Published online Apr 28, 2011. doi: 10.3748/wjg.v17.i16.2104
Revised: September 9, 2010
Accepted: September 16, 2010
Published online: April 28, 2011
AIM: To evaluate the impact of feeding colicky infants with an adapted formula on the hydrogen breath test and clinical symptoms.
METHODS: Hydrogen expiration was measured by SC MicroLyzer gas chromatography at inclusion and 15 d after treatment with an adapted low-lactose formula in 20 colicky infants.
RESULTS: All babies were symptomatic: 85% with excess gas, 75% with abnormal feeding pattern, and 85% with excessive crying. The hydrogen breath test at inclusion was abnormal: 35 ± 3.1 ppm. After 15 d feeding with an adapted low-lactose formula, crying and flatulence decreased in 85% of patients (P < 0.001). For infants in whom no decrease of gas was reported, crying was still reduced (P < 0.01). Moreover, the feeding pattern was improved in 50% of infants when it was initially considered as abnormal. Finally, the hydrogen breath test decreased significantly (10 ± 2.5 ppm, P < 0.01).
CONCLUSION: This study showed an association between clinical improvement and evidence of decreased levels of hydrogen when the infants were fed with a specially designed, low-lactose formula.
- Citation: Infante D, Segarra O, Luyer BL. Dietary treatment of colic caused by excess gas in infants: Biochemical evidence. World J Gastroenterol 2011; 17(16): 2104-2108
- URL: https://www.wjgnet.com/1007-9327/full/v17/i16/2104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i16.2104
Infant colic continues to be one of the most disconcerting issues in pediatric medicine. Wessel in 1954 established the famous “rule of three” criteria: “a symptomatic disorder characterized by paroxysms of fussing, agitation or crying, lasting more than 3 h a day and occurring more than 3 d a week for at least 3 wk”[1]. These criteria are now outdated and as there is no clear definition for the condition, studies on its causes, prevalence and treatment inevitably include a heterogeneous group of infants with different problems[2-4]. The definition of “excessive infant crying syndrome”[5] is preferred, although the word “colic” is still used and can be defined as an acronym standing for “Cause Obscure Lengthy Infant Crying”. It is characterized by paroxysms of excessive and inconsolable crying. The infant might present with a tense abdomen, flex the leg to the abdomen, and appear flushed. Symptoms typically start around the second week of life, peak around 3-6 wk and resolve by 3 mo[4,6]. This term now includes digestive disorders such as constipation, gastroesophageal reflux, allergy to cow milk proteins, and excess intestinal gas due to malabsorption of lactose, and its prevalence has been established[6,7].
These disorders, although not serious from a medical point of view, can be very distressing for the baby and his/her family, and can be associated with symptoms of depression in the mother in the first months after birth[8]. For most of these disorders, some dietary solutions have been developed in compliance with the international expert group coordinated by ESPGHAN (European Society for Paediatric Gastroenterology, Hepatology and Nutrition)[9].
The objective of this study was to provide clinical and biochemical evidence of the efficacy of an adapted formula in colic caused by excessive gas, due to physiological hypolactasia, which led to excessive infant crying syndrome.
We included formula-fed infants who were referred to their pediatrician and/or the Unit of Gastroenterology, Hepatology and Nutrition, Children’s Hospital, Vall d′Hebron, Barcelona, Spain because of excessive crying reported by their parents. Infants with vomiting/regurgitation, constipation or cutaneous rash were excluded. When the parents reported “rumbling tummies” excessive flatus, and frothy stools, we considered it as suggestive of carbohydrate malabsorption. Other symptoms were taken into account such as feeding difficulties (e.g. crying during meals) and excessive crying time per 24 h. The hydrogen breath test was performed in case of suspected excess intestinal gas, or infant crying for > 3 h/d to diagnose possible reduced lactose absorption.
Twenty consecutive infants with positive hydrogen breath test were included in this study. All infants were fed with an adapted formula, from various brands having a lactose content of 7 g/100 mL equivalent to 10.4 g of lactose/100 kcal (caloric density of formulas 67 kcal/100 mL).
All of them were eutrophic, Caucasian, healthy full term infants whose growth and development had been normal since birth. Infants were 3.7 wk old on average (range: 1.7-6 wk). They were switched to an adapted formula, Novalac AC (United Pharmaceuticals SA, Paris, France) during the intervention period (Table 1).
| Average composition for 100 mL | |
| Energy (kcal) | 65.7 |
| Proteins (g) | 1.4 |
| Carbohydrates (g) | 7.5 |
| Lactose (g) | 2.3 |
| Maltodextrin (g) | 5.2 |
| Fat (g) | 3.3 |
| C18:2 (mg) | 610.0 |
| C18:3 (mg) | 59.8 |
| Calcium (mg) | 50.7 |
| Phosphorus (mg) | 31.2 |
Duration of crying, intestinal bloating and behavior during feeding (such as interruption of the meal due to crying) were evaluated through a questionnaire at baseline and during a second consultation 15 d later. A second hydrogen breath test was also performed during the second visit in these 20 infants.
The persons legally responsible for the children were invited to give their informed consent for participation in the study. The study was approved by the local ethics committee.
Breath samples were collected before the start of feeding, as well as at 90, 120 and 180 min after the beginning of the meal. The feeding schedule was not modified. Samples were taken using face masks with a two-way valve and a pot system. Breath samples were collected in duplicate. The samples were injected in an SC MicroLyzer gas chromatograph (Quinton Instrument Company, USA) for simultaneous detection of hydrogen, CO2 and methane[10]. This model had an internal gas chromatographic column through which the sample was flushed. Material in the column retarded components which might have interfered with the measurement, and hydrogen thus appeared by itself at the detector and was accurately measured. The gas was inserted in a SvRite-10 cartridge before being analyzed. Prior calibration was performed with a standard gas that contained 102 ppm hydrogen, 23 ppm methane, and 5% CO2. The results were expressed as parts per million (ppm). The result was considered to be positive when there was an increase > 20 ppm; the normal level for methane being < 10 ppm applying a correction factor for CO2. The hydrogen breath data were analyzed as a nested factorial design by analysis of variance. For each infant, the maximum hydrogen value was defined as being the highest mean of the hydrogen breath test, because the actual time from the beginning of the feeding did not have a consistent impact on the value.
Hydrogen values were expressed as mean ± SE. The values of expired hydrogen were compared using Student’s t test. A χ2 test was used for categorical variables when both groups were compared. P < 0.05 was considered significant. The statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
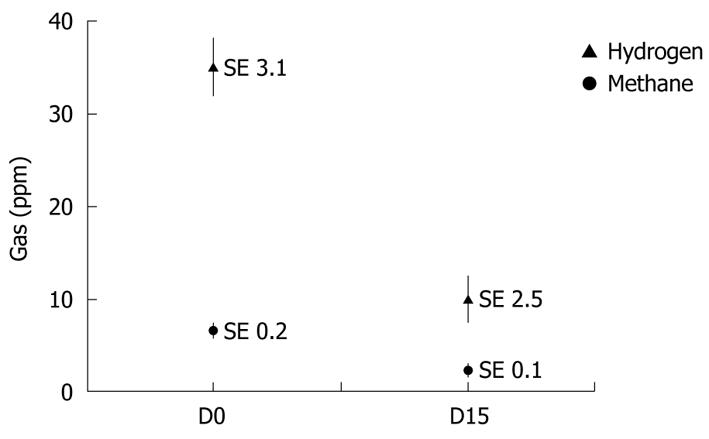
Table 2 shows the clinical evolution (data reported by relatives) of infants who were included because of crying secondary to excess gas. The duration of crying was reduced in all infants regardless of the initial duration, and 85% cried for < 1 h/d. Moreover, of the 85% of infants reported with excessive gas at inclusion, only 25% still had excessive gas at the end of the study period (P < 0.001). Four out of the five infants who were described by their parents as having excessive gas were crying for < 1 h/d. The proportion of infants for whom feeding was described as abnormal decreased from 75% at inclusion to 30% after 2 wk feeding with Novalac AC (P < 0.01). The level of hydrogen expired (biochemical evidence) decreased from 35 ± 3.1 ppm at inclusion to 10 ± 2.5 ppm (P < 0.01) after 2 wk feeding with Novalac AC. The methane excretion was unchanged (Figure 1).
| Inclusion | After 15 d | χ2 | P | |
| Excess gas | 17 (85) | 5 (25) | 14.56 | < 0.001 |
| Abnormal feeding | 15 (75) | 6 (30) | 8.22 | < 0.01 |
| Duration of crying | ||||
| < 1 h/d | 85% | |||
| 1-3 h/d | 13 (65) | 3 (15) | 10.41 | < 0.001 |
| > 3 h/d | 7 (35) | 0 (0) | 8.48 | < 0.01 |
Many authors tend to include digestive disorders in the etiology of “excessive infant crying syndrome” (COLIC), and recommend dietary treatment of this functional disorder[6,7]. Therefore, this study focused on transient lactose intolerance in infants with colic[11,12] and we do not discuss other etiologies such as food hypersensitivity, which has been reviewed by Hill et al[13].
As a result of the failure to break down all the lactose ingested, part of it enters the large bowel where it becomes a substrate for lactobacilli and bifidobacteria. This reaction (fermentation) produces hydrogen and other substances. Therefore, subsequent increases in breath hydrogen are accepted as an indirect sign of hypolactasia[6,11,12,14]. Miller et al[12] have shown that the expired hydrogen in 65 infants with colic was significantly higher than in control subjects (29 ppm vs 11 ppm, P < 0.001). Sixty-two percent of children with colic had expired hydrogen > 20 ppm, but 38% of control subjects also had abnormal levels of expired hydrogen. Similar results have been shown by Moore et al[14], with 80% of colicky infants having positive expired hydrogen vs 36% in normal infants. The assignment of infants to the colicky or control groups according to the mothers’ perception of the duration of crying might not distinguish clearly colicky or non-colicky infants. This might explain the dissociation between clinical and hydrogen values reported by some studies[12-14]. Moreover, among infants with positive expired hydrogen, individual response to abdominal distension might vary, and this is why some infants cry more than others. Therefore, the issue of personal susceptibility to stimuli and a lengthy crying response varies considerably between infants.
Our baseline data are close to those reported by Miller et al[12]. In this pilot study, we assessed the efficacy of the studied formula only in children younger than 6 wk who had a positive expired hydrogen test and clearly defined symptoms.
Mature breast milk contains 7 g lactose per 100 mL (10.2 g/100 kcal), as do several standard infants formulas. In the first few weeks, infants present a physiological or functional lactase deficiency that limits the amount of lactose that they can digest[15]. Twenty-seven point five percent of neonates present a positive hydrogen breath test (i.e. > 20 ppm) after lactose ingestion, regardless of sex or gestational age, with only weight playing a significant role: hydrogen expired is more important in infants with a birth weight < 2.5 kg than in those > 2.5 kg[16]. One study[17] has found that infants who weigh < 1.8 kg, fed with a low-lactose formula (< 5% lactose) ingested more calories, finished their bottles sooner, presented less milk residue in the stomach, and required feeding more often and with less interruptions than those fed with a formula with normal lactose content (7 g/100 mL). The study of Douwes et al[18] has indicated that abnormal expired hydrogen is more frequent in breast-fed infants or those fed with a 7.5% lactose formula, than in infants fed with a 1% lactose formula.
Furthermore, the concentration of hydrogen in the breath of healthy infants increases from birth to reach its highest level in the second month of life, and declines to low concentrations by 3-4 mo of age. This pattern is parallel to the evolution of crying in infants with COLIC[4]. Children selected in our study were almost as old as those in the clinical studies of Barr et al[4] or Moore et al[14] (3.7 wk vs 28.4 d and 2.6 wk, respectively) and younger than those studied by Miller et al[12] (median age: 8 wk).
Fermentation of the disaccharide generates osmotic active substances such as lactic acid, short chain fatty acids, and hydrogen and/or methane. Methane production in lactose malabsorbers is normal, and without significance[10].
Colic can result directly from the hyper-peristaltic stimulus of the fluid load imposed by the osmotic action of unabsorbed lactose in the small intestine, and gas or pharmacologically active metabolites might be responsible for the symptomatic signs. In many susceptible infants, this excess of gas is responsible for triggering colic.
Several studies have investigated lengthy crying and have related it to excess intestinal gas[14,19,20]. The rapid production of hydrogen in the lower bowel distends the colon, which causes different symptoms. This model of colic implies that symptoms could be relieved by reducing the lactose content of the infant feed. Four clinical trials that have used artificial lactase have been published[19,21-23]. When lactase is added to a formula 30 min before it is fed, 30% of the lactose is not hydrolyzed. In a double-blind, placebo-controlled crossover study, 10 colicky infants were fed breast milk and cows’ milk formulas, untreated and treated with lactase[19]. This study showed no evidence that low-lactose milk reduced the severity and amount of crying[19]. In another double-blind, placebo-controlled crossover trial in 12 infants, no effects on duration of crying and fussing were demonstrated[21]. In a third study with the same methodology, the lactase-treated formula reduced crying time by 1.14 h/d[22]. Yet another double-blind placebo-controlled crossover study was performed on 53 infants with colic who were treated with placebo or lactase added to their formula 4 h before they were fed. Data on 46 infants were available for crying time analysis and hydrogen breath tests were available in 34. Only 32 infants complied with treatment. In these infants, crying time and median expired hydrogen were significantly lower in the active group than in the placebo group[23]. The results of our study were in agreement with Kanabar’s study that also included an expired hydrogen measurement.
Differences in hydrogen breath excretion between colicky and control infants might also be associated with factors other than the amount or rate of delivery of lactose to the colon. The microbiota, the colonic bacterial metabolic pathways, the partial pressure of hydrogen in the colon, the buffering capacity of the colon, gut perfusion, and incomplete monosaccharide absorption might all play a part. Therefore, the volume of gas released by a fecal sample reflects the end result of a complex interaction of several factors.
This pathophysiological mechanism explains the clinical and biochemical response of these infants to an adapted, low-lactose diet[22,23]. However, when calcium absorption is enhanced by the presence of lactose[24], the formula has a lactose content (3 g/100 mL) that provides a daily amount close to absorption capacity in young infants of 4.5 g/kg per day)[25].
Even in the absence of a placebo control group, we believe that the clinical improvement observed in our study was related to dietary management. This clinical improvement appeared earlier (after 15 d feeding with the test formula only) than the usual resolution of colic. Moreover this improvement was endorsed by a decrease in expired hydrogen.
In conclusion this non-randomized, non-placebo-controlled pilot study demonstrates that the use of an adapted infant formula with a low lactose concentration leads to clinical improvement and a decrease in expired hydrogen in colicky infants. Thus, infants with colic might benefit from a switch from standard formula to this specific adapted formula. Larger randomized clinical trials on the efficacy of this formula are needed.
Infant colic is still one of the most disconcerting issues in pediatric medicine. This term now includes digestive disorders such as constipation, gastroesophageal reflux, allergy to cow milk proteins, and excess intestinal gas due to lactose malabsorption.
In infants with colic, the possibility of functional lactase deficiency has led to clinical trials with lactase supplementation of infant formulas. These studies have given conflicting results.
This article reports the clinical and biological efficiency of an adapted low lactose formula in infants with colic and positive hydrogen expiration.
In colicky infants with excess abdominal gas, a diet with an adapted low lactose formula can be tried for several days. If the results are positive, the diet can be continued for several months.
The experiments were well designed and the paper is well written. However, there is one major concern about the data analysis.
| 1. | Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics. 1954;14:421-435. |
| 2. | St James-Roberts I. What is distinct about infants' "colic" cries? Arch Dis Child. 1999;80:56-61; discussion 62. |
| 3. | Lucassen PL, Assendelft WJ, van Eijk JT, Gubbels JW, Douwes AC, van Geldrop WJ. Systematic review of the occurrence of infantile colic in the community. Arch Dis Child. 2001;84:398-403. |
| 4. | Barr RG, Rotman A, Yaremko J, Leduc D, Francoeur TE. The crying of infants with colic: a controlled empirical description. Pediatrics. 1992;90:14-21. |
| 5. | Poole SR. The infant with acute, unexplained, excessive crying. Pediatrics. 1991;88:450-455. |
| 6. | Savino F. Focus on infantile colic. Acta Paediatr. 2007;96:1259-1264. |
| 7. | Infante Pina D, Badia Llach X, Ariño-Armengol B, Villegas Iglesias V. Prevalence and dietetic management of mild gastrointestinal disorders in milk-fed infants. World J Gastroenterol. 2008;14:248-254. |
| 8. | Vik T, Grote V, Escribano J, Socha J, Verduci E, Fritsch M, Carlier C, von Kries R, Koletzko B. Infantile colic, prolonged crying and maternal postnatal depression. Acta Paediatr. 2009;98:1344-1348. |
| 9. | Koletzko B, Baker S, Cleghorn G, Neto UF, Gopalan S, Hernell O, Hock QS, Jirapinyo P, Lonnerdal B, Pencharz P. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr. 2005;41:584-599. |
| 10. | Tormo R, Bertaccini A, Conde M, Infante D, Cura I. Methane and hydrogen exhalation in normal children and in lactose malabsorption. Early Hum Dev. 2001;65 Suppl:S165-S172. |
| 11. | Hyams JS, Geertsma MA, Etienne NL, Treem WR. Colonic hydrogen production in infants with colic. J Pediatr. 1989;115:592-594. |
| 12. | Miller JJ, McVeagh P, Fleet GH, Petocz P, Brand JC. Breath hydrogen excretion in infants with colic. Arch Dis Child. 1989;64:725-729. |
| 13. | Hill DJ, Hosking CS. Infantile colic and food hypersensitivity. J Pediatr Gastroenterol Nutr. 2000;30 Suppl:S67-S76. |
| 14. | Moore DJ, Robb TA, Davidson GP. Breath hydrogen response to milk containing lactose in colicky and noncolicky infants. J Pediatr. 1988;113:979-984. |
| 15. | Barr RG, Hanley J, Patterson DK, Wooldridge J. Breath hydrogen excretion in normal newborn infants in response to usual feeding patterns: evidence for "functional lactase insufficiency" beyond the first month of life. J Pediatr. 1984;104:527-533. |
| 16. | Laforgia N, Benedetti G, Altavilla T, Baldassarre ME, Grassi A, Bonsante F, Mautone A. [Significance of lactose breath test in the newborn]. Minerva Pediatr. 1995;47:433-436. |
| 17. | Griffin MP, Hansen JW. Can the elimination of lactose from formula improve feeding tolerance in premature infants? J Pediatr. 1999;135:587-592. |
| 18. | Douwes AC, Oosterkamp RF, Fernandes J, Los T, Jongbloed AA. Sugar malabsorption in healthy neonates estimated by breath hydrogen. Arch Dis Child. 1980;55:512-515. |
| 19. | Ståhlberg MR, Savilahti E. Infantile colic and feeding. Arch Dis Child. 1986;61:1232-1233. |
| 20. | Barr RG, Wooldridge J, Hanley J. Effects of formula change on intestinal hydrogen production and crying and fussing behavior. J Dev Behav Pediatr. 1991;12:248-253. |
| 21. | Miller JJ, McVeagh P, Fleet GH, Petocz P, Brand JC. Effect of yeast lactase enzyme on "colic" in infants fed human milk. J Pediatr. 1990;117:261-263. |
| 22. | Kearney PJ, Malone AJ, Hayes T, Cole M, Hyland M. A trial of lactase in the management of infant colic. J Hum Nutr Diet. 1998;11:281-285. |
| 23. | Kanabar D, Randhawa M, Clayton P. Improvement of symptoms in infant colic following reduction of lactose load with lactase. J Hum Nutr Diet. 2001;14:359-363. |
| 24. | Abrams SA, Griffin IJ, Davila PM. Calcium and zinc absorption from lactose-containing and lactose-free infant formulas. Am J Clin Nutr. 2002;76:442-446. |
| 25. | Fomon S. Carbohydrate. Nutrition of Normal Infants. St. Louis: Mosby 1993; 178-180. |
Peer reviewers: Guang-Yin Xu, MD, PhD, Assistant Professor, Division of Gastroenterology, Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555-0655, United States; Loes van Keimpema, MSc, PhD, Deparment of Gastroenterology and Hepatology, Radboud University Nijmegen Medical Center, PO Box 9101, 6500 HB, Nijmegen,
The Netherlands
S- Editor Shi ZF L- Editor Kerr C E- Editor Zheng XM