Published online Apr 28, 2011. doi: 10.3748/wjg.v17.i16.2086
Revised: January 10, 2011
Accepted: January 17, 2011
Published online: April 28, 2011
AIM: To investigate the effect of α-mangostin on the growth and apoptosis induction of human colon cancer cells.
METHODS: The three colorectal adenocarcinoma cell lines tested (COLO 205, MIP-101 and SW 620) were treated with α-mangostin to determine the effect on cell proliferation by MTT assay, cell morphology, chromatin condensation, cell cycle analysis, DNA fragmentation, phosphatidylserine exposure and changing of mitochondrial membrane potential. The molecular mechanisms of α-mangostin mediated apoptosis were further investigated by Western blotting analysis including activation of caspase cascade, cytochrome c release, Bax, Bid, p53 and Bcl-2 modifying factor.
RESULTS: The highest inhibitory effect of α-mangostin on cell proliferation of COLO 205, MIP-101 and SW 620 were 9.74 ± 0.85 μg/mL, 11.35 ± 1.12 μg/mL and 19.6 ± 1.53 μg/mL, respectively. Further study showed that α-mangostin induced apoptotic cell death in COLO 205 cells as indicated by membrane blebbing, chromatin condensation, DNA fragmentation, cell cycle analysis, sub-G1 peak (P < 0.05) and phosphatidylserine exposure. The executioner caspase, caspase-3, the initiator caspase, caspase-8, and caspase-9 were expressed upon treatment with α-mangostin. Further studies of apoptotic proteins were determined by Western blotting analysis showing increased mitochondrial cytochrome c release, Bax, p53 and Bmf as well as reduced mitochondrial membrane potential (P < 0.05). In addition, up-regulation of tBid and Fas were evident upon treatment with α-mangostin (P < 0.01).
CONCLUSION: α-Mangostin may be effective as an anti-cancer agent that induced apoptotic cell death in COLO 205 via a link between extrinsic and intrinsic pathways.
- Citation: Watanapokasin R, Jarinthanan F, Nakamura Y, Sawasjirakij N, Jaratrungtawee A, Suksamrarn S. Effects of α-mangostin on apoptosis induction of human colon cancer. World J Gastroenterol 2011; 17(16): 2086-2095
- URL: https://www.wjgnet.com/1007-9327/full/v17/i16/2086.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i16.2086
Searching for new biologically active compounds, novel chemotherapeutic agents derived from active phytochemicals, could be used to improve the anti-carcinogenicity of standard drug treatment. A variety of tropical plants have useful biological activities and some offer potential therapeutic applications. Mangosteen (Garcinia mangostana L.) in the Clusiaceae family has been used in Southeast Asia as traditional medicine for treatment of wounds, skin infection, diarrhea and chronic ulcer[1]. Phytochemical studies showed that the fruit hull of mangosteen is rich in a variety of oxygenated and prenylated xanthones[2,3] which possess different biological properties, such as anti-mycobacterial[4], anti-fungal[5], anti-oxidant[6-8], cytotoxicity[9-12] and anti-inflammatory activities[13]. However, the underlying molecular mechanisms of α-mangostin in COLO 205 cells are not yet reported.
Apoptosis plays a vital role in controlling cell number in many physiological and developmental stages, tissue homeostasis, and regulation of immune system[14], while insufficient apoptosis is an integral part of cancer development[15]. Mammalian cells have two major apoptotic pathways. One pathway (extrinsic pathway) is triggered when ligands [Fas/CD95, tumor necrosis factor (TNF)-α] bind to receptors on cell surface leading to the activation of caspase-8 and -3[16], respectively. The other involves mitochondrial (intrinsic) pathway induced by anti-cancer drugs, prostaglandin, etc. resulting in disruption of mitochondrial membrane and release of various pro-apoptotic factors[17-19]. The pro-apoptotic and anti-apoptotic members of B-cell CLL/lymphoma 2 (Bcl-2) family regulate the release of cytochrome c, a mitochondrial protein that can activate caspases.
Fas/CD95 belongs to the TNF superfamily and is the prototype of death receptor that initiates an apoptotic cascade[20]. FasL, the tumor necrosis factor-related cytokine, is a ligand of Fas and synthesized as a type II membrane protein[21]. Upon FasL binding, activated death receptors engage the Fas associated death domain (FADD)[22], which in turn recruits caspase-8 and forming the death inducing signaling complex (DISC). The DISC then activates caspase-8 through a proximity-inducing dimerization mechanism. In type I cells, caspase-8 directly activates caspase-3, -6 and -7, leading to cell death. In contrast, in type II cells the small amount of active caspase-8 generated at the DISC is not sufficient to induce cell death, therefore the mitochondria-dependent apoptosis pathway is needed[23]. As such, the pro-apoptotic signal has to be amplified via cleavage of the BH3-only protein Bid, Bax/Bak-assisted release of cytochrome c from the mitochondria, an activation of caspase-9 and subsequently caspase-3[24].
The objective of the present study was to purify the α-mangostin from the fruit hull of Garcinia mangostana L. and explore its effect on apoptosis induction and mechanisms involved in COLO 205 cells.
Mangosteen fruit (G. mangostana) was collected from Kombang District, Chantaburi Province, Thailand in April, 2007. A voucher specimen (Ms Porntip Wongnapa No. 002) was deposited at the Faculty of Science, Ramkhamhaeng University. The dried and pulverized fruit hull of G. mangostana (0.5 kg) was thoroughly extracted with ethyl acetate (EtOAc) at 50°C. The combined extract after filtration was concentrated under reduced pressure to yield the extract as a yellowish solid (285 g). A portion of the extract was subjected to repeated column chromatography over silica gel using a gradient of hexane/acetone which yielded the pure major compound, α-mangostin, including other minor xanthones. Purity of α-mangostin exceeded 98% as determined by LC analysis and its spectroscopic data (NMR and MS) was consistent with the reported values[12].
Three human colorectal cancer cell lines were used: COLO 205 (colorectal adenocarcinoma), MIP-101 (colorectal carcinoma) and SW620 (colorectal adenocarcinoma). COLO 205 and SW620 were obtained from the American Type Culture Collection (Manassas, VA). MIP-101 was a generous gift from Peter Thomas, Boston University School of Medicine, Boston, MA. COLO 205 and MIP-101 were maintained in the RPMI 1640 medium (Invitrogen), supplemented with 10% fetal calf serum (Invitrogen). SW620 were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen), supplemented with 10% fetal calf serum. All cell lines were maintained in culture at 37°C in an atmosphere of 5% CO2.
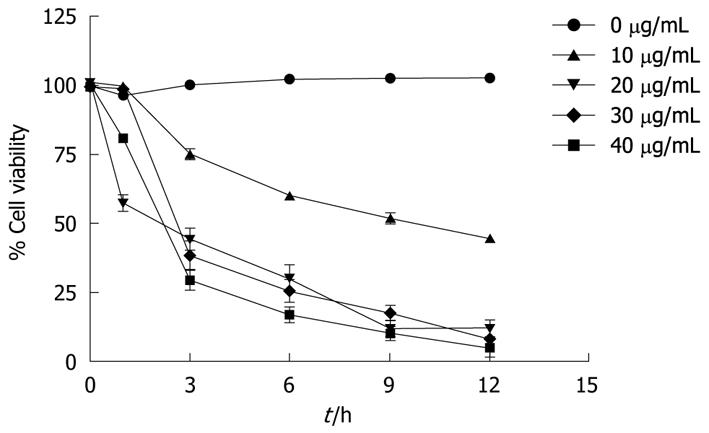
The cytotoxic activity of α-mangostin was determined by cell proliferation analysis using MTT assays as previously described[25]. Briefly, cells were cultured in 96-well plates at a density of 1 × 104/well in complete medium. Then the cells were treated with varying concentrations of α-mangostin and incubated at 37°C for 24 h. The final DMSO concentration in each well was 0.05%, at which concentration no appreciable effect on cell proliferation was seen. Then, 100 μL of 5.0 mg/mL MTT in culture media was added to each well and incubated at 37°C for 2 h. The metabolic product of MTT, formazan, in each well was dissolved in DMSO, and the absorbance was determined at 595 nm. Effect of α-mangostin on the viability of COLO 205 cells was analyzed by using a trypan blue exclusion method. Briefly, cells were cultured in 96-well plates at cell density of 1 × 104/well at 37°C for 24 h. α-mangostin was then added to culture wells at 0, 10, 20, 30 and 40 μg/mL or vehicle (in DMSO), incubated at 37°C then collected periodically (0, 3, 6, 9 and 12 h). The number of viable cells was determined with hemocytometers under a light microscope. Cell viability was expressed as a percentage of the number of viable cells to that of the control, to which no α-mangostin was applied.
Characterization of the anticancer activity of α-mangostin in COLO 205 cells was further conducted. Based on the preliminary experiments, 20 μg/mL of α-mangostin was used to study cell and nuclear morphology, cytochrome c release, mitochondrial transmembrane potential, expression of pro-apoptotic proteins, Fas and truncated-Bid (t-Bid). However, a selective range of test concentrations, ranging from 10 to 30 μg/mL was used to study DNA fragmentation and for cell cycle analysis and annexin V-FITC assays.
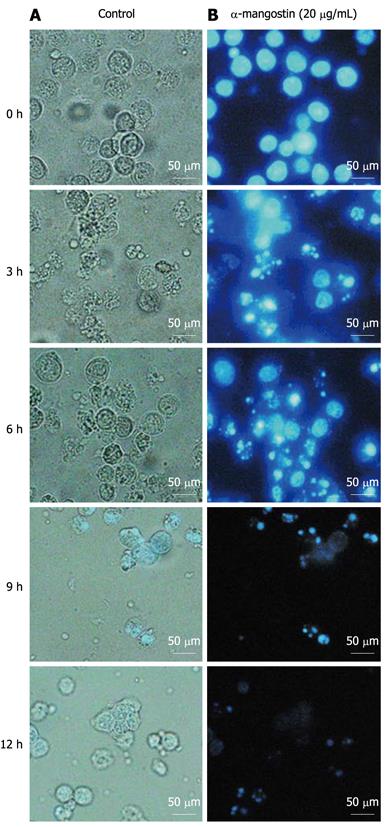
COLO 205 cells were cultured in 24-well culture plates at the initial number of 2 × 104/well in the presence of 20 μg/mL α-mangostin for 3, 6, 9 and 12 h. As controls, cells were cultured in the same fashion in the absence of α-mangostin. The cells were examined under a phase-contrast inverted microscope (model CKX31/CKX41, Olympus) for cell morphology.
The nuclear morphology was analyzed by treatment of COLO 205 cells with 20 μg/mL α-mangostin for 3, 6, 9 and 12 h. Control cells were grown in the same manner in the absence of α-mangostin. Cells were trypsinized and fixed with methanol. Then, cell nuclei were stained by treatment with 1 μg/mL Hoechst 33342 (Sigma) at 37°C for 15 min in the dark. Stained cells were examined under a fluorescence inverted microscope (model BX50, Olympus).
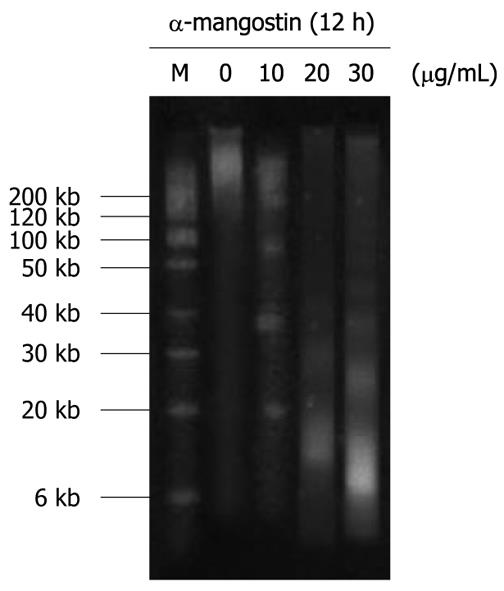
COLO 205 cells were treated with varying concentrations, 10, 20 and 30 μg/mL, of α-mangostin for 12 h and then lysed in 500 μL of lysis solution, consisting of 5 mmol/L Tris-Cl (pH 8.0), 0.5% Triton X-100, and 20 mmol/L ethylenediaminetetraacetic acid (EDTA). The cells were then treated with RNase A (0.5 mg/mL) for 1 h at 37°C. DNA fractions were prepared using phenol-chloroform-isoamyl alcohol (25:24:1) and electrophoresed on 1.8% agarose gels. Approximately 20 μg of DNA was loaded in each well and the agarose gels were run at 50 V for 2 h in Tris-borate/EDTA electrophoresis buffer. DNA was stained with ethidium bromide and visualized under a UV light trans-illuminator and photographed.
The cell cycle analysis, annexin V binding and mitochondrial transmembrane potential were investigated by flow cytometric analysis (FACScan, Becton Dickinson). The proper filters and optimal setting of the instrument were chosen, the histograms generated by FACS were analyzed by Cell Quest™ software (Becton Dickinson).
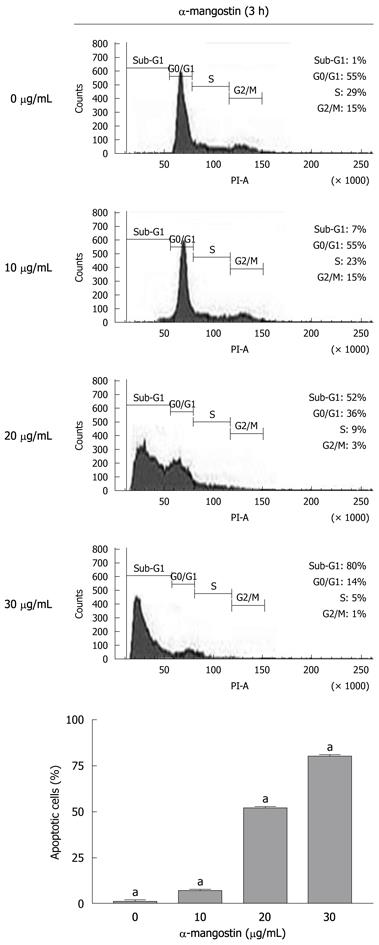
COLO 205 cells were cultured in 6-well culture plates at 4 × 106 cells/well and treated with 0, 10, 20 and 30 μg/mL of α-mangostin for 3 h. The cells were then harvested, washed with PBS and resuspended in 200 μL PBS and fixed in 800 μL of ice-cold 70% ethanol at -20°C, overnight. The cells were stained with 1 mL of 50 μg/mL propidium iodide solution (containing 0.1% Triton X-100, and 0.1% sodium citrate) for 30 min at 37°C. The samples were then analyzed by a flow cytometer (FACScan, Becton Dickinson). Excitation was done at 488 nm, and emission filter at 600 nm. Histograms generated by FACS were analyzed by Cell Quest™ software (Becton Dickinson) to determine the percentage of cells in each phase.
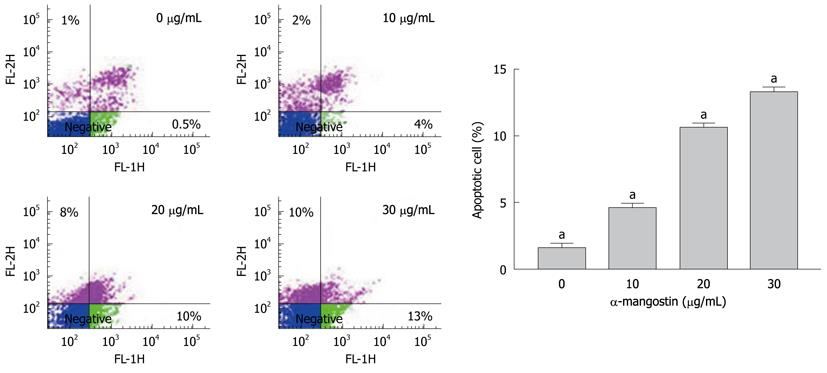
Percentage of α-mangostin-treated cells undergoing apoptosis was determined using an annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD) Bioscience, San Jose, CA). COLO 205 cells at 1 × 105 cells/mL were treated with 0, 10, 20 and 30 μg/mL of α-mangostin for 3 h and resuspended in 100 μL of annexin V binding buffer (10 mmol/L HEPES, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2), then incubated with 5 μL of 1 μg/mL FITC-conjugated annexin V and 1 μg/mL propidium iodide for 15 min at room temperature prior to analysis on a FACScan, Becton Dickinson.
COLO 205 cells at 1 × 106 cells/mL were treated with 20 μg/mL of α-mangostin for 3 h, and then incubated with 10 μg/mL JC-1 (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolcarbocyanine iodide) at 37°C for 10 min in darkness. Stained cells were washed with PBS, followed by FACS analysis. The mitochondrial function was assessed as JC-1 green (uncoupled mitochondria) or red (contact mitochondria).
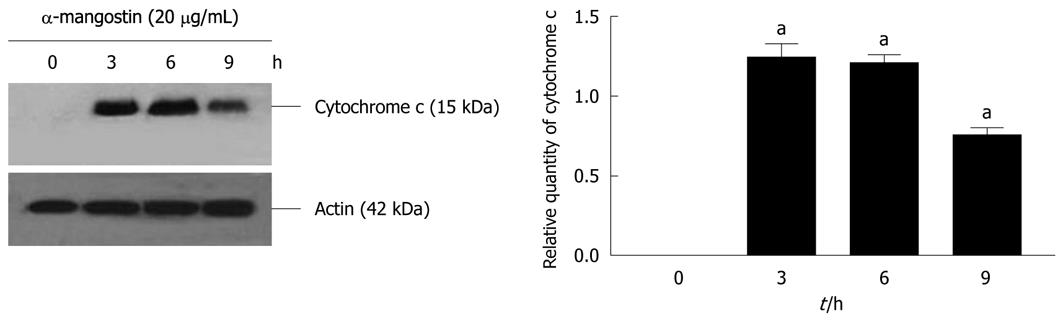
COLO 205 cells were cultured in 6-well culture plates at 4 × 106 cells/well and then incubated in the absence or presence of 20 μg/mL α-mangostin for 3, 6, and 9 h. Cell lysates was prepared as previously described[25]. Briefly, cell suspensions were sonicated for 10 s and the cell lysates was centrifuged at 4°C at 10 000 ×g for 30 min. The supernatants (cytosol fractions) were subjected to SDS-PAGE using 12% polyacrylamide gels, and transferred onto Immobilon P membrane and subjected to immuno-detection of cytochrome c using a mouse monoclonal antibody against human cytochrome c (7H8, mouse monoclonal Ig G 2b, Santa Cruz, Biotechnology) with goat anti-mouse IgG conjugates to horse radish peroxidase (Cell Signaling Technology) and detected using an ECL Plus Western Blotting Detection System (Amersham Biosciences).
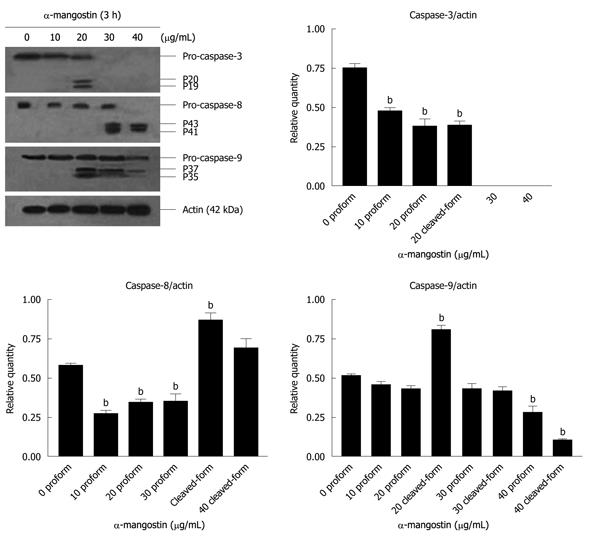
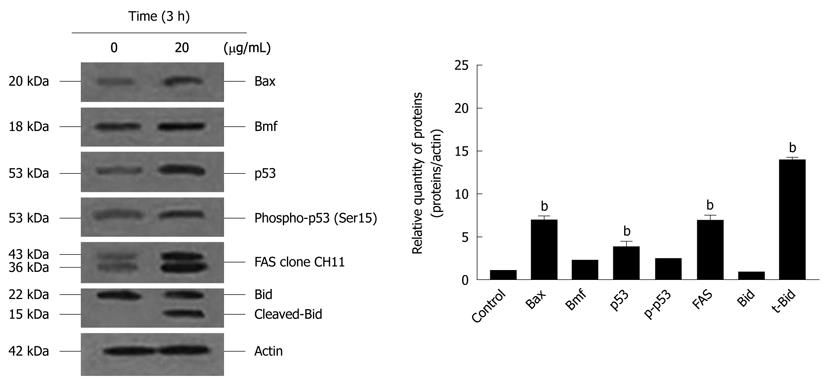
COLO 205 cells were treated with 20 μg/mL α-mangostin for 3 h, lysed in lysis buffer. Cell lysates were subjected to SDS-PAGE using 12% Tris/HCl ready gels (BioRad), The transfered proteins were incubated with appropriate antibodies at 4°C overnight: rabbit polyclonal anti-caspase-3 (8G10); mouse polyclonal anti-caspase-8 (1C12); mouse monoclonal anti-caspase-9 (C9); rabbit polyclonal anti-Bid; mouse monoclonal anti-p53; rabbit polyclonal anti-Bax, anti-Bmf (Cell Signaling Technology) and mouse monoclonal anti-Fas (CD 95) (CH 11, MBL international). After the removal of unbound primary antibodies, the blots were incubated with a secondary antibody (goat anti-rabbit IgG and goat anti-mouse IgG, each of which was conjugated with horse radish peroxidase; (Cell Signaling Technology) as described for Western blotting analysis of cytochrome c above.
Data were expressed as mean ± SD. Statistical comparisons were performed by using one-way analysis of variance. A P value less than 0.05 was considered statistically significance.
The IC50 value of the three human colonic cancer cell lines, after 24 h incubation with serial dilutions of α-mangostin, COLO 205, MIP-101 and SW 620 were 9.74 ± 0.85 μg/mL, 11.35 ± 1.12 μg/mL and 19.6 ± 1.53 μg/mL, respectively. Among the three cell lines tested, the highest inhibitory effect of α-mangostin on cell proliferation was detected with COLO 205. Thus, COLO 205 cells were used as the primary target in subsequent experiments.
The viability of COLO 205 cells decreased by the treatment with α-mangostin in both concentration- and time-dependent fashions (Figure 1). Treatment of COLO 205 cells with α-mangostin at 20 μg/mL or higher for 12 h reduced the number of viable cells to approximately 5%-10% of the control cells, to which no α-mangostin was applied.
Cells treated with 20 μg/mL α-mangostin for 3, 6, 9 and 12 h showed evident morphological changes including rounding and blebbing as well as the presence of apoptotic bodies (Figure 2A, 3, 6, 9 and 12 h). Such morphological changes were not seen with control cells (without the α-mangostin treatment) (Figure 2A, 0 h). For nuclei staining with Hoechst 33342, chromatin condensation and destructive fragmentation of the nucleus with intact cell membrane was seen with COLO 205 cells which had been treated with 20 μg/mL of α-mangostin for 3, 6, 9 and 12 h (Figure 2B, 3, 6, 9 and 12 h), indicated early apoptosis while the nuclei of control cells without α-mangostin treatment showed normal morphology (Figure 2B, 0 h).
The early apoptosis was detected upon treatment of COLO 205 cells with 0, 10, 20 and 30 μg/mL of α-mangostin for 3 h using Annexin V-FITC assay. The results indicated significant increases in apoptotic populations in COLO 205 cells approximately 0.50% ± 0.02%, 4.00% ± 0.25%, 10.00% ± 0.50% and 13.00% ± 0.30%, (P < 0.05) respectively (Figure 3). Exposure of COLO 205 cells to increasing concentrations (0, 10, 20 and 30 μg/mL) of α-mangostin for 3 h resulted in increased percentage of cells arrested in sub G-1 phase (apoptotic cell death) of 1.03% ± 0.15%, 7.00% ± 0.20%, 51.00% ± 1.53% and 80.00% ± 2.08% (P < 0.05), respectively (Figure 4). It is evident that the formation of apoptotic cells at sub-G1 phase was directly proportional to the increased concentration of α-mangostin.
Fragmentation of genomic DNA was apparent by the presence of DNA ladders when COLO 205 cells were treated with 10, 20 and 30 μg/mL α-mangostin for 12 h (Figure 5). The characteristic ladder pattern of discontinuous DNA fragments was observed only in the treated cells, whereas the untreated control showed no DNA fragmentation.
The activation of caspases-3, -8 and -9 was detected (Figure 6). The amount of the pro-enzyme form of caspase-3 (pro-caspase-3, 35 kDa), pro-caspase-8 (57 kDa) and pro-caspase-9) (47 kDa) decreased with increasing concentration of α-mangostin (10, 20, 30 and 40 μg/mL). Accordingly, cleaved activated forms of caspase-3 (19 and 20 kDa), caspase-8 (41 and 43 kDa) and caspase-9 (35 and 37 kDa) (P < 0.01) became apparent upon treatment of α-mangostin at 20 μg/mL or higher. Activated caspase-3 was apparent upon treatment with 20 μg/mL α-mangostin but not at higher concentrations (30 and 40 μg/mL α-mangostin) due to cell death at higher concentrations, as the effector caspase-3 is the late event of the pathways. On the other hand, caspase-8 and -9 were also detected implying the consecutive activation of caspases of the intrinsic pathway. In addition, induction of apoptosis by α-mangostin was accompanied by increased phospho-p53, pro-apoptotic Bax and Bmf (P < 0.01) (Figure 7). The release of cytochrome c from mitochondria to cytosol was evident upon treatment of COLO 205 cells with 20 μg/mL α-mangostin for 3 h and 6 h (Figure 8). The non-cytosolic fraction (pellet) of the treated cells showed no cytochrome c expression (data not shown). At 9 h of α-mangostin treatment, cytochrome c was reduced due to increasing cell death. No appreciable amount of cytochrome c was detected in the cytosol fraction of control COLO 205 cells, to which no α-mangostin was applied (Figure 8). This implied that α-mangostin mediated apoptosis is accompanied by mitochondrial dysfunction, which could be further strengthened by mitochondrial membrane depolarization detected by a carbocyanine fluorescence dye, JC-1, upon treatment with α-mangostin. The results indicated the increased percentage of cells with depolarized mitochondrial membrane potential (red to green) approximately 82% after α-mangostin treatment for 3 h (Figure 9). These results further confirmed that α-mangostin is an efficient inducer of apoptosis that both extrinsic and intrinsic pathway may be involved. Thus, we further conducted the Western blotting analysis of Bid, t-Bid, the linker between extrinsic and intrinsic pathway, and Fas receptor and found that they were up-regulated upon α-mangostin treatment (P < 0.01) (Figure 7).
The evident goal of medical research is to be able to manipulate the machinery of cell death. Regulation of apoptosis might also lead to new possibilities for cancer therapy[26]. In the present study, we showed that α-mangostin treatment of human colon COLO 205 induced cytotoxic effects in a dose and time dependent fashion. We then investigated the apoptotic effects of α-mangostin in COLO 205 cells to advance our knowledge of its biological functions and also health advantages. The membrane shrinkage, chromatin condensation and fragmentation were detected. As cancer growth is associated with the loss of cell cycle checkpoints, which regulate the DNA integrity and ensure that the genes are co-ordinately expressed[27]. The sub-G1 fraction and phosphatidylserine translocation is an indication of apoptosis cell death that naturally occurs in cells and is beneficial for cancer therapy[28]. Therefore, to characterize apoptotic cells upon treatment of COLO 205 cells with α-mangostin, a biparametric cytofluorimetric analysis was performed using PI and annexin V-FITC, which stained DNA and phosphatidylserine residues, respectively. In the early apoptotic process, a phosphatidylserine residue became exposed on the cell surface by flipping from the inner to outer leaflet of the cytoplasmic membrane[29,30]. Our results demonstrated the increased sub-G1 population and numbers of early apoptotic cells upon treatment of COLO 205 cells with 20 μg/mL α-mangostin for 3 h as compared to untreated control. The Bcl-2 family of proteins regulates apoptosis and it has been shown that the gene products of Bcl-2 and Bax play important roles in apoptotic cell death[14]. The Bcl-2 family comprises of both pro-apoptotic and anti-apoptotic proteins that elicit opposite effects on mitochondria. Anti-apoptotic members include Bcl-2, Bcl-xL, Bcl-W, Mcl-1, whereas pro-apoptotic members are Bid, Bax, Bakm, Bmf and others. Several pathways involve p53-mediated apoptosis, and one of these is the Bcl-2 and Bax proteins. The Bax protein is a p53 target and known to promote cytochrome c release from mitochondria which in turn activates caspase-3. Regulation of Bax/Bcl-2 and caspases activity becomes important targets for cancer intervention. Caspase-3 being the major executioner caspase[31], thus we examined whether activated caspase-3, -8 and -9 is involved in apoptotic induction and found evident expression of activated caspase-3, -8 and -9.
Under the influence of α-mangostin treatment in COLO 205 cells, a cell death pathway both via death receptor pathway and mitochondrial pathway may be involved, as caspase-8 and -9 were expressed. We further demonstrated the loss of mitochondrial membrane potential, release of cytochrome c into the cytosol and DNA fragmentation. Our results are in agreement with a study showing that apoptosis was associated with a loss of mitochondrial membrane potential, which may correspond to the opening of an outer membrane pore, leading to cytochrome c release from mitochondria into the cytosol. The released cytochrome c later triggered the cleavage and activation of caspases and onset of apoptosis[32]. The expression of Fas and caspase-8 implies the death receptor pathway, while regulation of mitochondrial membrane permeability by upregulation of Bax and p-Bad triggering the release of cytochrome c from mitochondria to cytosol. Our further investigation showed the expression of Fas, Bid, t-Bid, p-53, phospho-p53 and the proteins in Bcl-2 family (Bax and Bmf). Expression of t-Bid is the important linkage between death receptor and mitochondrial pathway. When Bid is cleaved into t-Bid by the activated caspase-8 and translocated towards the mitochondria to activate Bax and Bak, then changing the mitochondrial membrane permeability and the release of cytochrome c[33] which form a complex with apoptotic enzyme activators and caspase-9. It activates and starts a caspase cascade reaction, which further activates the downstream caspase-3 and other caspase family members for apoptosis induction. We also noticed that α-mangostin up-regulated the expression of Bax in COLO 205 cells at protein level, suggesting that Bcl-2 family protein regulate α-mangostin mediated apoptotic cell death.
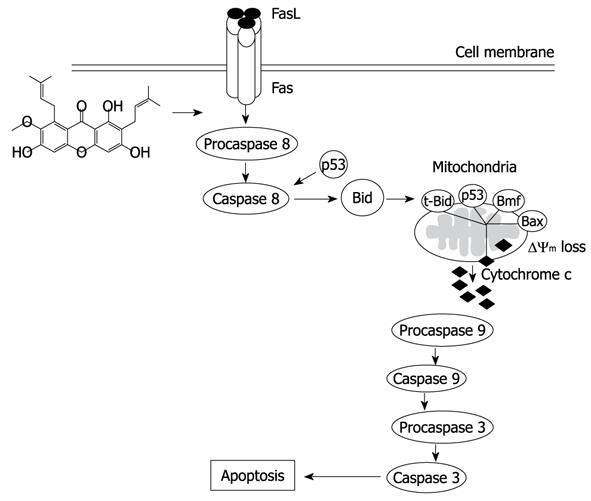
Taken together our results evaluating the molecular mechanism that α-mangostin induced apoptosis cell death in COLO 205 cells may occur via caspase-8 dependent cleavage of Bid to tBid providing a link between extrinsic and intrinsic pathways (Figure 10). This could be a promising chemotherapeutic agent and may also serve as a model to develop and design new derivatives which may be more potent.
Cancer causes significant morbidity and mortality and is a major public health problem worldwide. Globally, colorectal cancer is one of the most common types of cancer in both men and women. Phytochemical studies showed that a variety of tropical plants have useful biological activities and some offer potential therapeutic applications. However, the molecular mechanisms underlying α-mangostin induced apoptosis in human colorectal adenocarcinoma have not yet been fully understood.
Apoptosis plays a vital role in controlling cell number in many physiological and developmental stages, tissue homeostasis, and regulation of the immune system, while insufficient apoptosis is an integral part of cancer development. The main function of apoptosis is to dispose of a cell without causing damage or stress to neighbouring cells. Thus, the anti-cancer drug that induces apoptotic cell death would be more suitable for use in patients and should be further developed. Therefore, the effect of α-mangostin on the growth and apoptosis induction of human colon cancer cells was investigated in this study.
The results showed that α-mangostin induced apoptotic cell death in COLO 205 cells indicating by membrane blebbing, chromatin condensation, DNA fragmentation, cell cycle analysis, sub-G1 peak, and phosphatidylserine exposure. The expression of caspase-3, caspase-8 and caspase-9, cytochrome c release, Bax, p53 and Bcl-2 modifying factor (Bmf) as well as reduced mitochondrial membrane potential were demonstrated. In addition, upon treatment with α-mangostin, up-regulation of tBid and Fas were evident. Therefore, α-mangostin may be effective as an anti-cancer agent that induces apoptotic cell death in COLO 205 via a link between extrinsic and intrinsic pathways.
α-mangostin could be used as a future promising anti-cancer agent for the treatment of colorectal adenocarcinoma cells.
The fruit hull of mangosteen (Garcinia mangostana L.) in the Clusiaceae family is rich in a variety of oxygenated and prenylated xanthones which possess different biological properties, such as anti-mycobacterial, anti-fungal, anti-oxidant, cytotoxicity and anti-inflammatory activities.
In this study the authors examined the effect of α-mangostin on the growth and apoptosis induction of human colon cancer cells. They showed that α-mangostin induced apoptotic cell death in COLO 205 cells, activated caspase-3, -8, and -9, increased the mitochondrial cytochrome c release, Bax, p53 and Bmf, reduced mitochondrial membrane potential, and up-regulated tBid and Fas. From these results, the authors suggested that α-mangostin may be effective as an anti-cancer agent via a link between extrinsic and intrinsic pathways. It is interesting that the authors clearly showed the mechanism through which α-mangostin induced apoptosis in colon cancer cells.
| 1. | Mahabusarakam W, Wiriyachitra P, Taylor WC. Chemical constituents of garcinia mangostana. J Nat Prod. 1987;50:474-478. |
| 2. | Peres V, Nagem TJ. Trioxygenated naturally occurring xanthones. Phytochemistry. 1997;44:191-214. |
| 3. | Peres V, Nagem TJ, de Oliveira FF. Tetraoxygenated naturally occurring xanthones. Phytochemistry. 2000;55:683-710. |
| 4. | Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, Suksamrarn A. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem Pharm Bull (Tokyo). 2003;51:857-859. |
| 5. | Gopalakrishnan G, Banumathi B, Suresh G. Evaluation of the antifungal activity of natural xanthones from Garcinia mangostana and their synthetic derivatives. J Nat Prod. 1997;60:519-524. |
| 6. | Williams P, Ongsakul M, Proudfoot J, Croft K, Beilin L. Mangostin inhibits the oxidative modification of human low density lipoprotein. Free Radic Res. 1995;23:175-184. |
| 7. | Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem. 2006;54:2077-2082. |
| 8. | Mahabusarakam W, Proudfoot J, Taylor W, Croft K. Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radic Res. 2000;33:643-659. |
| 9. | Ho CK, Huang YL, Chen CC. Garcinone E, a xanthone derivative, has potent cytotoxic effect against hepatocellular carcinoma cell lines. Planta Med. 2002;68:975-979. |
| 10. | Matsumoto K, Akao Y, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorg Med Chem. 2005;13:6064-6069. |
| 11. | Nakagawa Y, Iinuma M, Naoe T, Nozawa Y, Akao Y. Characterized mechanism of alpha-mangostin-induced cell death: caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem. 2007;15:5620-5628. |
| 12. | Suksamrarn S, Komutiban O, Ratananukul P, Chimnoi N, Lartpornmatulee N, Suksamrarn A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull (Tokyo). 2006;54:301-305. |
| 13. | Chen CN, Hsieh FJ, Cheng YM, Chang KJ, Lee PH. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in angiogenesis and clinical outcome of human gastric cancer. J Surg Oncol. 2006;94:226-233. |
| 14. | Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347-354. |
| 15. | Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462. |
| 16. | Leist M, Jäättelä M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589-598. |
| 17. | Rudner J, Lepple-Wienhues A, Budach W, Berschauer J, Friedrich B, Wesselborg S, Schulze-Osthoff K, Belka C. Wild-type, mitochondrial and ER-restricted Bcl-2 inhibit DNA damage-induced apoptosis but do not affect death receptor-induced apoptosis. J Cell Sci. 2001;114:4161-4172. |
| 18. | Nencioni A, Lauber K, Grünebach F, Van Parijs L, Denzlinger C, Wesselborg S, Brossart P. Cyclopentenone prostaglandins induce lymphocyte apoptosis by activating the mitochondrial apoptosis pathway independent of external death receptor signaling. J Immunol. 2003;171:5148-5156. |
| 19. | Castedo M, Ferri K, Roumier T, Métivier D, Zamzami N, Kroemer G. Quantitation of mitochondrial alterations associated with apoptosis. J Immunol Methods. 2002;265:39-47. |
| 20. | Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci USA. 1999;96:10964-10967. |
| 21. | Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S. Fas ligand in human serum. Nat Med. 1996;2:317-322. |
| 22. | Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, Lenardo MJ. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354-2357. |
| 23. | Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675-1687. |
| 24. | Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491-501. |
| 25. | Watanapokasin R, Jarinthanan F, Jerusalmi A, Suksamrarn S, Nakamura Y, Sukseree S, Uthaisang-Tanethpongtamb W, Ratananukul P, Sano T. Potential of xanthones from tropical fruit mangosteen as anti-cancer agents: caspase-dependent apoptosis induction in vitro and in mice. Appl Biochem Biotechnol. 2010;162:1080-1094. |
| 26. | Tamatani T, Azuma M, Motegi K, Takamaru N, Kawashima Y, Bando T. Cepharanthin-enhanced radiosensitivity through the inhibition of radiation-induced nuclear factor-kappaB activity in human oral squamous cell carcinoma cells. Int J Oncol. 2007;31:761-768. |
| 27. | Nojima H. Cell cycle checkpoints, chromosome stability and the progression of cancer. Hum Cell. 1997;10:221-230. |
| 28. | Lopéz L, Villavicencio MA, Albores A, Martínez M, de la Garza J, Meléndez-Zajgla J, Maldonado V. Cupressus lusitanica (Cupressaceae) leaf extract induces apoptosis in cancer cells. J. Ethnopharmacol. 2002;80:115-120. |
| 29. | Diaz C, Schroit AJ. Role of translocases in the generation of phosphatidylserine asymmetry. J Membr Biol. 1996;151:1-9. |
| 30. | Earnshaw WC. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995;7:337-343. |
| 32. | Kantrow SP, Piantadosi CA. Release of cytochrome c from liver mitochondria during permeability transition. Biochem Biophys Res Commun. 1997;232:669-671. |
| 33. | Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM, Herrmann A, Basañez G. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J Biol Chem. 2004;279:30081-30091. |
Peer reviewer: Masahiro Iizuka, MD, PhD, Director, Akita Health Care Center, Akita Red Cross Hospital, 3-4-23, Nakadori, Akita, 010-0001, Japan
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM