Published online Apr 28, 2011. doi: 10.3748/wjg.v17.i16.2126
Revised: December 10, 2010
Accepted: December 17, 2010
Published online: April 28, 2011
AIM: To investigate the markers of pancreatic diseases and provide basic data and experimental methods for the diagnosis of pancreatic diseases.
METHODS: There were 15 patients in the present study, among whom 10 had pancreatic cancer and 5, chronic pancreatitis. In all patients, pancreatic cancer or chronic pancreatitis was located on the head of the pancreas. Pathology data of all patients was confirmed by biopsy and surgery. Among the 10 patients with pancreatic cancer, 3 people had a medical history of long-term alcohol consumption. Of 5 patients with chronic pancreatitis, 4 men suffered from alcoholic chronic pancreatitis. Pancreatic juice samples were obtained from patients by endoscopic retrograde cholangio-pancreatography. Magnetic resonance spectroscopyn was performed on an 11.7-T scanner (Bruker DRX-500) using Call-Purcell-Meiboom-Gill pulse sequences. The parameters were as follows: spectral width, 15 KHz; time domain, 64 K; number of scans, 512; and acquisition time, 2.128 s.
RESULTS: The main component of pancreatic juice included leucine, iso-leucine, valine, lactate, alanine, acetate, aspartate, lysine, glycine, threonine, tyrosine, histidine, tryptophan, and phenylalanine. On performing 1D 1H and 2D total correlation spectroscopy, we found a triplet peak at the chemical shift of 1.19 ppm, which only appeared in the spectra of pancreatic juice obtained from patients with alcoholic chronic pancreatitis. This triplet peak was considered the resonance of the methyl of ethoxy group, which may be associated with the metabolism of alcohol in the pancreas.
CONCLUSION: The triplet peak, at the chemical shift of 1.19 ppm is likely to be the characteristic metabolite of alcoholic chronic pancreatitis.
- Citation: Wang J, Ma C, Liao Z, Tian B, Lu JP. Study on chronic pancreatitis and pancreatic cancer using MRS and pancreatic juice samples. World J Gastroenterol 2011; 17(16): 2126-2130
- URL: https://www.wjgnet.com/1007-9327/full/v17/i16/2126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i16.2126
Pancreatic cancer accounts for about 2% of all cancer cases, but it has the worst prognosis of all cancers with a 5-year survival rate of less than 3%[1,2]. Because of the deep-seated location of the pancreas and no apparent symptoms at the initial stages of pancreatic cancer, it is difficult to diagnose this disease in the early stages. Chronic pancreatitis is a kind of localized or diffuse inflammation, and is caused by many factors. One of the medical dilemmas is to distinguish pancreatic cancer from chronic pancreatitis with a mass in the head of the pancreas; both these diseases have similar clinical behavior and imaging features[3]. A puncture biopsy is usually preferred over an operation when diagnosing the disease, but this is an injurious procedure and may lead to some complications[4].
Magnetic resonance imaging (MRI) is the most common procedure used in the diagnosis of pancreatic cancer. An MRI helps obtain images of the pancreas and its surrounding structures[5]. The deep-seated location of the pancreas and similar clinical manifestations of chronic pancreatitis and pancreatic cancer are the main barriers in differentiating between these two diseases even with advanced MRI techniques. Magnetic resonance spectroscopy (MRS) has high sensitivity and resolution, allows in vitro testing of metabolites, and has been widely used in the field of metabolomics[6-12]. MRS will be the most potent tool to help differentiate between pancreatic cancer and chronic pancreatitis, and it will be the most effective tool for the early diagnosis of a pancreatic tumor.
Usually, in the process of cancerization, gene and metabolite abnormalities appear before tissue structure transformation. Detection of abnormalities in metabolites facilitates early diagnoses of tumors. Clinically, the serum marker CA19-9[13] and gene tumor markers such as the K-ras gene[14,15], p53 anti-oncogene, and p53 protein[16-19] are widely used as markers of pancreatic cancer; however, these markers are not sensitive, show low specificity, and are used as auxiliary tools[20]. Beger et al[10] had successfully used MRS and mass spectrum (MS) to analyze blood constituents of patients with pancreatic cancer and of healthy volunteers; they were able to make a good distinction between pancreatic cancer and the control group by performing lipid profiling of the blood. Pancreatic juice is the exocrine of the pancreas, and is closely related with pancreatic tissues. We wanted to investigate whether it is possible to obtain some information to help differentiate between chronic pancreatitis and pancreatic cancer by analyzing pancreatic juice samples. In the present study, we used MRS technology to analyze the pancreatic juice of patients with pancreatic cancer or chronic pancreatitis with a mass in the head of the pancreas and tried to explore the markers of these diseases. We provided basic data and experimental methods for the study of pancreatopathy.
The initial subject population comprised 35 patients with pancreatic cancer (24 men and 11 women; mean age, 67.2 years; age range, 47-85 years) recruited between January 2006 and June 2009. We selected 10 subjects (7 men and 3 women; mean age, 67.7 years; age range, 57-74 years) with surgically confirmed pancreatic cancer. The mean maximum lesion diameter was 26.1 mm (range, 11-51 mm), and all lesions were located in the head of the pancreas. We chose 5 patients (4 men and 1 woman; mean age, 58.3 years) with chronic pancreatitis, confirmed by in vivo biopsy, and lesions located at the head of the pancreas. The medical history of every patient was recorded in detail. All study protocols were approved by our Institutional Review Board, and informed consent was obtained from all patients before they were enrolled in this study.
Pancreatic juice samples were obtained from patients by endoscopic retrograde cholangio-pancreatography (ERCP) in frozen tubes and immediately placed in liquid nitrogen before storing the tubes in a -80°C refrigerator for MRS experiments. All patients were diagnosed by biopsy analyses of pathology data. Both the tumor marker CA19-9 and the cancer gene maker p53 were detected in patients with pancreatic cancer, whereas these markers were not detected in patients with chronic pancreatitis.
MRS experiments were performed on a Bruker DRX-500 spectrometer (1H frequency, 500.13 MHz; Bruker Biospin, Rheinstetten, Germany). Pancreatic juice samples were diluted with phosphate buffer in D2O and placed in sample tubes (diameter, 5 mm), which are used in MRS experiments. Spectra were acquired at 300.0 K using Call-Purcell-Meiboom-Gill (CPMG) pulse sequence[21,22] along with water presaturation during the relaxation delay of 2 s. The CPMG pulse sequence was applied as a T2 filter to suppress signals from molecules with short T2 values (such as macromolecules and lipids), using a total echo time (TE) time of 320 ms. The main parameters for the 1D 1H-MRS spectra were as follows: spectral width (SW), 15 KHz; time domain (TD), 64 K; number of scans (NS), 512; and acquisition time (AQ), 2.128 s. Spectral assignments were confirmed by 2D 1H-1H TOCSY[23] and J-resolved (JRES) along with the values obtained from the literature[24]. The main parameters used for TOCSY were as follows: TD (F1-dimensional), 512; TD, (F2-dimensional), 1 K; SW (F1 and F2-dimensional), 5 KHz; and NS, 32. The main parameters used for JRES were as follows: TD (F1-dimensional), 256; TD (F2-dimensional), 8 K; SW (F1-dimensional), 78 Hz; SW (F2-dimensional), 8 KHz; and NS, 32. In both the 2D MRS experiments, the delay time was 2 s. The stability of the pancreatic juice samples was evaluated by repeating a 1D MRS experiment after overall acquisition. No biochemical degradation of any of the pancreatic juice samples was observed.
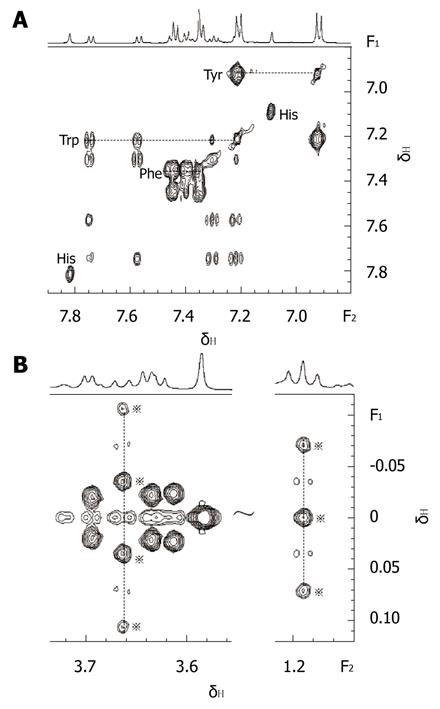
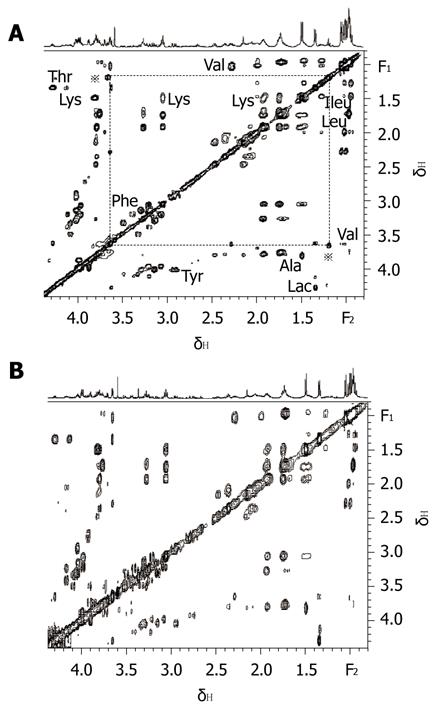
On combining 2D MRS experimental results (Figure 1) obtained in the present study with results from related literature[25-27] we identified the main resonances in 1H MRS spectra of pancreatic juice. TOCSY is a useful 2D MRS technology, it can be used to distinguish the frequencies in the total spin system and improve the sensitivity of detecting small J couplings[28]. On the basis of TOCSY data, the resonances in 1D 1H MRS spectra (Figure 2) of some amino acids were identified. The components, including leucine (Leu), iso-leucine (Ileu), valine (Val), lactate (Lac), alanine (Ala), acetate (Ace), aspartate (Asp), lysine (Lys), glycine (Gly), threonine (Thr), tyrosine (Tyr), histidine (His), tryptophan (Trp) and phenylalanine (Phe), and the locations were also identified.
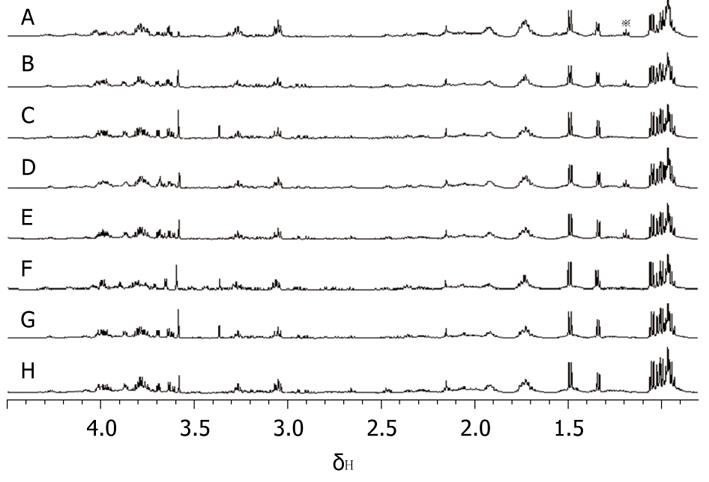
From the analysis of 1D 1H MRS spectra of all pancreatic juice samples, it was easy to find a triplet peak at the chemical shift of 1.19 ppm (Figure 3), which only appeared in some spectra. Four of the pancreatic juice samples of patients with chronic pancreatitis showed a triplet peak at the chemical shift of 1.19 ppm on 1H MRS, whereas the spectra of the pancreatic juice of patients with pancreatic cancer did not show a peak at the chemical shift of 1.19 ppm. When subjected to 2D TOCSY, the spectra of pancreatic juice samples of patients with chronic pancreatitis only showed one correlation peak at the chemical shift of 1.19 ppm and 3.36 ppm (Figure 4). By chemical shift and J coupling constant, we found that the peak at the chemical shift of 1.19 ppm was the 1H peak of the methyl of ethoxy group (CH3CH2O-). The 1D 1H spectra of the 4 pancreatic juice samples of patients with chronic pancreatitis showed a triplet peak at the chemical shift of 1.19 ppm; further, these patients had a history of drinking, which was found from the analysis of pathology data. The 1D 1H spectra of pancreatic juice obtained from the female patient with chronic pancreatitis did not show a triplet peak at the chemical shift of 1.19 ppm.
Among the 10 patients with pancreatic cancer, diagnosed by postoperative analyses of pathology data, 3 people had a medical history of long-term alcohol consumption. Of 5 patients with chronic pancreatitis, 4 men had a medical history of long-term alcohol consumption, and they were typical patients with alcoholic chronic pancreatitis. The female patient suffered from auto-immune chronic pancreatitis by analyses of the pathology data.
In recent years, there has been much debate on the relation between chronic pancreatitis and pancreatic cancer. While some scholars[29] believe that both diseases have a close connection, others[30] disagree. The results of our study show that there is no apparent difference between the components of pancreatic juice obtained from patients with chronic pancreatitis and pancreatic cancer, except that 1D 1H spectra of pancreatic juice obtained from the former group of patients who suffered from alcoholic chronic pancreatitis shows a triplet peak of the methyl of ethoxy group (CH3CH2O-) at the chemical shift of 1.19 ppm. This finding may be used to differentiate pancreatic cancer from alcoholic chronic pancreatitis with a mass in the head of the pancreas.
In the present study of the 10 patients with pancreatic cancer, 3 had a medical history of long-term alcohol consumption, but the 1D 1H spectra of their pancreatic juice did not show the triplet peak of the ethoxy group (CH3CH2O-); further, the pathology data of these patients did not show symptoms related to chronic pancreatitis. Hence, we can conclude that alcohol is not the main factor that causes pancreatic cancer. It is controversial whether long-term alcohol consumption can cause pancreatic cancer. Riediger et al[31] found that the association between alcohol and pancreatic cancer was not apparent during epidemiological investigation, which is in accord with our results.
We applied MRS to study pancreatic juice obtained from patients with chronic pancreatitis and pancreatic cancer, and separated the various components of different amino acids in human pancreatic juice by 1D and 2D 1H spectra. Recently, many analyses on the components of pancreatic juice have focused on the aspect of proteomics. Our study in the field of metabolomics is auxiliary to proteomics, and goes a step further in the study of pancreatopathy.
It is difficult to differentiate between pancreatic cancer and chronic pancreatitis with a mass in the head of the pancreas solely by MRI, because both these diseases have similar clinical behaviors and imaging features. There is no noninvasive method that can be successfully used to diagnose these diseases. Biopsy is frequently used to diagnose pancreatic cancer or chronic pancreatitis with a mass in the head of the pancreas but has some disadvantages, e.g. false negative results, many complications (bleeding, seepage of bile and pancreatic juice), and risk of tumor metastasis. ERCP causes some injury, but it is better than biopsy, because it causes fewer complications. ERCP, when combined with MRS technology, helps obtain more information on metabolites, which cannot be obtained from biopsy or MRI, and is likely to be used to distinguish pancreatic cancer from alcoholic chronic pancreatitis with a mass in the head of pancreas on the basis of 1H MRS spectra of pancreatic juice.
There are some limitations to this study. Lack of control groups and the content of metabolites in the pancreatic juice being small meant we could not perform a quantitative analysis, and only obtained some qualitative results. The excreta of patients with pancreatic cancer or chronic pancreatitis may not only have different components but also differ in quantity.
In conclusions, MRS is a powerful tool that can be applied to the study of pancreatic juice obtained from patients by ERCP, and does not cause injury. The triplet peak, which is at the chemical shift of 1.19 ppm in 1D 1H-MRS data of pancreatic juice obtained from the patients with alcoholic chronic pancreatitis, was identified as resonance of the methyl of ethoxy group (CH3CH2O-). The ethoxy group may be associated with alcohol metabolism in the pancreas, and is likely to be used to distinguish pancreatic cancer from alcoholic chronic pancreatitis with a mass in the head of the pancreas. In view of small numbers, further confirmation of the results in a larger number of patients is required.
Pancreatic cancer is a malignant neoplasm of the pancreas, and it has a high death rate. One of the medical dilemmas is to distinguish pancreatic cancer from chronic pancreatitis with a mass in the head of the pancreas; both diseases have similar clinical behavior and imaging features. Exploring the markers to distinguish the diseases is very important to the therapies of patients in clinic.
CA19-9, K-ras gene, p53 anti-oncogene, and p53 protein are widely used as markers of pancreatic cancer, but they are not sensitive and show low specificity. Searching characteristic markers of the diseases is still the goal of tireless pursuit. Magnetic resonance spectroscopy (MRS) has high sensitivity and resolution, allows in vitro testing of metabolites, and has been widely used in the field of metabolomics. MRS will be the most potent tool to help differentiate between pancreatic cancer and chronic pancreatitis.
It is difficult to differentiate between pancreatic cancer and chronic pancreatitis with a mass in the head of the pancreas solely by Magnetic Resonance Imaging (MRI). Biopsy is frequently used to diagnose the diseases but has many complications and risk of tumor metastasis. Endoscopic retrograde cholangio-pancreatography (ERCP) combined with MRS helps obtain more information on metabolites, which cannot be obtained from biopsy or MRI. We separated the various components of different amino acids in human pancreatic juice. The triplet peak which is at the chemical shift of 1.19 ppm in 1D 1H MRS spectra was identified as resonance of the methyl of ethoxy group (CH3CH2O-), and it may be the characteristic metabolites of the patients with alcoholic chronic pancreatitis.
This study provides basic data and experimental methods for the diagnosis of pancreatic diseases. The ethoxy group is likely to be used to distinguish pancreatic cancer from alcoholic chronic pancreatitis with a mass in the head of the pancreas.
Chemical shift is a basic concept in MRS, the pick appearing in the 1H MRS spectra with different chemical shift means the nuclear of proton spins with different frequency. The same rotation frequency of nuclear of protons only shows one peak in the 1H MRS spectra. The triplet peak at the chemical shift of 1.19 ppm is caused by the interaction of nearby nuclear of protons. In fact, it is split by one peak.
In my opinion, the article is acceptable for publication.
| 1. | Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7-33. |
| 2. | Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465. |
| 3. | Shimosegawa T, Kume K, Satoh K. Chronic pancreatitis and pancreatic cancer: prediction and mechanism. Clin Gastroenterol Hepatol. 2009;7:S23-S28. |
| 4. | Al-Haddad M, Eloubeidi MA. Diagnostic and therapeutic applications of endoscopic ultrasound-guided punctures. Dig Dis. 2008;26:390-397. |
| 5. | Bolog N, Constantinescu G, Oancea I, Beuran M, Albu R, Tanţă M, Nicolau E, Iordache F. Magnetic resonance imaging of bile and pancreatic ducts: a retrospective study. Rom J Gastroenterol. 2004;13:91-97. |
| 6. | Parsons HM, Ludwig C, Viant MR. Line-shape analysis of J-resolved NMR spectra: application to metabolomics and quantification of intensity errors from signal processing and high signal congestion. Magn Reson Chem. 2009;47 Suppl 1:S86-S95. |
| 7. | Beckwith-Hall BM, Thompson NA, Nicholson JK, Lindon JC, Holmes E. A metabonomic investigation of hepatotoxicity using diffusion-edited 1H NMR spectroscopy of blood serum. Analyst. 2003;128:814-818. |
| 8. | Tang H, Wang Y, Nicholson JK, Lindon JC. Use of relaxation-edited one-dimensional and two dimensional nuclear magnetic resonance spectroscopy to improve detection of small metabolites in blood plasma. Anal Biochem. 2004;325:260-272. |
| 9. | Potts BC, Deese AJ, Stevens GJ, Reily MD, Robertson DG, Theiss J. NMR of biofluids and pattern recognition: assessing the impact of NMR parameters on the principal component analysis of urine from rat and mouse. J Pharm Biomed Anal. 2001;26:463-476. |
| 10. | Beger RD, Schnackenberg LK, Holland RD, Li DH, Dragan Y. Metabonomic models of human pancreatic cancer using 1D proton NMR spectra of lipids in plasma. Metabolomics. 2006;2:125-134. |
| 11. | Lenz EM, Bright J, Wilson ID, Morgan SR, Nash AF. A 1H NMR-based metabonomic study of urine and plasma samples obtained from healthy human subjects. J Pharm Biomed Anal. 2003;33:1103-1115. |
| 12. | Duarte IF, Goodfellow BJ, Barros A, Jones JG, Barosa C, Diogo L, Garcia P, Gil AM. Metabolic characterisation of plasma in juveniles with glycogen storage disease type 1a (GSD1a) by high-resolution (1)H NMR spectroscopy. NMR Biomed. 2007;20:401-412. |
| 13. | Balzano G, Di Carlo V. Is CA 19-9 useful in the management of pancreatic cancer? Lancet Oncol. 2008;9:89-91. |
| 14. | Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549-554. |
| 15. | Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988;16:7773-7782. |
| 16. | Algül H, Schmid RM. Pancreatic cancer: a plea for good and comprehensive morphological studies. Eur J Gastroenterol Hepatol. 2008;20:713-715. |
| 17. | Redston MS, Caldas C, Seymour AB, Hruban RH, da Costa L, Yeo CJ, Kern SE. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994;54:3025-3033. |
| 18. | Kirsch DG, Kastan MB. Tumor-suppressor p53: implications for tumor development and prognosis. J Clin Oncol. 1998;16:3158-3168. |
| 19. | Yokoyama M, Yamanaka Y, Friess H, Buchler M, Korc M. p53 expression in human pancreatic cancer correlates with enhanced biological aggressiveness. Anticancer Res. 1994;14:2477-2483. |
| 20. | Fry LC, Mönkemüller K, Malfertheiner P. Molecular markers of pancreatic cancer: development and clinical relevance. Langenbecks Arch Surg. 2008;393:883-890. |
| 21. | Carr HY, Purcell EM. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954;94:630-638. |
| 22. | Meiboom S, Gill D. Modified spin-echo method for measuring nuclear relaxation times. Rev Sci Instrum. 1958;29:688-691. |
| 23. | Polshakov VI, Frenkiel TA, Westley B, Chadwick M, May F, Carr MD, Feeney J. NMR-based structural studies of the pNR-2/pS2 single domain trefoil peptide. Similarities to porcine spasmolytic peptide and evidence for a monomeric structure. Eur J Biochem. 1995;233:847-855. |
| 24. | Gheysen K, Mihai C, Conrath K, Martins JC. Rapid identification of common hexapyranose monosaccharide units by a simple TOCSY matching approach. Chemistry. 2008;14:8869-8878. |
| 25. | Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129-153. |
| 26. | Waters NJ, Holmes E, Waterfield CJ, Farrant RD, Nicholson JK. NMR and pattern recognition studies on liver extracts and intact livers from rats treated with alpha-naphthylisothiocyanate. Biochem Pharmacol. 2002;64:67-77. |
| 27. | Commodari F, Khiat A, Ibrahimi S, Brizius AR, Kalkstein N. Comparison of the phytoestrogen trans-resveratrol (3, 4', 5-trihydroxystilbene) structures from x-ray diffraction and solution NMR. Magn Reson Chem. 2005;43:567-572. |
| 28. | Mandelshtam VA. The multidimensional filter diagonalization method. J Magn Reson. 2000;144:343-356. |
| 29. | Beger HG, Rau BM, Gansauge F, Poch B. Duodenum-preserving subtotal and total pancreatic head resections for inflammatory and cystic neoplastic lesions of the pancreas. J Gastrointest Surg. 2008;12:1127-1132. |
| 30. | Nair RJ, Lawler L, Miller MR. Chronic pancreatitis. Am Fam Physician. 2007;76:1679-1688. |
| 31. | Riediger H, Adam U, Fischer E, Keck T, Pfeffer F, Hopt UT, Makowiec F. Long-term outcome after resection for chronic pancreatitis in 224 patients. J Gastrointest Surg. 2007;11:949-959; discussion 959-960. |
Peer reviewer: Vinay Kumar Kapoor, Professor, Department of Surgical Gastroenterology, Sanjay Gandhi Post-Graduate Institute of Medical Sciences, Lucknow 226014, India
S- Editor Sun H L- Editor O’Neill M E- Editor Ma WH