Published online May 7, 2011. doi: 10.3748/wjg.v17.i17.2223
Revised: September 18, 2010
Accepted: September 25, 2010
Published online: May 7, 2011
AIM: To examine the efficacy of glycyrrhizin preparation (GL-p) in the treatment of a rat model of ulcerative colitis (UC).
METHODS: Experimental colitis was induced by oral administration of dextran sodium sulfate. Rats with colitis were intrarectally administered GL-p or saline. The extent of colitis was evaluated based on body weight gain, colon wet weight, and macroscopic damage score. The expression levels of pro-inflammatory cytokines and chemokines in the inflamed mucosa were measured by cytokine antibody array analysis. The effect of GL-p on myeloperoxidase (MPO) activity in the inflamed mucosa and purified enzyme was assayed.
RESULTS: GL-p treatment significantly ameliorated the extent of colitis compared to sham treatment with saline. Cytokine antibody array analysis showed that GL-p treatment significantly decreased the expression levels of pro-inflammatory cytokines and chemokines, including interleukin (IL)-1β, IL-6, tumor necrosis factor-α, cytokine-induced neutrophil chemoattractant-2, and monocyte chemoattractant protein-1 in the inflamed mucosa. Furthermore, GL-p inhibited the oxidative activity of mucosal and purified MPO.
CONCLUSION: GL-p enema has a therapeutic effect on experimental colitis in rats and may be useful in the treatment of UC.
- Citation: Kudo T, Okamura S, Zhang Y, Masuo T, Mori M. Topical application of glycyrrhizin preparation ameliorates experimentally induced colitis in rats. World J Gastroenterol 2011; 17(17): 2223-2228
- URL: https://www.wjgnet.com/1007-9327/full/v17/i17/2223.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i17.2223
Ulcerative colitis (UC) is a chronic inflammation of the large intestine, which is characterized by pronounced infiltration of neutrophils into colonic lesions with hyperemia, hemorrhage, and ulcerations. Its etiology is not fully understood but immunological mechanisms seem to be involved[1,2]. Increased pro-inflammatory mediators, including cytokines and chemokines, are observed in patients with colitis[3]. An efficacious treatment strategy comprises controlling the expression and neutralizing the function of these mediators. Agents such as aminosalicylates, corticosteroids, and immunosuppressants inhibit the production of these factors and are used in the treatment of UC; for example, antibodies that target tumor necrosis factor (TNF)-α are clinically efficacious to neutralize TNF-α actions[1,2]. However, these agents may cause serious adverse effects, such as agranulocytosis, severe infections, osteoporosis, and malignant lymphoid tumors.
Glycyrrhizin (GL), a natural compound extracted from the roots of the Chinese herb Glycyrrhiza glabra, has been used for several centuries in traditional Chinese medicine. Its preparation (GL-p) has been clinically used as an anti-allergic agent and also as treatment for chronic hepatitis for more than 30 years[4]. GL-p has useful pharmacological properties, such as anti-inflammatory, immunomodulatory, and cytoprotective effects[5]. Furthermore, treatment with GL-p is rarely accompanied by severe adverse effects, even during long-term use. However, the efficacy of GL-p in the treatment of other inflammatory conditions including UC is not well known.
Several models of experimentally induced colitis have been developed to investigate the mechanisms of inflammation and immunological disturbances[6]. Among them, an animal model with dextran sulfate sodium (DSS)-induced colitis exhibits several symptoms similar to those seen in human UC; therefore, they are considered reliable for studying the pathogenesis of UC[6,7].
To confirm the hypothesis that GL-p ameliorates colitis through its pharmacological effects, we have conducted experiments to investigate the therapeutic efficacy of GL-p for DSS-induced colitis in rats.
Male Wistar rats (approximately 250 g, 8 wk old) were used for the experiments. All rats were housed in specific pathogen-free conditions in the animal facility of Gunma University and fed standard laboratory chow and tap water ad libitum. This study was approved by the Animal Care and Experimentation Committee at Gunma University.
To induce colitis, rats were orally administered 3% solution of DSS (molecular weight, 5000; Wako Pure Chemical Industries, Ltd., Osaka, Japan) via drinking water for 7 d (from day 0 to day 6).
GL-p is commercially supplied by Minophagen Pharmaceutical (Tokyo, Japan) as a solution (Stronger Neo-Minophagen C®), which contains 2 mg GL monoammonium, 1 mg L-cysteine, and 20 mg glycine per mL in physiological saline solution. Rats in the GL-p and GL groups were respectively administered 1 mL GL-p or 0.2% GL solution transanally under diethyl ether anesthesia, once daily for four consecutive days (day 3 to day 6). Control rats were administered 1 mL saline. Body weight was measured throughout the experiments. All rats were killed on day 7 under excess diethyl ether anesthesia.
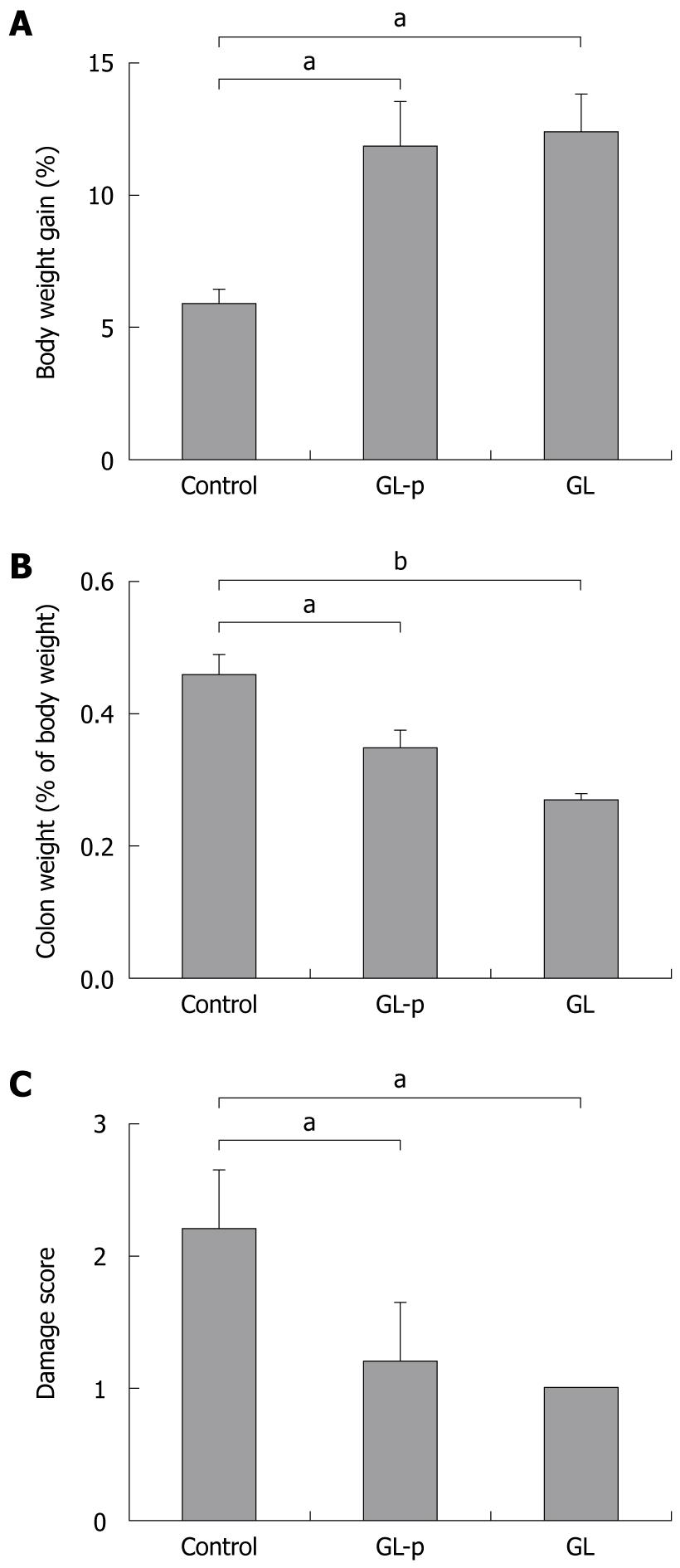
Body weight gain was calculated by subtracting the body weight at the beginning of the treatment from that at autopsy. An 8-cm long biopsy specimen of the distal colon was resected at autopsy and opened by longitudinal incision. The wet weight of this biopsy specimen was measured followed by observation of the gross appearance of the mucosa. Mucosal damage was measured and macroscopically scored on a scale of 0 to 10 according to the following criteria[8]: 0, no damage; 1, hyperemia without ulcers; 2, hyperemia and thickening of the bowel wall without ulcers; 3, one ulcer without thickening of the bowel wall; 4, two or more sites of ulceration and inflammation; 5, two or more major sites of ulceration and inflammation or one site of ulceration and inflammation extending > 1 cm along the length of the colon; 6-10, one point being added for each additional centimeter of involvement beyond an initial 2 cm. Tissue specimens were kept at -80°C until cytokines were evaluated.
Myeloperoxidase (MPO) activity was measured according to the modified method of Bradley et al[9]. The mucosal scrapings were homogenized with a Diax 600 homogenizer (Heidolph, Germany) in 1 mL buffer that contained 0.5% hexadecyltrimethylammonium bromide and 50 mmol/L potassium phosphate (pH 6.0). The homogenates were sonicated for 10 s, freeze-thawed three times, and centrifuged at 40 000 ×g for 15 min at 4°C. From each sample, 100 μL was added to 2.9 mL 50 mmol/L phosphate buffer (pH 6.0) that contained 0.167 mg/mL o-dianisidine hydrochloride and 0.0005% hydrogen peroxide. MPO activity was measured colorimetrically using a spectrometer with a change of absorbance of 460 nm during a 30-min interval at 25°C. One unit of MPO activity was defined as 1 mmol H2O2 broken down to H2O and results were expressed as units per gram mucosal tissue. The MPO activities in the supernatant of mucosal homogenate and in the purified human MPO enzyme (Sigma-Aldrich Japan, Tokyo, Japan) were measured in the presence and absence of various amounts of GL-p.
The Cytokine Array (Raybiotech Inc., Norcross, GA, USA)[10] was used to detect 19 different cytokines and chemokines in the supernatant of homogenized colonic mucosal scrapings according to the manufacturer’s recommended protocol. After the membranes were exposed to X-ray film (GE Healthcare Bioscience Co. Ltd.), the exposed films were digitized and the relative cytokine levels were compared by densitometrical analysis using ImageJ ver. 1.38x software (National Institute of Health, Rockville Pike, Bethesda, MD, USA). The relative cytokine levels were obtained by subtracting the background staining and normalizing from the positive controls on the same membrane.
All data are presented as mean ± SE. Student’s t test was used for comparison between the data in the two groups. One-way ANOVA followed by Tukey’s post hoc test was used to analyze the data for multiple groups. For evaluation of the damage score, the non-parametric Kruskal-Wallis test followed by the Steel-Dwass test was used. P < 0.05 was considered statistically significant. KyPlot 5.0 (KyensLab. Inc., Tokyo, Japan) was used for the statistical analyses.
After treatment with 3% DSS for 7 d, all the rats developed symptoms of colitis. Diarrhea was first observed on day 4 after the onset of treatment, followed by rectal bleeding and body weight loss. Compared to the control group, the GL-p and GL groups experienced significantly increased body weight gain (P < 0.05), decreased colon wet weight (P < 0.05), and reduced macroscopic damage score (P < 0.05) (Figure 1). Significant differences between GL-p and GL groups were not detected (Figure 1).
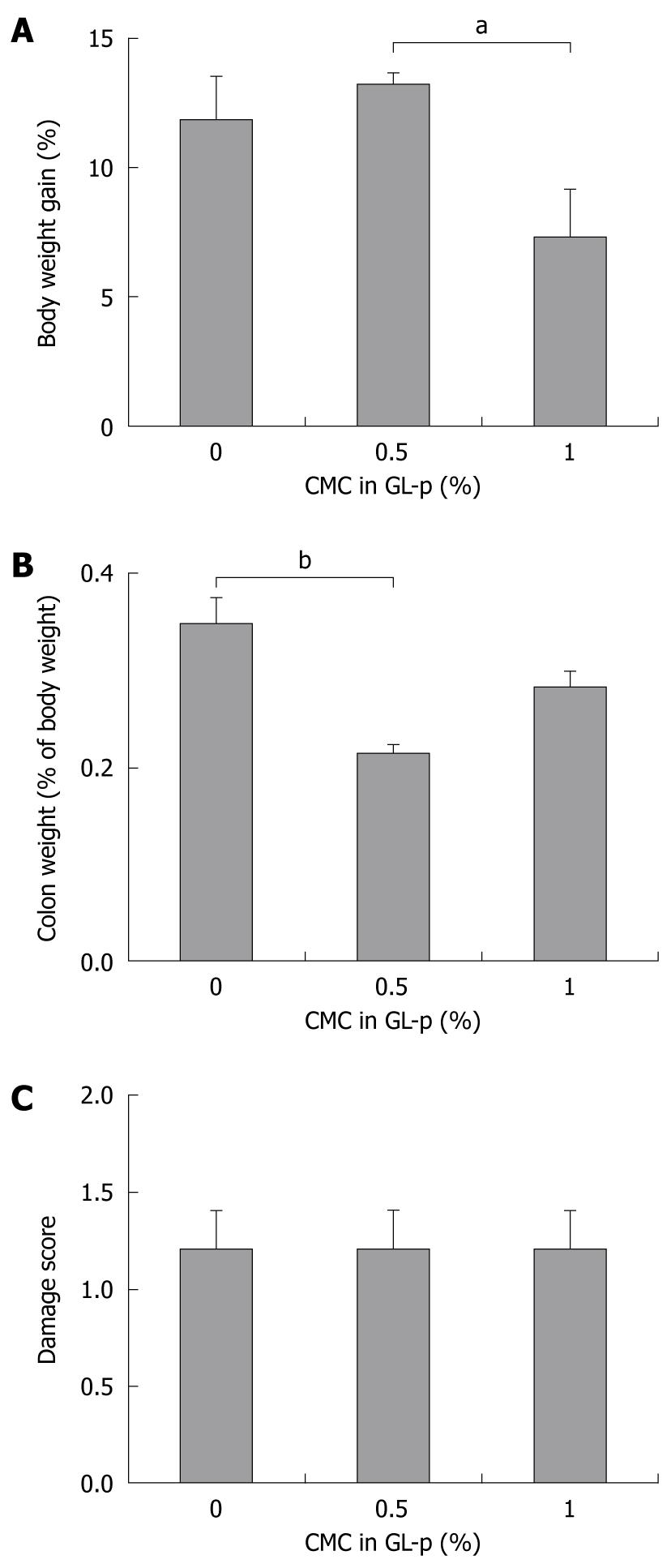
To determine whether the addition of carboxymethylcellulose (CMC) as a viscosity modifier augments the therapeutic effects of GL-p enema, we compared the group treated with GL-p alone to those treated with a combination of GL-p and CMC. The addition of 0.5% CMC seemed to increase body weight gain and significantly decrease the colon wet weight compared to GL-p alone (P < 0.01), whereas addition of CMC did not result in any changes in the macroscopic damage score (Figure 2).
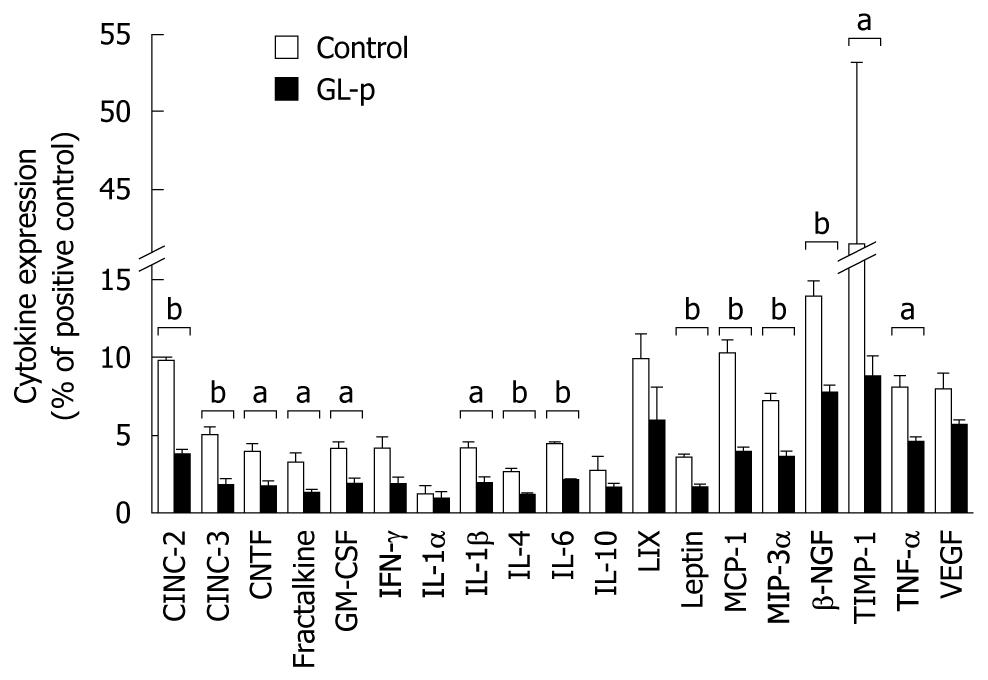
Using cytokine antibody array analysis, the 19 cytokine levels between the GL-p and control groups were compared (Figure 3). The levels of pro-inflammatory cytokines including interleukin (IL)-1β, IL-6 and TNF-α were significantly decreased in the GL-p group, and those of chemokines, such as cytokine-induced chemoattractant (CINC)-2 and -3, granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein (MCP)-1, and macrophage inflammatory protein (MIP)-3α, were also significantly inhibited in the GL-p group. Among Th1 cytokines, interferon (IFN)-γ was not significantly inhibited. Among Th2 cytokines, IL-4 and IL-6 decreased while IL-10 did not change. The levels of leptin, tissue inhibitor of metalloproteinase (TIMP)-1, fractalkine, and ciliary neurotrophic factor (CNTF) were decreased in the GL-p group.
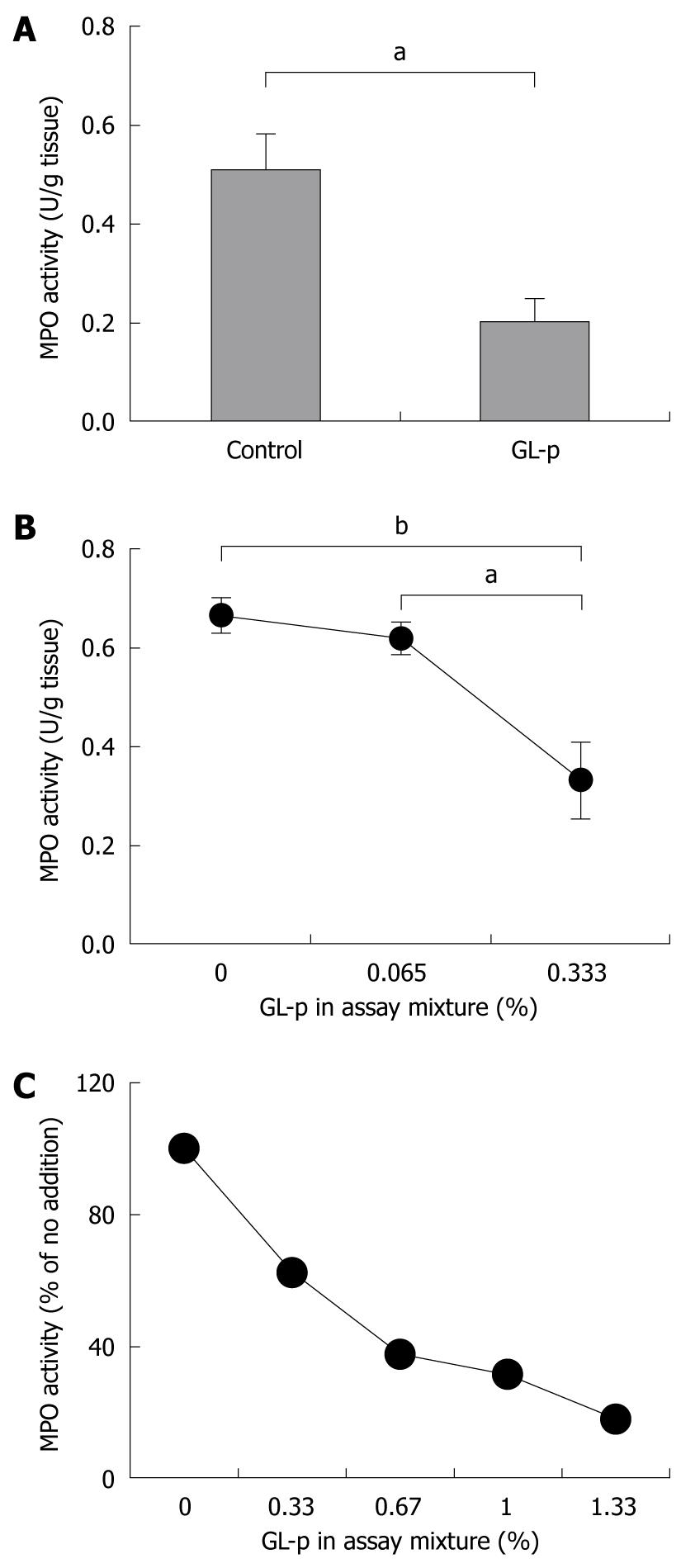
To evaluate the effect of GL-p on the number of neutrophils infiltrating the colon, levels of mucosal MPO activity were measured. Rats treated with GL-p showed a significantly low level of mucosal MPO activity compared to control rats (P < 0.05) (Figure 4A). We conducted in vitro experiments to assess the presence of an inhibitory effect of GL-p on MPO activity, and found that GL-p dose-dependently inhibited MPO activity in mucosal tissue (Figure 4B), as well as purified human enzyme (Figure 4C).
In the present study, we showed that GL-p ameliorated the extent of DSS-induced colitis in rats. We used cytokine antibody array analyses and revealed that GL-p reduced the level of pro-inflammatory cytokines and chemokines in the colonic mucosa. Furthermore, GL-p was shown to inhibit MPO activity.
We demonstrated that GL-p administration ameliorated the production of pro-inflammatory cytokines and chemokines, such as IL-1β, TNF-α, IL-6, CINC-2 and -3, MCP-1, MIP-3α, TIMP-1, fractalkine, CNTF, leptin, and GM-CSF. The relevance of pro-inflammatory cytokines and chemokines to the pathophysiology of chronic inflammation in inflammatory bowel disease (IBD) has recently been elucidated, and their manipulation has successfully reduced disease severity and maintained remission. IL-1β has been well characterized as a potent inflammatory cytokine that is produced by inflammatory and mucosal epithelial cells during colonic inflammation[11]. Its neutralization with antibodies suppresses the development of DSS-induced colitis[12]. IL-6 is a pleiotropic cytokine that exerts its pro-inflammatory effects mainly by means of its soluble IL-6 receptor. The anti-IL-6 receptor monoclonal antibody abrogates murine colitis[13]. TNF-α is a key inflammatory cytokine produced mainly from macrophages and is a target of IBD treatment. Anti-TNF-α antibody and soluble TNF-α receptor are used clinically with significant benefits. Collectively, these findings suggest the efficacy of GL-p in the treatment of human UC.
GL-p used in this study was a combination of GL with 2% of glycine and 0.4% of L-cysteine. A diet that contains 5% glycine has recently been reported to prevent colitis in rats, which is chemically induced by DSS or 2,4,6-trinitrobenzene sulfonic acid (TNBS)[14]. Glycine inhibits the induction of pro-inflammatory cytokines and chemokines, such as IL-1β, TNF-α, CINC and MIP-2. On the contrary, dietary L-cysteine supplementation is also reported to have a therapeutic effect on DSS-induced porcine colitis[15]. However, no significant therapeutic differences were observed between GL alone and GL-p in the present study (Figure 1). Although the difference in the experimental settings, such as the route of administration, may be relevant to this contradiction, the precise reasons for this are unknown. One possibility is that the effect of amino acids in GL-p was masked by that of GL in our experimental settings. To evaluate this possibility, further experiments including a control group treated with amino acids alone is required.
Our study demonstrated that GL was beneficial in the treatment of rat colitis if administered intrarectally (Figure 1). However, GL was not proven to be efficacious in a previous study[16]. Although the beneficial effects of oral administration of Hange-shashin-to (HST), a combination of seven herbs[16], on DSS-induced colitis in rats has been demonstrated, the previous study did not detect the effects of oral administration of a single constituent included in HST, such as GL. One possible explanation for the contradiction between their results and ours may be the difference in the type of experimental colitis used. They used a model of colitis induced by intracolonic instillation of TNBS, which is known as a model of Crohn’s disease, and has distinct immunological features from those of the DSS-induced colitis that we used[6]. This seems to have caused conflicting responses to GL administration, which suggests that Crohn’s disease and UC should be distinguished at the point of clinical application of GL treatment. Another possibility is the differences in dose and route of administration. They administered an oral dose of 2.67 or 5.37 mg/kg GL. We administered a dose of 8 mg/kg (2 mg GL per rat weighing 250 g) intrarectally. We were subsequently able to deliver higher concentrations of GL to the inflamed colon compared to the previous study.
Regarding GL administration for colitis, Yuan et al[17] recently have reported the results of experiments using colitis induced by acetic acid installation, which is a model of acute colitis[6]. They found that GL reduced colonic injury with the suppression of nuclear factor-κB, TNF-α, and intercellular adhesion molecule-1 in the affected mucosa. They administered GL at a dose of 40 mg/kg, which was five times greater than that in our study. Furthermore, they chose the intraperitoneal route for administration. Use of this route may raise the systemic concentration of GL sufficiently high to cause adverse effects such as severe hypertension, hypokalemia, and other signs of mineralocorticoid excess, because the incidence of adverse effects is dose-dependent[4,5]. We administered GL and GL-p intrarectally to maintain low systemic concentrations of the agents despite high local concentrations[18]. For clinical application, rectal administration is preferable as it may maximize quality of life by reducing the necessity for frequent visits to outpatient clinics to receive parenteral administration of GL-p.
We demonstrated that GL-p treatment decreased MPO activity in the inflamed mucosa (Figure 4A), which indicates the inhibition of neutrophil accumulation at the site of injury through GL-p administration. MPO is mainly produced by neutrophils that infiltrate the inflammatory sites, therefore, MPO activity in specimens is believed to reflect the number of infiltrating neutrophils[9]. Furthermore, in this study, we demonstrated for the first time that GL-p suppressed MPO activity in a dose-dependent manner in experiments using the inflamed mucosa (Figure 4B), as well as purified human enzyme (Figure 4C). This appears to be a new mechanism in the therapeutic actions of this agent. Although the main function of MPO is to destroy phagocytosed microorganisms by strong oxidative species within the phagosome, the excessive amount and activation of MPO at inflamed sites may cause tissue injury by modification of lipids and proteins through reactive oxidative species[19]. Therefore, modulation of MPO oxidation activity by GL-p has a therapeutic potential for inflammation.
In the treatment of UC, development of colorectal carcinoma is a serious complication. It is noticeable that long-term use of GL-p is effective in preventing development of hepatocellular carcinoma in patients with chronic hepatitis C[20,21]. Amelioration of chronic inflammation is suggested to reduce the possibility of oncogenesis, therefore, GL-p enema may prevent the development of colon cancer.
In conclusion, our study suggests a therapeutic effect of GL-p enemas on experimental colitis in rats. Further studies are necessary to determine an effective administration strategy with higher efficacy and fewer adverse effects in the treatment of patients with UC.
Ulcerative colitis (UC) affects many people worldwide. Its pathophysiology involves immunological mechanisms. Glycyrrhizin preparation (GL-p) may be useful in the treatment of UC because it has anti-inflammatory and immunomodulatory effects.
Although a previous study has demonstrated that oral administration of a combination of seven herbs is beneficial in the treatment of colitis in rats, the effect of GL, one of its constituents, was not detected.
The study demonstrated that GL-p was beneficial in the treatment of rat colitis if administered intrarectally. GL-p decreased the expression of pro-inflammatory cytokines and inhibited myeloperoxidase activity.
The results of this study suggest a therapeutic effect of GL-p enemas on experimental colitis in rats. Further studies are required to apply this treatment in patients with UC.
GL is a natural compound extracted from the roots of the Chinese herb Glycyrrhiza glabra and GL-p has been clinically used as an anti-allergic agent and as a treatment for chronic hepatitis in Japan.
GL, an active ingredient from licorice root, has long been used for traditional medicinal purposes. Very little work on this subject has been described in the mainstream literature, and this work is a welcome addition. The authors have done a commendable job of comparing their study with the only (to my knowledge) previously published work in the field, primarily that of Yuan et al.
Peer reviewer: Dr. William R Parker, PhD, Assistant Professor, Department of Surgery, Duke University Medical Center, Box 2605, Durham, NC 27710, United States
S- Editor Sun H L- Editor Kerr C E- Editor Zheng XM
| 1. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. |
| 2. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. |
| 3. | Hirata I, Murano M, Nitta M, Sasaki S, Toshina K, Maemura K, Katsu K. Estimation of mucosal inflammatory mediators in rat DSS-induced colitis. Possible role of PGE(2) in protection against mucosal damage. Digestion. 2001;63 Suppl 1:73-80. |
| 4. | van Rossum TG, Vulto AG, de Man RA, Brouwer JT, Schalm SW. Review article: glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther. 1998;12:199-205. |
| 5. | Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709-724. |
| 6. | Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344-1367. |
| 7. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. |
| 8. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. |
| 9. | Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206-209. |
| 10. | Watanabe M, Guo W, Zou S, Sugiyo S, Dubner R, Ren K. Antibody array analysis of peripheral and blood cytokine levels in rats after masseter inflammation. Neurosci Lett. 2005;382:128-133. |
| 11. | Radema SA, van Deventer SJ, Cerami A. Interleukin 1 beta is expressed predominantly by enterocytes in experimental colitis. Gastroenterology. 1991;100:1180-1186. |
| 12. | Arai Y, Takanashi H, Kitagawa H, Okayasu I. Involvement of interleukin-1 in the development of ulcerative colitis induced by dextran sulfate sodium in mice. Cytokine. 1998;10:890-896. |
| 13. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. |
| 14. | Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, Sato N. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125:775-785. |
| 15. | Kim CJ, Kovacs-Nolan J, Yang C, Archbold T, Fan MZ, Mine Y. L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochim Biophys Acta. 2009;1790:1161-1169. |
| 16. | Kawashima K, Nomura A, Makino T, Saito K, Kano Y. Pharmacological properties of traditional medicine (XXIX): effect of Hange-shashin-to and the combinations of its herbal constituents on rat experimental colitis. Biol Pharm Bull. 2004;27:1599-1603. |
| 17. | Yuan H, Ji WS, Wu KX, Jiao JX, Sun LH, Feng YT. Anti-inflammatory effect of Diammonium Glycyrrhizinate in a rat model of ulcerative colitis. World J Gastroenterol. 2006;12:4578-4581. |
| 18. | Campieri M, Paoluzi P, D'Albasio G, Brunetti G, Pera A, Barbara L. Better quality of therapy with 5-ASA colonic foam in active ulcerative colitis. A multicenter comparative trial with 5-ASA enema. Dig Dis Sci. 1993;38:1843-1850. |
| 19. | Hope HR, Remsen EE, Lewis C Jr, Heuvelman DM, Walker MC, Jennings M, Connolly DT. Large-scale purification of myeloperoxidase from HL60 promyelocytic cells: characterization and comparison to human neutrophil myeloperoxidase. Protein Expr Purif. 2000;18:269-276. |
| 20. | Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494-1500. |
| 21. | Ikeda K, Arase Y, Kobayashi M, Saitoh S, Someya T, Hosaka T, Sezaki H, Akuta N, Suzuki Y, Suzuki F. A long-term glycyrrhizin injection therapy reduces hepatocellular carcinogenesis rate in patients with interferon-resistant active chronic hepatitis C: a cohort study of 1249 patients. Dig Dis Sci. 2006;51:603-609. |