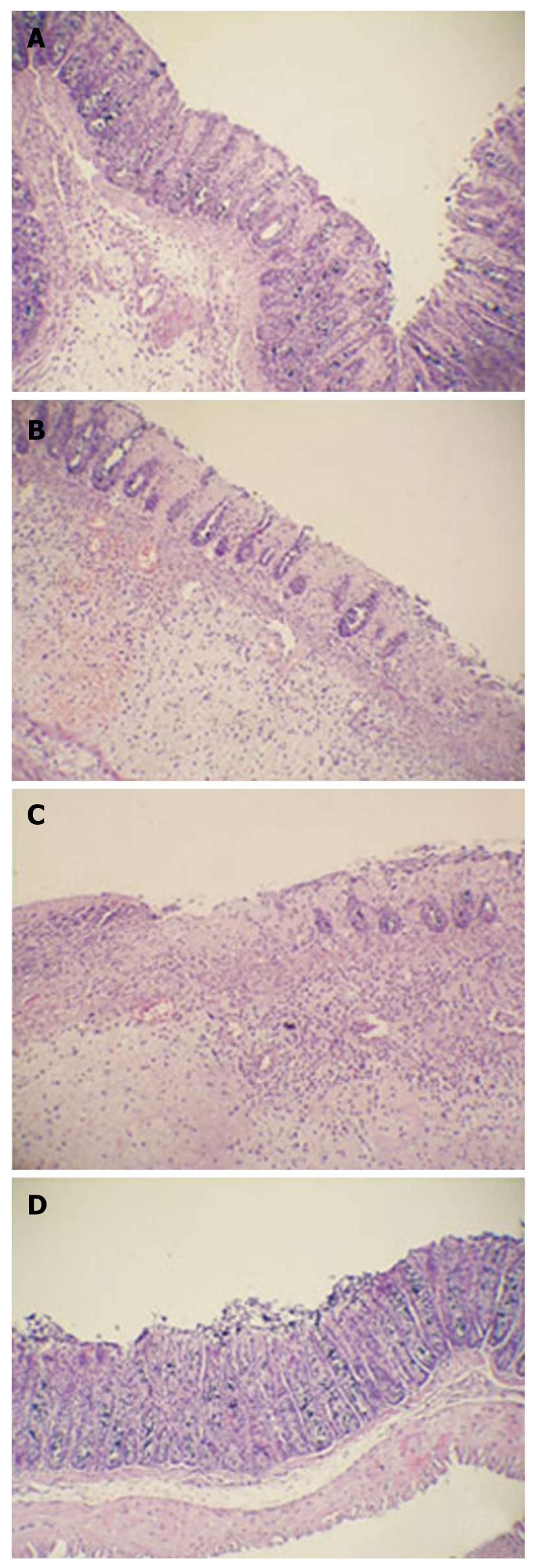
Histological analysis of rat tissues was performed on day 10. The colon tissues were fixed in 10% formalin, embedded in paraffin and sectioned at a thickness of 6 μm. Sections were stained with hematoxylin and eosin (HE) and evaluated under light microscope.
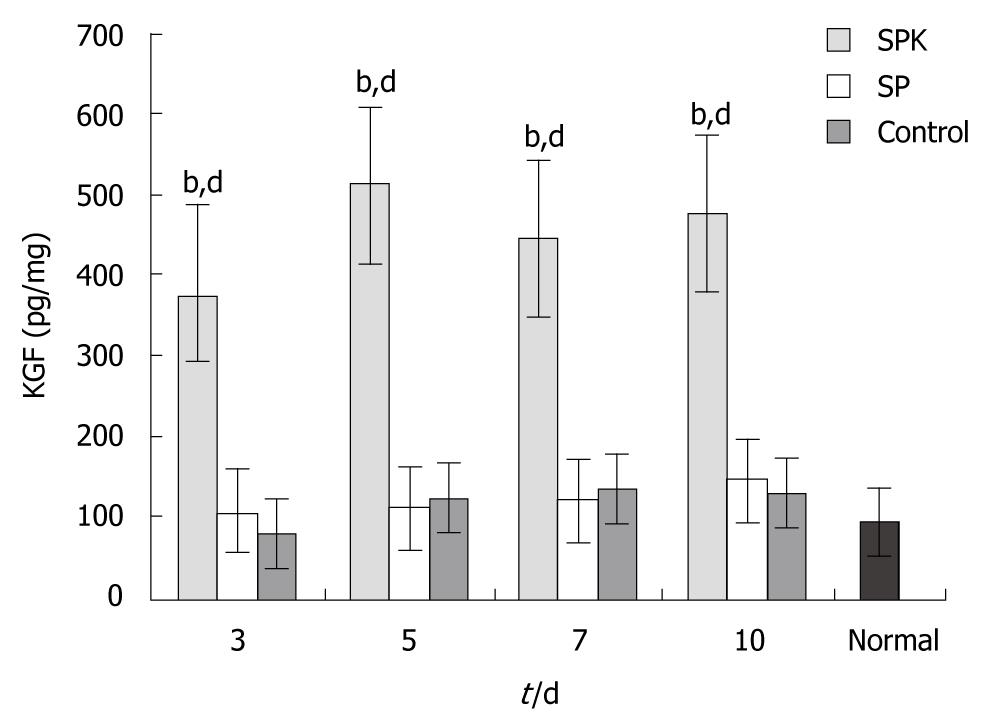
The colon tissues were homogenized with nine-fold volume of ice-cold phosphate-buffered saline (PBS) using a glass homogenizer at 4°C. The homogenate was centrifuged at 855 ×g for 10 min at 4°C to remove the cell debris and the supernatant was obtained to determine the KGF and TNF-α content with Enzyme-linked immunosorbent assay (ELISA) kits (R and D, Minneapolis, MN, USA) according to the manufacturer’s instructions. The optical density was detected at 450 nm with a microplate reader (Thermo, Pittsburgh, PA, USA).
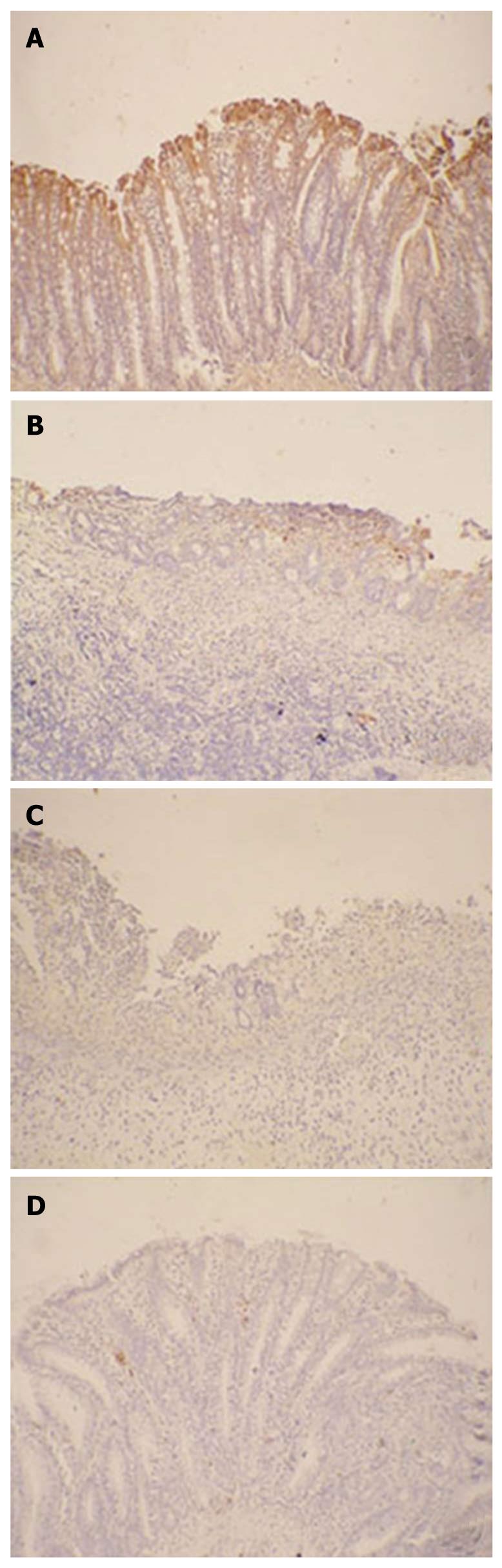
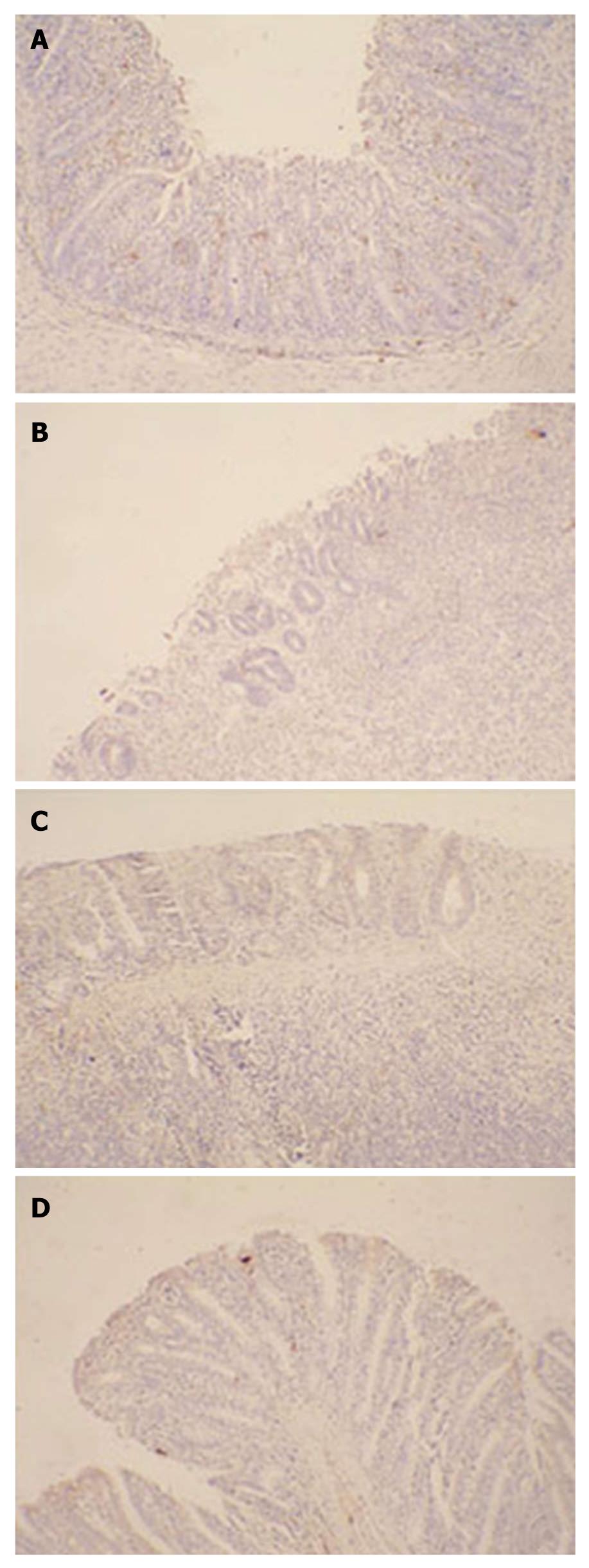
Immunohistochemistry for KGFR and Ki67 was performed as follows. The slides were incubated with a rabbit anti-rat primary antibody to KGFR (1:500, R and D, Minneapolis, MN, USA) or Ki67 (1:500, R and D, Minneapolis, MN, USA) in PBS at 4°C overnight. After 3 washes in PBS, the sections were incubated with a horse radish peroxidase (HRP)-conjugated goat anti-rabbit antibody (Beijing Zhongshan Biotechnology, Beijing, China) at a 1:1000 dilution in PBS at 37°C for 2 h. The sections were then incubated with an avidin-biotin-peroxidase complex (Beijing Zhongshan Biotechnology, Beijing, China), followed by DAB (Beijing Zhongshan Biotechnology, Beijing, China) to induce a color reaction. The expression and localization of the KGFR and Ki67 were examined under light microscope (Olympus, Japan), and a brown color was indicated as positive.
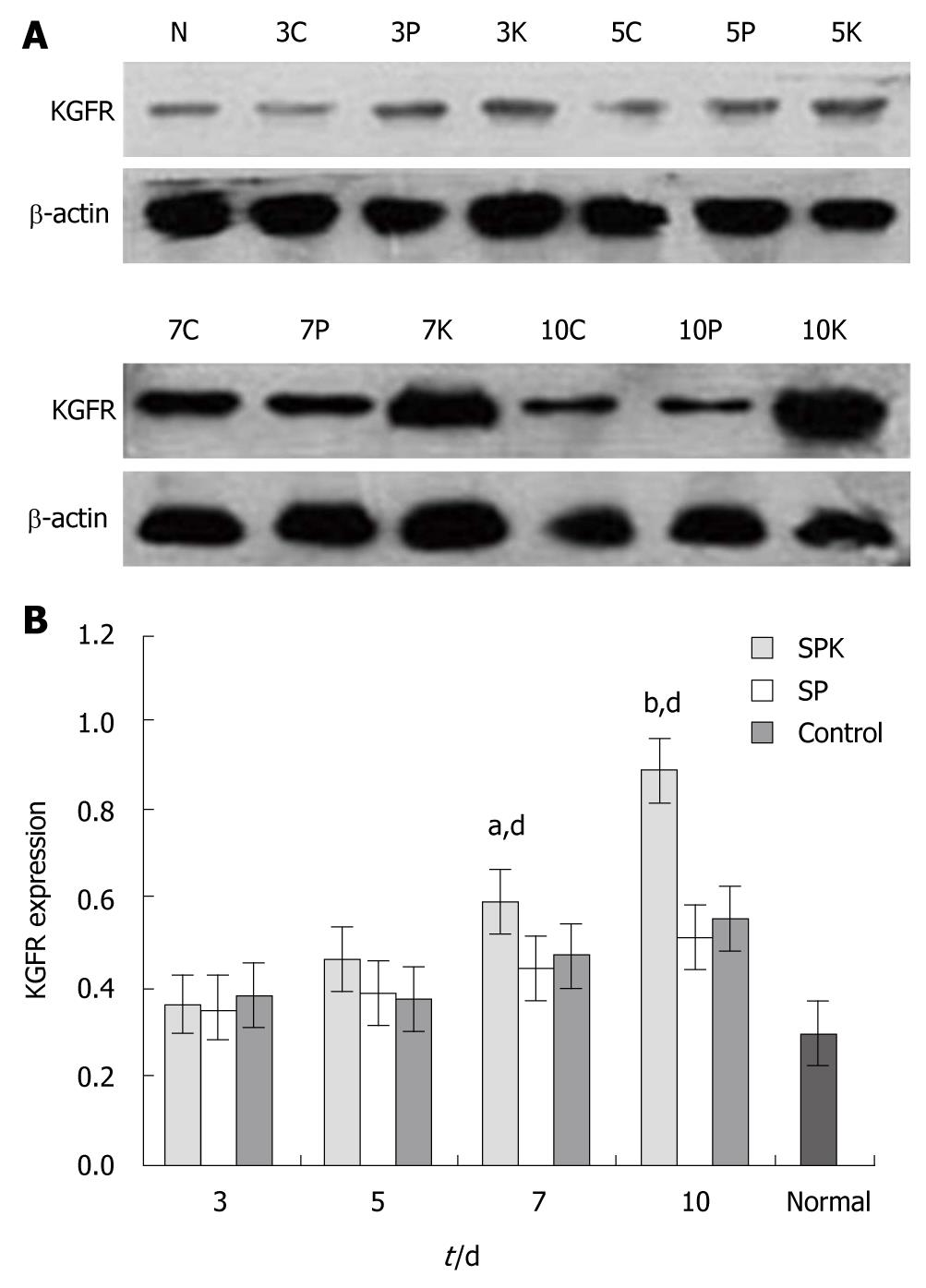
To determine the effect of SPK strain on the KGFR in colon tissues, we detected the expression of KGFR using Western blotting techniques. The colonic homogenates were obtained as previously described, and the total protein in homogenates was quantified with the bicinchoninic acid (BCA) protein assay kit (Beijing Biosynthesis Biotechnology Beijing, China). Twenty μg of the total protein was resolved in SDS-PAGE, and protein was transferred onto PVDF membrane (Milipore, Temecula, CA, USA) electrophoretically at 80 mA for 2 h at 4°C. The membranes were blocked in 5% skim milk (w/v, in Tris-Buffered Saline with Tween-20 (TBST) for 2 h at room temperature. After 3 washes with TBST, the membranes were incubated with a rabbit anti-rat KGFR (1:500, R and D, Minneapolis, MN USA) and a rabbit anti-rat β-actin antibody (1:1000, R and D, Minneapolis, MN USA) overnight at 4°C, respectively. After 3 washes with TBST, the membranes were incubated with a HRP-conjugated goat anti-rabbit antibody (Beijing Zhongshan Biotechnology, Beijing, China) at a 1:2000 dilution for 3 h at 37°C. Bands on the membranes were imaged by X-ray with chemoluminescence reagents (Beijing Solarbio Science and Technology, Beijing, China). Finally, the bands were scanned and quantified using the Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
The colonic homogenates were performed as mentioned above, and superoxide dismutase (SOD) activity and malondialdehyde (MDA) contents were determined by spectrophotometry according to the manufacturer’s protocol. SOD and MDA detection kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
The data from different groups at various time points were analyzed using one-way ANOVA with SPSS 11.5 software (IBM, Chicago, IL, USA). P < 0.05 was considered statistically significant, and data were presented as mean ± SD.
The rat body weight on day 10, as shown in Table 1, was significantly reduced (P < 0.05) in the SP and control groups, compared with the normal group. However, the body weight loss almost recovered in SPK group. The increase of the colonic weight/length ratio, an index of colonic edema associated with acetic acid-induced inflammatory reaction, was inhibited in SPK group compared with the SP and control groups (P < 0.01, Table 1). However, it was still higher than that in normal group (P < 0.05, Table 1).
Diarrhea is one of the clinical signs of intestinal inflammation, so the effect of administration of SPK on the stool score was investigated. After treatment with acetic acid, diarrhea occurred in all groups on day 1. However, administration of SPK strain resulted in an obvious improvement of stool score on day 5 (P < 0.05, Figure 1), and a normal level was achieved on day 9 in SPK group.
Histological sections were examined separately by two experienced observers. In SP and control groups, remarkable inflammatory cell infiltration and edema of the lamina propria were observed. The number of goblet cells dramatically decreased. Moreover, severely damaged mucous glands and thinner mucous layer were found in these two groups, and local hyperemia with thicker lamina propria was presented as well (Figure 2B and C). In contrast, the histological damage induced by acetic acid was improved by administration of SPK strain. Hyperemia was not found in the lamina propria, and inflammatory cell infiltration and edema were decreased in SPK group. The intestinal architecture recovered and glands arranged more regularly, as compared with the SP and control groups (Figure 2A).
To investigate whether KGF could be expressed effectively in this gene therapy, the KGF concentration in the homogenates was measured by ELISA. In SPK group, the KGF concentration increased significantly at all time points, and peaked on day 5 (514.73 ± 103.30 pg/mg, Figure 3). The high level of KGF expression maintained between days 3 and 10. The result of high expression of KGF in SPK group demonstrated that Salmonella typhimurium Ty21a was an effective vector in the gene therapy on ulcerative colitis.
The effect of SPK on the expression of KGFR was confirmed by both Western blotting and immunohistochemistry. After exposure to acetic acid, the expression of KGFR in the colon tissues increased by more than 2-folds of the normal level (Figure 4). In SPK group, the expression of KGFR increased significantly on day 7 compared with the SP and control groups (P < 0.05, Figure 4B), and it increased by approximately 5-folds of the normal level on day 10 (P < 0.01, Figure 4B). As shown by immunohistochemistry, the expression of KGFR was located mainly in the epithelial lamina, and it was kept high in all groups, which was consistent with that detected by Western blotting (Figure 5).
To detect whether administration of SPK strain could promote intestinal epithelial cells proliferation, Ki67 expression, one of the markers of cell proliferation, was investigated by immunohistochemistry. Ten days after administration of SPK, the Ki67 expression in the epithelial lamina increased significantly compared with the SP and control groups (Figure 6).
As revealed in Table 2, the decreased SOD activity induced by acetic acid was inhibited significantly by administration of SPK strain on day 5 (P < 0.01), and the SOD activity of SPK group became similar to the normal group on the 10th d. MDA contents increased significantly due to the inhibition of SOD activity induced by acetic acid, and reached to 4-folds approximately of the normal level (Table 3). However, administration of SPK strain resulted in a remarkable decrease of MDA contents (P < 0.01, Table 3) on day 7, compared with the SP group and control group. The decrease of MDA contents maintained until day 10, although they were still higher than normal levels (P < 0.01, Table 3).
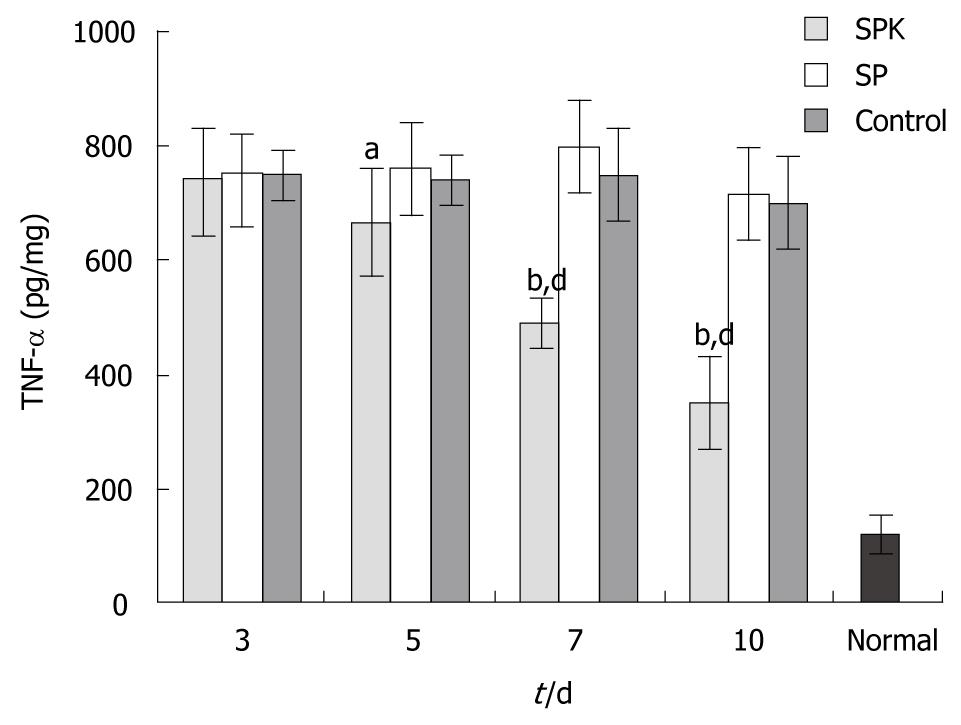
To examine the effect of SPK strain on the expression of proinflammatory cytokine, the TNF-α level in colon was measured by ELISA. The TNF-α level of colon tissues elevated dramatically after exposure to acetic acid (Figure 7). However, administration of SPK strain significantly inhibited the overexpression of TNF-α between days 5 and 10, as compared with the SP and control groups (P < 0.01, Figure 7).
Ulcerative colitis is one of the refractory diseases, and there are still no specific and ideal drugs and therapies for this disease. In this study, we focused on the gene therapy of KGF mediated by attenuated Salmonella typhimurium Ty21a. Our results showed that oral administration of SPK strain ameliorated the colitis induced by acetic acid in rat model. Furthermore, no side effects induced by administration Salmonella typhimurium Ty21a, were detected. This gene therapy is potential for the treatment of ulcerative colitis.
Keratinocyte growth factor receptor (KGFR/FGFR2-IIIb), a splice variant of FGFR2, is a transmembrane tyrosine kinase receptor encoded by the bek gene[15]. KGFR, with a high affinity to KGF, expresses mainly in epithelial cell lineages, so KGF specifically stimulates epithelial cells via binding with KGFR. KGF promotes the intestinal epithelial cell proliferation and differentiation in a paracrine fashion[11]. KGF may help maintain and restore the integrity of the intestinal mucosa after injury[16]. And local administration of KGF could alleviate the inflammatory and promote the re-epithelialization and adaptative process after proctocolectomy[10]. In addition, the expression of KGF and KGFR in the intestinal tissues of IBD patients was enhanced significantly [16], suggesting that the interaction of KGF and KGFR may play a crucial role in the progress of IBD. In this study, we found that the expression of KGF increased remarkably compared with the control group at all time points after administration of SPK strain, and the expression of KGFR in SPK group also elevated obviously on day 7. The expression of Ki67, one of the markers of cell proliferation[17], in the epithelial lamina increased in SPK group, which indicated that KGF might directly promote the restoration of intestinal epithelial cells via binding with KGFR in the ulcerative colitis.
Many factors are involved in the process of the pathogenesis of IBD, although the accurate mechanisms are still unclear. The damage induced by reactive oxygen species (ROS) is one of the important factors. Accumulation of ROS in ulcerative colon tissues stimulates inflammation responses and secretion of proinflammatory cytokines, such as TNF-α, IL-1 and IL-6[18,19]. ROS also impairs the integrity of the intestinal epithelial cells and increases the intestinal mucosal permeability, which subsequently attenuates the barrier function and host defense to exogenous bacteria and microorganisms[20,21]. In addition, ROS could induce DNA damage and stimulate activation of NF-κB that plays an important role in inflammation responses[19,22]. As it was reported previously, ROS levels increased significantly in IBD patients[23]. There are two main reasons for the ROS increase. The first one is that the release of ROS from lymphocytes, macrophages and neutrophils increases in the process of IBD; and the second one is the endogenous antioxidant enzymes, such as SOD and GSH, reduce and lead to the accumulation of ROS[22]. SOD is able to counteract ROS by catalyzing ROS to oxygen (O2) and hydrogen peroxide (H2O2) which is subsequently decomposed to water (H2O) and oxygen (O2)[24]. At present, many therapies based on SOD have been applied in treatment of IBD. Suzuki et al[25] reported that PC-SOD (40 mg or 80 mg daily) is able to improve UC rapidly. In addition, SOD gene therapy mediated by Lactobacillus gasser also ameliorates the IBD in IL10-deficient mice via reducing the infiltration[26].
The present study showed that the SOD activity increased significantly on the 5th d after administration of SPK strain compared with the SP and control groups (P < 0.01, Table 2). The increase of SOD activity may be associated with KGF-induced promotion of the proliferation and repairing of intestinal epithelial cells[16], which secreted SOD in regenerative intestinal tissues[26]. MDA, one of the direct products of ROS, induces lipid peroxidation and its contents are usually used as a marker for free radicals-induced lipid peroxidation[19]. In this study, MDA contents in SPK group decreased significantly on day 7, accompanied with the increase of SOD activity. These results demonstrated that administration of SPK strain could protect intestinal tissues from damages induced by ROS.
TNF-α, mainly from activated macrophages in the gastrointestinal tract, plays an important role in the pathogenesis of IBD. It directly induces apoptosis of epithelial cells, promotes production of oxygen free radicals (ROS), interferes with the intestinal epithelial barrier, activates neutrophils and macrophages, and causes inflammation as well[27,28]. Moreover, by activating transcription factor nuclear factor κB (NF-κB) signal pathway, TNF-α indirectly induces production and secretion of many inflammatory cytokines and chemokines involving IL-6, IL-12, IL-18, IFN-γ and MMPs[27,29,30]. The expression of TNF-α in IBD patients increases dramatically, and Infliximab, an anti-TNF-α monoclonal antibody, has been applied clinically to treat Crohn’s disease and ulcerative colitis[31]. The TNF-α levels in colon tissues are often used as a biomarker of the severity of colitis[29]. Similarly, the expression of TNF-α increased dramatically in the damaged colonic tissues induced by acetic acids in this study. However, in SPK group, TNF-α level decreased significantly on day 7, which demonstrated that administration of SPK strain could ameliorate rat ulcerative colitis resulting from inhibition of TNF-α secretion. It has been reported that TNF-α could induce excessive expression of KGF in the intestinal tissues[32]. KGF, in return, could down-regulate the TNF-α expression in the lung or intestinal tract injury induced by bone marrow transplantation[33,34]. This is consistent with the results in this study. All these suggest that there may be a negative feedback mechanism between TNF-α and KGF.
Attenuated Salmonella typhimurium is one of the prevalent bacterial vectors in gene therapy and oral DNA vaccines, which could transfer multiple exogenous gene into host cells effectively[35,36]. In vivo, live attenuated Salmonella strains penetrate into the intestinal epithelial barrier via M cells and macrophages, and reach the Peyer’s patches, where the targeting gene would be expressed. The exogenous gene would subsequently be expressed in the host cells[37]. Attenuated Salmonella has been proved to be a simple, effective and safe vector in gene therapy[36,38]. In addition, production of bacteria is relatively simple and low in cost compared with other vectors, such as virus and liposome. We chose the attenuated Salmonella typhimurium Ty21a as the vector of KGF gene therapy, an aroA mutant strain that is safe and effective for human and has been approved for human use by the U.S. FDA[39,40]. In the present study, no adverse effects were observed in the animals administrated orally with SPK and SP strain. Therefore, this attenuated Salmonella strain is safe for treatment of ulcerative colitis.
In conclusion, the oral gene therapy of KGF could improve the colitis physiologically and pathologically in rat model and this therapy may be a potential, safe and efficient for ulcerative colitis.
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder in gastrointestinal tract mainly involving ulcerative colitis (UC) and Crohn’s disease (CD). It is characterized by tissue edema, inflammation and diarrhea. Although conventional drugs, such as corticosteroids and 5-aminosalicylate preparations, are effective in the treatment of IBD, long-term medication would induce severe side effects, affecting the life quality of patients. Keratinocyte growth factor (KGF), a member of the fibroblast growth factor family (FGFs), could specifically stimulate proliferation and differentiation of various epithelial cells of different organs including skin, lung, intestine and bladder. So, the authors hypothesized that KGF could repair the damaged intestinal mucosa and relieve the inflammation.
Previous studies indicated that KGF could promote proliferation and differentiation of intestinal epithelial cells both in vivo and in vitro, and protect the intestine. However, the recombinant proteins of cytokines are limited in clinical usage because of their instability.
To avoid the disadvantages of protein drugs, the authors attempted to develop an oral gene therapy of KGF mediated by attenuated Salmonella typhimurium to improve the ulcerative colitis in rats with acetic acid-induced colitis.
The oral gene therapy of KGF could improve the colitis physiologically and pathologically in rat model, and this therapy may be potential, safe and efficient for ulcerative colitis.
Oral administration of the attenuated Salmonella typhimurium Ty21a strain carrying KGF gene could effectively relieve the ulcerative colitis induced by acetic acid, through promoting the proliferation of intestinal mucosal cells, inhibiting the expression of tumor necrosis factor-α, a inflammatory factor, and protecting the intestinal tissues from the damage of reactive oxygen species.
| 1. | Akcan A, Kucuk C, Sozuer E, Esel D, Akyildiz H, Akgun H, Muhtaroglu S, Aritas Y. Melatonin reduces bacterial translocation and apoptosis in trinitrobenzene sulphonic acid-induced colitis of rats. World J Gastroenterol. 2008;14:918-924. |
| 2. | Zhang HQ, Ding TT, Zhao JS, Yang X, Zhang HX, Zhang JJ, Cui YL. Therapeutic effects of Clostridium butyricum on experimental colitis induced by oxazolone in rats. World J Gastroenterol. 2009;15:1821-1828. |
| 3. | Luchini AC, Rodrigues-Orsi P, Cestari SH, Seito LN, Witaicenis A, Pellizzon CH, Di Stasi LC. Intestinal anti-inflammatory activity of coumarin and 4-hydroxycoumarin in the trinitrobenzenesulphonic acid model of rat colitis. Biol Pharm Bull. 2008;31:1343-1350. |
| 4. | Konturek PC, Brzozowski T, Engel M, Burnat G, Gaca P, Kwiecien S, Pajdo R, Konturek SJ. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J Physiol Pharmacol. 2009;60:41-47. |
| 5. | Dong WG, Liu SP, Yu BP, Wu DF, Luo HS, Yu JP. Ameliorative effects of sodium ferulate on experimental colitis and their mechanisms in rats. World J Gastroenterol. 2003;9:2533-2538. |
| 6. | Kang JW, Kim TW, La JH, Sung TS, Kim HJ, Kwon YB, Kim JY, Yang IS. Electroacupuncture ameliorates experimental colitis induced by acetic acid in rat. J Vet Sci. 2004;5:189-195. |
| 7. | Lindberg A, Eberhardson M, Karlsson M, Karlén P. Long-term follow-up with Granulocyte and Monocyte Apheresis re-treatment in patients with chronically active inflammatory bowel disease. BMC Gastroenterol. 2010;10:73. |
| 8. | Prince LS, Karp PH, Moninger TO, Welsh MJ. KGF alters gene expression in human airway epithelia: potential regulation of the inflammatory response. Physiol Genomics. 2001;6:81-89. |
| 9. | Finch PW, Cheng AL. Analysis of the cellular basis of keratinocyte growth factor overexpression in inflammatory bowel disease. Gut. 1999;45:848-855. |
| 10. | Otte JM, Boser S, Brunke G, Kiehne K, Schmitz F, Banasiewicz T, Drews M, Schmidt WE, Herzig KH. Expression of keratinocyte growth factor and its receptor in adaptive changes of ileorectal pouch mucosa. Scand J Gastroenterol. 2005;40:1066-1075. |
| 11. | Visco V, Bava FA, d'Alessandro F, Cavallini M, Ziparo V, Torrisi MR. Human colon fibroblasts induce differentiation and proliferation of intestinal epithelial cells through the direct paracrine action of keratinocyte growth factor. J Cell Physiol. 2009;220:204-213. |
| 12. | Sasaki M, FitzGerald AJ, Mandir N, Berlanga-Acosta J, Goodlad RA. Keratinocyte growth factor and epidermal growth factor can reverse the intestinal atrophy associated with elemental diets in mice. Exp Physiol. 2003;88:261-267. |
| 13. | Hamady ZZ, Scott N, Farrar MD, Lodge JP, Holland KT, Whitehead T, Carding SR. Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut. 2010;59:461-469. |
| 14. | Sung TS, La JH, Kim TW, Yang IS. Alteration of nitrergic neuromuscular transmission as a result of acute experimental colitis in rat. J Vet Sci. 2006;7:143-150. |
| 15. | Liu JJ, Shay JW, Wilson SE. Characterization of a soluble KGF receptor cDNA from human corneal and breast epithelial cells. Invest Ophthalmol Vis Sci. 1998;39:2584-2593. |
| 16. | Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 2002;99:14338-14343. |
| 17. | Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736-750. |
| 18. | Ishihara T, Tanaka K, Tasaka Y, Namba T, Suzuki J, Ishihara T, Okamoto S, Hibi T, Takenaga M, Igarashi R. Therapeutic effect of lecithinized superoxide dismutase against colitis. J Pharmacol Exp Ther. 2009;328:152-164. |
| 19. | Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M, Lv P, An P, Liu SQ, Yu HG. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm. 2006;2006:92642. |
| 20. | Liu XC, Mei Q, Xu JM, Hu J. Balsalazine decreases intestinal mucosal permeability of dextran sulfate sodium-induced colitis in mice. Acta Pharmacol Sin. 2009;30:987-993. |
| 21. | Kurutas EB, Cetinkaya A, Bulbuloglu E, Kantarceken B. Effects of antioxidant therapy on leukocyte myeloperoxidase and Cu/Zn-superoxide dismutase and plasma malondialdehyde levels in experimental colitis. Mediators Inflamm. 2005;2005:390-394. |
| 22. | Liu LN, Mei QB, Liu L, Zhang F, Liu ZG, Wang ZP, Wang RT. Protective effects of Rheum tanguticum polysaccharide against hydrogen peroxide-induced intestinal epithelial cell injury. World J Gastroenterol. 2005;11:1503-1507. |
| 23. | Oku T, Iyama S, Sato T, Sato Y, Tanaka M, Sagawa T, Kuribayashi K, Sumiyosi T, Murase K, Machida T. Amelioration of murine dextran sulfate sodium-induced colitis by ex vivo extracellular superoxide dismutase gene transfer. Inflamm Bowel Dis. 2006;12:630-640. |
| 24. | Krieglstein CF, Cerwinka WH, Laroux FS, Salter JW, Russell JM, Schuermann G, Grisham MB, Ross CR, Granger DN. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med. 2001;194:1207-1218. |
| 25. | Suzuki Y, Matsumoto T, Okamoto S, Hibi T. A lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Colorectal Dis. 2008;10:931-934. |
| 26. | Carroll IM, Andrus JM, Bruno-Bárcena JM, Klaenhammer TR, Hassan HM, Threadgill DS. Anti-inflammatory properties of Lactobacillus gasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G729-G738. |
| 27. | Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77-97. |
| 28. | Liu SP, Dong WG, Wu DF, Luo HS, Yu JP. Protective effect of angelica sinensis polysaccharide on experimental immunological colon injury in rats. World J Gastroenterol. 2003;9:2786-2790. |
| 29. | Li JH, Yu JP, Yu HG, Xu XM, Yu LL, Liu J, Luo HS. Melatonin reduces inflammatory injury through inhibiting NF-kappaB activation in rats with colitis. Mediators Inflamm. 2005;2005:185-193. |
| 30. | Horie A, Nagai K, Ohkura S, Ohama T, Komatsu H, Sato K. Proinflammatory cytokines suppress the expression level of protease-activated receptor-2 through the induction of iNOS in rat colon. J Vet Med Sci. 2009;71:1609-1615. |
| 32. | MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2-9. |
| 33. | Haddad IY, Panoskaltsis-Mortari A, Ingbar DH, Resnik ER, Yang S, Farrell CL, Lacey DL, Cornfield DN, Blazar BR. Interactions of keratinocyte growth factor with a nitrating species after marrow transplantation in mice. Am J Physiol. 1999;277:L391-L400. |
| 34. | Krijanovski OI, Hill GR, Cooke KR, Teshima T, Crawford JM, Brinson YS, Ferrara JL. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood. 1999;94:825-831. |
| 35. | Fu W, Lan H, Li S, Han X, Gao T, Ren D. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2'-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther. 2008;15:474-484. |
| 36. | Qi H, Li YH, Zheng SB. [Oral gene therapy via live attenuated Salmonella leads to tumor regression and survival prolongation in mice]. Nanfang Yikedaxue Xuebao. 2006;26:1738-1741. |
| 37. | Yuhua L, Kunyuan G, Hui C, Yongmei X, Chaoyang S, Xun T, Daming R. Oral cytokine gene therapy against murine tumor using attenuated Salmonella typhimurium. Int J Cancer. 2001;94:438-443. |
| 38. | Ryan RM, Green J, Williams PJ, Tazzyman S, Hunt S, Harmey JH, Kehoe SC, Lewis CE. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther. 2009;16:329-339. |
| 39. | Walker MJ, Rohde M, Timmis KN, Guzmán CA. Specific lung mucosal and systemic immune responses after oral immunization of mice with Salmonella typhimurium aroA, Salmonella typhi Ty21a, and invasive Escherichia coli expressing recombinant pertussis toxin S1 subunit. Infect Immun. 1992;60:4260-4268. |
Peer reviewer: Yuji Naito, Associate Professor, Department of Gastroenterology and Hepatology, Kyoto Prefectural University of Medicine, 465-Kajiicho, Kamigyoku, Kyoto 602-8566, Japan
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH