INTRODUCTION
Nitric oxide (NO), a multifunctional endogenous gas molecule, is metabolized from L-arginine by enzymatic reaction in the presence of NO synthase (NOS)[1]. It is accepted as the most critical messenger and functional executant in a variety of biologic activities. Numerous researches have reported the beneficial effects of NO, including endothelium protection, inhibition of local inflammation and cell pathoproliferation[2]. NO bioavailability is most directly regulated by the abundance and/or activity of NOS. NOS can be competed by substrates, endogenous L-arginine and asymmetric dimethylarginine (ADMA)[1]. The function of ADMA is to decrease NO production via inhibiting the NOS activity. ADMA is derived from the catabolism of proteins containing methylated arginine residues[3]. Arginine methylation of cellular proteins is catalyzed by protein arginine methyltransferases[3]. ADMA is hydrolyzed by dimethylarginine dimethylaminohydrolase (DDAH)[3].
Growing evidence has shown that excessive accumulation of ADMA can decrease NO bioavailability in cells/tissues, induce domain dysfunctions or produce detrimental effects in multiple systems[4]. Besides inhibiting NO synthesis, ADMA can directly induce oxidative stress and cell apoptosis, and participate in the inflammation reactions[5,6]. It is well documented that ADMA is involved in a wide range of cardiovascular disorders. ADMA impairs endothelial functions, thus leading to hypertension, atherosclerosis, coronary heart disease, diabetes mellitus, pulmonary hypertension and renal failure[7,8]. Although it is well established that ADMA is an independent risk factor of cardiovascular diseases, the pathological role of ADMA in other diseases is still unknown, including gastric diseases.
NO AND GASTRIC MUCOSAL PROTECTION
Gastric mucosal diseases, including gastric ulcers, affect 25%-30% of the world’s population. The mechanism of gastric mucosal damage and protection is yet unclear. NO, an important gas signaling molecule, is a gastric mucosal protective factor that contributes significantly to maintaining normal gastric mucosal integrity[9]. Gastric mucosa is composed of gastric epithelial cells and glands. Gastric epithelial cells can secrete multiple active substances, including NO. There is substantial evidence that NO may be an important defendant factor of gastric mucosa[10]. In the animal models of gastric mucosal injury induced by ethanol, endothelin, ischemia/reperfusion, cold-stress, water immersion restraint, indomethacin and aspirin, NO exerts protective effects on gastric mucosal integrity[11-13]. The mechanisms underlying the protective effects of NO could be explained as follows: (1) NO increases gastric mucosal blood flow; (2) NO regulates the secretion of mucus and bicarbonate, which compose the first level of gastric tissue defense; and (3) NO inhibits the secretion of gastric juice[14,15].
ADMA AND GASTRIC MUCOSAL DAMAGE
Helicobacter pylori (H. pylori) infection is a definitive cause of gastroduodenal ulcer. Epidemiological analysis has shown that 70%-80% gastric ulcers and 95%-100% duodenal ulcers are attributed to H. pylori infection. In a clinical investigation of H. pylori-positive and -negative volunteers, H. pylori infection was found to increase the tissue contents of ADMA[16]. More importantly, a laboratory research has shown that proteolysis of H. pylori extract also results in a substantial accumulation of ADMA, indicating that H. pylori infection must be taken into account as a cause of increased ADMA levels[17]. Strong associations have also been found between ADMA and gastric mucosal dysfunction. Exogenous administration with ADMA inhibits the mucosal alkaline response to acid exposure, which is regarded as one of the important beneficial factors of gastric mucosa complaint. And an increase in ADMA mediates the water extract of H. pylori-induced acid-base imbalance in duodenal tissues[17]. In our in vitro experiment, we found that intervention of H. pylori stimulated ADMA release from cultured gastric epithelial cells[18]. We also observed that H. pylori infection markedly exacerbated ADMA-produced gastric tissue injury in rats[19].
Besides H. pylori invasion, gastric mucosal function is also disturbed by other factors, such as ethanol, indomethacin and cold-stress. Our recent studies have explored the role of ADMA in gastric mucosal injury induced by other causes in addition to H. pylori infection. The results have shown that ADMA levels are increased in gastric juice in three separate gastric mucosal models, including ethanol, indomethacin and cold-stress initiated mucosal injury, accompanied with a decrease in NO level and DDAH activity[19]. Pretreatment with L-arginine, an antagonist of ADMA, markedly reduces the degree of gastric injury and elevates NO bioavailability. Administration of exogenous ADMA significantly exacerbates gastric injury, concomitantly with a decrease in plasma level of NO. These findings indicate that ADMA may participate in the gastric mucosal injury process induced by various deleterious factors. Multiple exogenous NOS inhibitors, such as L-NMA, L-NAME and L-NMMA intensify gastric mucosal injury by decreasing gastric mucosal blood flow, while NO donor (nitroprusside sodium) or substrate (L-arginine) reverses such effects[13,20].
Cigarette smoking is one of the risk factors provoking gastroduodenal ulceration[21]. It increases both the incidence and relapse rate of peptic ulcer diseases and delays ulcer healing. Besides reducing bioavailability of NO, nicotine, a major component of tobacco, aggravates gastric mucosal injury due to some factors such as ethanol or stress[22]. Recently, we have found that in the rats treated with ethanol, the ulcer index and ADMA level were increased and the NO level was decreased, and these effects of ethanol were augmented by pre-treatment with nicotine[23]. Nicotine alone did not show significant impact on ulcer index, ADMA level and NO level. In vitro incubation of epithelial cells with ethanol induced cell injury and increased ADMA level in the cultural medium, an effect that was amplified in the presence of nicotine. Therefore, the accelerated effect of nicotine on gastric mucosal dysfunction is associated with endogenous ADMA.
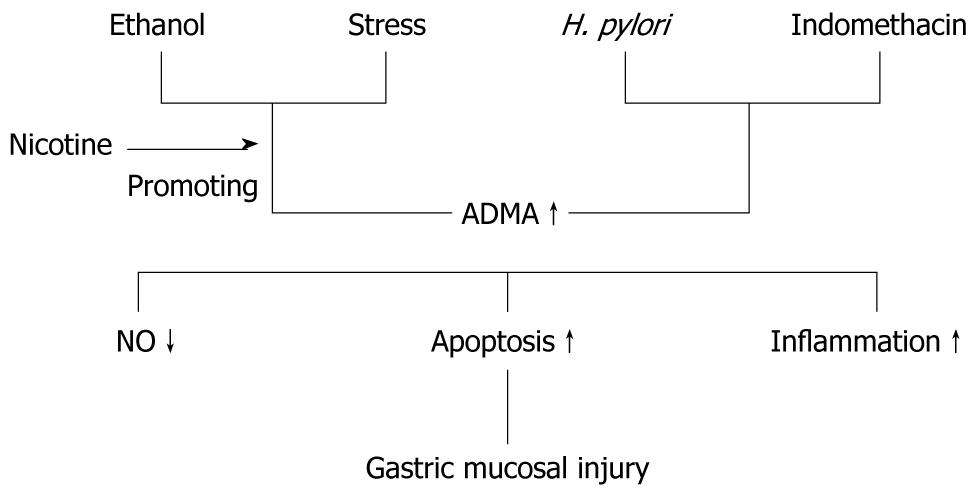
The mechanism of ADMA directly producing adverse effect in gastric mucosa is incompletely understood. It is widely accepted that NO bioavailability decrease is the major reason. Apoptosis reaction produced by ADMA has been observed in different types of cells, such as endothelial cell and smooth muscle cell[6,24]. Cell apoptosis has also been observed in cultured gastric epithelial cells when exposed to an exorbitant concentration of ADMA[23]. ADMA could directly induce the production of inflammatory cytokines tumor necrosis factor (TNF)-α and soluble intercellular adhesion molecule-1 via activation of p38 MAPK and ERK1/2 pathways in cultured endothelial cells[25]. We deduce that inflammatory reaction may be another important factor for ADMA-induced gastric injury because TNF-α level, the marker of inflammation, is significantly elevated when cells are damaged by ADMA in gastric mucosal epithelial cells. The hypothesized role of ADMA in gastric mucosal injury and the underlying mechanisms are summarized in Figure 1.
Figure 1 The hypothesized role of asymmetric dimethylarginine in gastric mucosal injury and the underlying mechanisms.
ADMA: Asymmetric dimethylarginine; NO: Nitric oxide.
PERSPECTIVES AND CLINICAL IMPLICATIONS
ADMA might be a novel clinical and experimental biomarker related to gastric mucosal disorder. The mechanisms of impairment involve NO generation inhibition, inflammatory reaction and apoptosis promotion. Although the therapeutic tool targeting ADMA is available in multiple cardiovascular diseases, it is unknown in gastrointestinal diseases. The strategy to inhibit ADMA is beneficial to the treatment of gastric ulcer induced by ethanol in rats[26]. Thus, ADMA might be a candidate of therapeutic target in gastric mucosal damage.
Peer reviewer: Bruno Bonaz, Professor, Clinique Universitaire d'Hépato-Gastroentérologie, CHU de Grenoble, BP217, 38043 Grenoble Cedex 09, France
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM