Published online Apr 21, 2011. doi: 10.3748/wjg.v17.i15.1982
Revised: January 4, 2011
Accepted: January 11, 2011
Published online: April 21, 2011
AIM: To study the effect of viscosity on axial force in the esophagus during primary peristalsis using a newly validated impedance-based axial force recording technique.
METHODS: A probe able to simultaneously measure both axial force and manometry was positioned above the lower esophageal sphincter. Potable tap water and three thickened fluids were used to create boluses of different viscosities. Water has a viscosity of 1 mPa·s. The three thickened fluids were made with different concentrations of Clinutren Instant thickener. The viscous fluids were in appearance comparable to pudding (2 kPa·s), yogurt (6 kPa·s) and slush ice (10 kPa·s). Six healthy volunteers swallowed 5 and 10 mL of boluses multiple times.
RESULTS: The pressure amplitude did not increase with the bolus viscosity nor with the bolus volume whereas the axial force increased marginally with bolus volume (0.1 > P > 0.05). Both techniques showed that contraction duration increased with bolus viscosity (P < 0.01). Association was found between axial force and pressure but the association became weaker with increasing viscosity. The pressure amplitude did not increase with the viscosity or bolus volume whereas the axial force increased marginally with the bolus size.
CONCLUSION: This indicates a discrepancy between the physiological functions that can be recorded with axial force measurements and pressure measurements.
- Citation: Gravesen F, Behan N, Drewes A, Gregersen H. Viscosity of food boluses affects the axial force in the esophagus. World J Gastroenterol 2011; 17(15): 1982-1988
- URL: https://www.wjgnet.com/1007-9327/full/v17/i15/1982.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i15.1982
The primary function of the esophagus is to transport swallowed material from the pharynx to the stomach. A voluntary swallow initiates coordinated neuro-motor activity resulting in an aborally propagating contraction termed primary peristalsis. The primary effect of peristalsis is to develop force in the axial direction to pass the food into the stomach. Esophageal peristalsis depends on several factors such as body position, gravity, bolus size, and bolus viscosity[1,2].
The most common method to study esophageal peristalsis is manometry using low-compliance perfused catheters or more recently high-resolution manometry using multiple solid state transducers mounted on the catheter. It has been shown that bolus volume affects the peristaltic contraction velocity and duration[3,4]. The interval between swallows[5,6], body position[2,4] and temperature of the bolus[7] also affect peristalsis. However, the pressure amplitude is not affected by increasing bolus viscosity whereas the duration and velocity is reduced[7,8].
Since the swallowed material is transported in the axial (longitudinal) direction of the esophagus, axial force measurements from a theoretical and practical standpoint better reflect esophageal function than pressure recordings do. Manometry measures the radial pressure which merely is an indirect measurement of the radial force (perpendicular to the axial force direction). Video-fluoroscopy is used to visually assess esophageal motor flow but it does not provide quantitative information on force in either radial or axial directions[9,10]. Several attempts have been made to develop techniques to measure the axial force in the esophagus[11-14]. Despite promising initial results based on strain gauge technology, this method has never been thoroughly tested or never made a breakthrough in clinical studies. Thus, only scarce data exist on the axial force in the esophagus. To the best of our knowledge, no studies have been published on the effect of bolus viscosity with axial force recordings.
The aim of this paper was to study the effect of bolus viscosity on axial force in the esophagus during primary peristalsis using a newly validated impedance-based force recording technique[15,16] and to compare these results with manometric recordings.
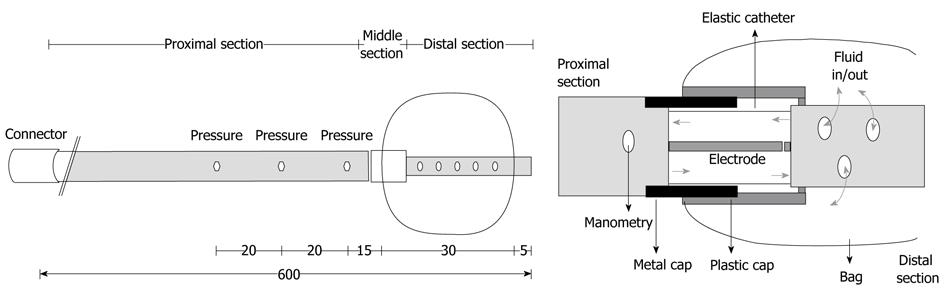
The probe was custom-made to measure axial force and pressure simultaneously at different positions on the probe (Ditens A/S, Aalborg, Denmark). The probe was 60 cm long and constructed from three different catheters. The proximal catheter of the probe was used for manometry, the middle catheter contained the transducer for axial force measurement, and the distal catheter was 2.5 cm long and contained a small bag (Figure 1).
The proximal catheter of the probe was made from an 8-lumen polyurethane catheter with an outer diameter of 4.6 mm. The 8 channels had different diameters. Three channels (diameters of 0.5 mm) were used for manometric measurements using a low compliance perfusion system. The side holes for manometric measurements were placed 6, 8 and 10 cm proximal to the tip of the catheter. Steel threads were placed in a 1.0 mm lumen to avoid elongation of the proximal catheter. One 0.5 mm lumen contained two wires connected to the force transducer electrodes in the middle catheter. Two channels with diameters of 2.0 mm were used to re-circulate saline (0.09%) in the middle catheter and to inflate the bag. The last lumen with a diameter of 1.3 mm contained a temperature sensor (TC Ltd., Uxbridge, England) for measurement 0.5 cm proximal to the force transducer. It was used to temperature compensate the impedance signal.
The middle catheter was 2 cm long and consisted of three single-lumen catheters/cylinders inside each other[15,17]. The innermost catheter was 3 mm in diameter and made of elastic Natvar catheter (Colorite Polymers, Belfast, Ireland). Two electrodes for electrical impedance measurement were placed inside this catheter. One electrode was mounted on the distal catheter and the other on the proximal catheter. Two overlapping rigid cylinders (outer diameters of 4 and 5 mm) surrounded the inner catheter. They protected the elastic catheter from radial forces and from bending, factors that would introduce errors.
The distal catheter was a non-stretchable catheter with an outer diameter of 1.5 mm through which inflation of the bag was done. The cylindrical shaped bag was made of 25 μm thick polyurethane (Ditens A/S, Aalborg, Denmark) and contained up to 13 mL. It was mounted on the outer rigid cylinder and the distal catheter. Tensile axial force applied to the bag made the rigid cylinders and electrodes move apart, resulting in increased electrical impedance. The electrical impedance, measured as the electrical potential difference (voltage), was calibrated to axial force [g] by applying precision weights in the range of 0-200 g.
Six healthy men were included in the study (mean 38.3 years, range 25-61). Oral and written informed consent was obtained from all subjects. Ethics approval for the study was obtained from the Local Ethics Committee (Protocol No. VN 2003/120 mch).
The catheter was first inserted through the mouth and esophagus into the stomach. The lower esophageal sphincter (LES) was located by the proximal pressure recordings. The probe was further retracted, placing the middle of the bag 5 cm proximal to the LES. Pressure was recorded 8, 10 and 12 cm proximal to the LES. Axial force was recorded 6.5 cm proximal to the LES.
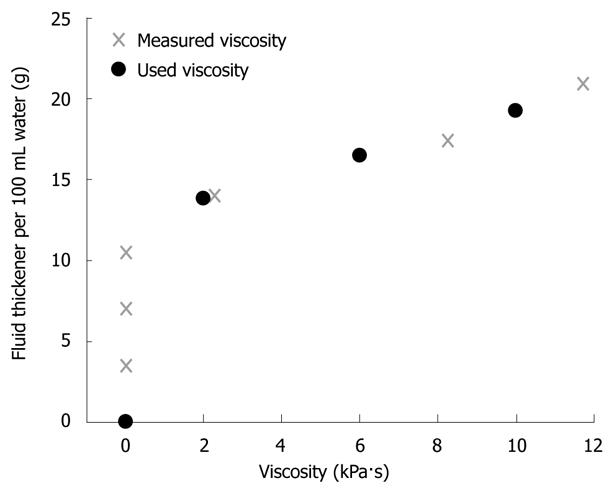
Potable tap water and three thickened fluids were used to create boluses of different viscosities. Water has a viscosity of 1 mPa·s. The three thickened fluids (TF1-TF3) were made by mixing 100 mL tab water with 13.8, 16.5 and 19.3 g Clinutren Instant thickener (Nestlé, Vevey, Switzerland), respectively. An analysis of the instant thickener product was generated prior to the study by Vysera Biomedical Ltd., showing the viscosity as a function of the concentration in water (Figure 2). The selected viscosities of the thickened fluids were 2 kPa·s, 6 kPa·s and 10 kPa·s. The viscous fluids were in appearance comparable to pudding (2 kPa·s), yogurt (6 kPa·s) and slush ice (10 kPa·s). The bag was inflated with 2 mL of fluid throughout the experiments to enable peristalsis to grip the bag. The protocol included four series of five dry swallows, five 5 mL swallows and five 10 mL swallows. Each series tested a different fluid. At the end of each series the fluid used for the next series was mixed. All boluses were given at room temperature (23-26°C). The interval between swallows was at least 45 s. All subjects were studied in upright position with the upper body tilted 30 degrees posterior and instructed to swallow as normally as possible. The volunteers drank 65 mL water between each series (after the five consecutive swallows) to clear the esophagus from any excess fluids of high viscosity.
Contraction amplitude and duration were analyzed for both force and pressure measurements. The start of a contraction was defined as the interception with the x-axis for the linear fit of the steep incline of a contraction wave. The end of a contraction was defined as the interception with the x-axis for the linear fit to the steep decline of a contraction. This definition was used because the bolus itself affected the measures before the arrival of a peristaltic contraction. This definition has previously been verified by video fluoroscopy[18] and used in different manometric studies[8,19]. The linear fit was calculated using a semi-automatic program custom made for the purpose using MatLab® version 7 (Mathworks, Natick, MA, USA).
Complete absence of motor activity (manometry < 15 mmHg[20,21], force < 10 g) at a given site was termed “failure of contraction”. Double-peaked, triple-peaked and repetitive waves were quantified manually during the semi-automatic analysis.
The results are given as grand mean ± SD. The correlation coefficient ρ between the force and pressure measurements was computed with Pearson’s correlation test for duration and amplitude at individual bolus size. Two-way analysis of variance (ANOVA) was used for the analysis of axial force and pressure. ANOVA was also used to analyze differences between bolus size. The axial force and pressure amplitude cannot be compared directly. Therefore, normalization was done by division with the overall mean of the axial force amplitude and pressure amplitude, respectively. The overall mean was computed from all the contraction amplitudes. P < 0.05 was considered significant.
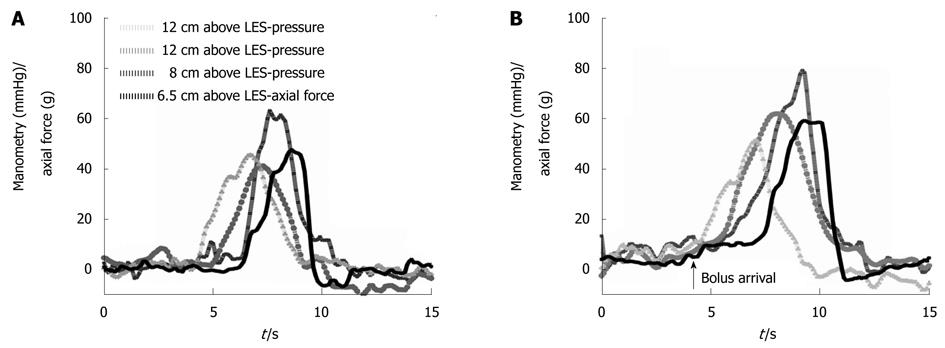
The study was conducted without adverse events for the subjects. The age of the subjects was 27.7 ± 4.2 years. Representative recordings obtained in a subject swallowing 5 mL of water and 10 mL of thickened fluid (10 kPa·s) are shown in Figure 3. The arrival of the bolus in front of the peristaltic wave can be seen in the recording from the 10 mL swallow (marked with an arrow). The number of peristaltic contractions was higher during 5 and 10 mL swallows when compared to dry swallows; that is fewer wet swallows failed to induce contraction. The number of contractions was lower for multi-peaked contractions for both manometry and axial force during 5 mL swallows compared to dry swallows. Manometry and axial force showed an equal number of contractions. Only contractions, but not the events that did not fulfill the contraction criteria, were included in the subsequent analysis. No qualitative changes in the shape of the peristaltic contraction were found in association with the quantitative changes described. No increase or decrease in contractile amplitude or duration was found during the five subsequent swallows in a series. Thus, in the following analysis the averages were used.
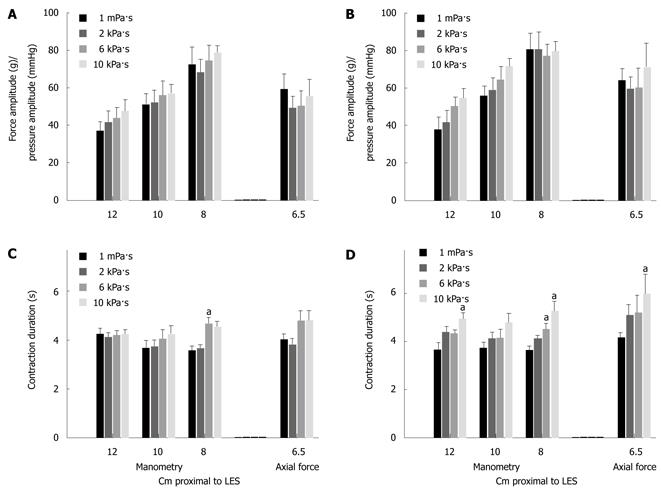
The most distal pressure recording site had the biggest amplitude for both 5 mL swallows (F = 22.5, P < 0.001) and 10 mL swallows (F = 26.3, P < 0.001). Thus, the manometric amplitude increased distally when comparing recordings at different levels.
The pressure amplitude did not depend on viscosity or bolus size at any recordings site (Figure 4). The axial force amplitude was 38.7 ± 17.2 g during dry swallows (not shown). The axial force amplitude was marginally influenced by bolus size (F = 3.5, P = 0.069) but did not increase with the bolus viscosity (Figure 4). Using 2-way ANOVA no difference was found when normalized amplitudes for pressure recorded 8 cm proximal to LES was compared to normalized axial force amplitudes.
The contraction duration recorded with manometry was not influenced by the recording site (Figure 4). The duration increased with viscosity for pressure recorded 8 cm above LES (F = 12.3, P < 0.01) (Figure 4).
The contraction duration measured with axial force increased with increasing viscosity (F = 4.3, P = 0.01) and bolus size (5 mL versus 10 mL) (F = 4.9, P = 0.03). The pressure duration recorded 8 cm proximal to the LES was lower than that for axial force for 10 mL swallows (F = 4.9, P = 0.033). Pressure recorded 8 cm proximal to the LES showed a change with viscosity (F = 12.3, P < 0.001) and the post hoc analysis showed difference between water and 10 kPa·s fluid (P < 0.001) and between water and 6 kPa·s fluid (P = 0.001). Contrary to the amplitude the duration did not change when comparing the different manometric recording sites.
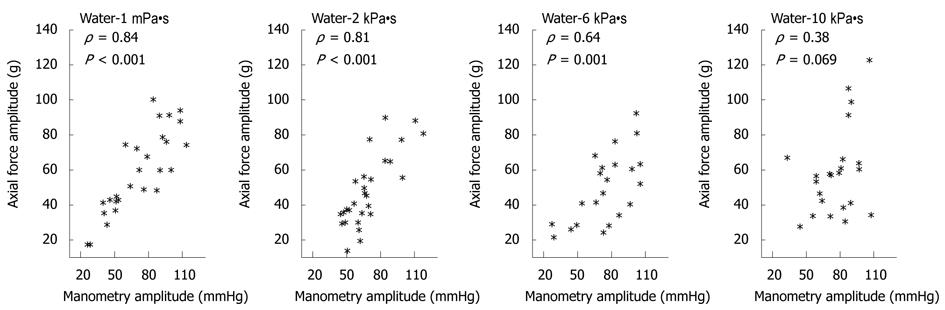
The association between the pressure amplitude measured 8 cm proximal to the LES and force amplitude recorded during 5 mL swallows decreased with increasing bolus viscosity (Figure 5 and Table 1). The correlation coefficients for amplitudes during 10 mL swallows were lower compared to 5 mL swallows. Association was not found at any viscosity or bolus volume when comparing axial force amplitudes to pressure amplitudes recorded 12 cm proximal to the LES (all P > 0.05). With regard to contraction duration no association was found for 5 mL swallows except in one case (Table 1). A weak association between axial force and manometry 8 cm proximal to LES was found for 10 mL swallows (P < 0.58).
| Volume | 5 mL | 5 mL | 5 mL | 5 mL | 10 mL | 10 mL | 10 mL | 10 mL | |
| Viscosity (Pa•s) | 1 m | 2 k | 6 k | 10 k | 1 m | 2 k | 6 k | 10 k | |
| Amplitude | |||||||||
| 8 cm | ρ = | 0.84a | 0.81a | 0.64a | 0.38 | 0.7a | 0.52a | 0.46a | 0.7a |
| P = | < 0.001a | < 0.001a | 0.001a | 0.069 | < 0.001a | 0.008a | 0.02a | < 0.001a | |
| 10 cm | ρ = | 0.49a | 0.68a | 0.57a | 0.41 | 0.27 | 0.08 | -0.7 | 0.26 |
| P = | 0.016a | < 0.001a | 0.021a | 0.063 | 0.163 | 0.695 | 0.749 | 0.29 | |
| 12 cm | ρ = | 0.41 | 0.39 | 0.48 | -0.32 | -0.25 | -0.06 | -0.15 | 0.27 |
| P = | 0.06 | 0.058 | 0.062 | 0.157 | 0.244 | 0.785 | 0.494 | 0.255 | |
| Duration | |||||||||
| 8 cm | ρ = | 0.36 | 0.34 | 0.47a | 0.32 | 0.55a | 0.4a | 0.58a | 0.53a |
| P = | 0.061 | 0.08 | 0.027a | 0.133 | 0.002a | 0.048a | 0.002a | 0.014a | |
| 10 cm | ρ = | -0.23 | 0.17 | 0.48 | 0.33 | 0.34 | 0.29 | 0.32 | 0.25 |
| P = | 0.292 | 0.433 | 0.062 | 0.138 | 0.075 | 0.159 | 0.122 | 0.307 | |
| 12 cm | ρ = | -0.32 | 0.31 | -0.28 | 0 | -0.35 | 0.03 | -0.07 | -0.25 |
| P = | 0.14 | 0.136 | 0.287 | 0.997 | 0.104 | 0.908 | 0.766 | 0.293 | |
The major results were that the pressure and force amplitude did not increase with the viscosity. The axial force amplitude depended on the bolus size. This was not the case for the pressure amplitude. The association between pressure and force amplitudes was weak at large bolus size (10 mL), indicating that pressure will be a less exact measure of the esophageal function.
The swallow sequence was not randomized or blinded since pilot experiments showed that the subjects could easily tell the difference between dry swallows, 5 mL and 10 mL volumes in the mouth. The same accounted for the fluids of different viscosity. However, the lack of blinding is of minor importance as a previous study did not show a learning effect regarding the swallowed bolus size[22].
The viscous fluids did not influence subsequent recordings by accumulating around the probe or bag between swallows since the parameters for dry swallows did not change and the number of contractions remained constant. Individual successive contractions showed no increase in amplitude or duration in five succeeding swallows.
In the current study the viscosity ranged from 1 mPa·s to 10 kPa·s. A previous study used somewhat lower viscosities between 1 mPa·s to 860 mPa·s[8]. Since the viscosity curve is highly non-linear this may not affect the appearance of the fluid to the same degree. However, it is important to emphasize that the exact viscosity should be given in scientific publications.
The association between manometric data recorded 8 cm proximal to the LES and axial force recorded 6.5 cm proximal to the LES was calculated. However, the distance between recording sites is of minor importance because the amplitude and duration were calculated for each curve individually. One may argue that it is important to evaluate intrabolus pressure since it will be influenced by viscosity[18]. However, we believe that the contraction pressure is more important.
This study confirms the results of Dooley and coworkers[7] that the contraction pressure amplitude is not affected by changes in bolus viscosity. We further show that the axial force amplitude does not depend on the viscosity.
Previous studies found a poor correlation between the axial force and manometry[23-25]. Pope and Horton found that swallowing 10 mL of salad oil reduced the axial force amplitude by 50% in the subsequent swallows[13]. This shows the frictional force is an important factor and the esophagus ability to “grip” the bolus is of great importance to a powerful forward-moving peristaltic wave. In the present study the pressure amplitude was not affected in the same way as axial force amplitude when the frictional force is changed. We believe that one of the reasons that the association between pressure amplitudes and axial force amplitudes became weaker as viscosity increased was due to a change in the frictional resistance (the frictional force resisting fluid movements). It is suspected that the range in frictional resistance between water and the viscous fluids was too low to reveal any difference when comparing axial force amplitudes and manometry amplitudes. Frictional resistance is a complex mechanism and will change depending on e.g. the bolus content, amount of mixed saliva, bolus velocity[26]. Thus the axial force amplitude (or function) generated by the esophagus may be affected.
The duration of the peristaltic wave was affected by bolus viscosity, especially for 10 mL swallows. This confirms previous manometric studies[7,8] showing a difference in the whole range of viscosities compared to water. It has been suggested that the increased duration is due to that the bolus reside longer time at the recording site[7]. However, this is not likely to happen even with very viscous fluids. As seen in the raw tracings the bolus reached the axial force transducer before the actual wave arrived. This implies that the bolus has passed the pressure recordings sites.
In conclusion, the study provided information about the pressure and axial force during peristaltic contractions when exposed to change in bolus viscosity and volume. Though the contractile patterns appeared the same for pressure and force measurements, clear differences were found between the recordings, especially at high volumes and high viscosities. It is expected that profound differences between manometry and axial force will be found between patient groups with esophageal diseases. For example it is well known that some achalasia patients show a “common cavity phenomenon” with aperistalsis. This will create a radial pressure without changes in axial force. The same may account for other esophageal diseases. Thus, it is expected that axial force measurements will have clinical relevance. This will be a subject for subsequent studies.
Development of diagnostic tools within esophageal motility diseases have for a long time been based on manometry. The development has mainly focused on use of multiple pressure sensors (high resolution manometry). Several authors have previously shown that relying on manometry alone can lead to erroneous conclusions.
Axial force measurements, also known as traction force, provide additional information not currently available through manometry examinations alone. Axial force provides a more physiological measurement. However few studies have compared simultaneous manometry and axial force.
This is the first study of its kind to examine axial force in relation to viscosity. The data suggests that axial force can provide additional information in relation to motility.
These data are in accordance with other studies relying on axial force measurements though the areas of interest have been bolus size, temperature etc. The data, together with other papers, suggests that using axial force to assists manometry in motility examinations will provide additional information important to provide a valid diagnosis.
This is a very interesting experimental study with important clinical implications in the diagnosis of esophageal disease.
| 1. | Kim CH, Hsu JJ, O'Connor MK, Weaver AL, Brown ML, Zinsmeister AR. Effect of viscosity on oropharyngeal and esophageal emptying in man. Dig Dis Sci. 1994;39:189-192. |
| 2. | Kaye MD, Wexler RM. Alteration of esophageal peristalsis by body position. Dig Dis Sci. 1981;26:897-901. |
| 3. | Hollis JB, Castell DO. Effect of dry swallows and wet swallows of different volumes on esophageal peristalsis. J Appl Physiol. 1975;38:1161-1164. |
| 4. | Weihrauch TR, Brummer A, Biewener H, Ewe K. Assessment of various factors influencing esophageal pressure measurement. I. Significance of methodical factors in intraluminal manometry. Klin Wochenschr. 1980;58:279-285. |
| 5. | Vanek AW, Diamant NE. Responses of the human esophagus to paired swallows. Gastroenterology. 1987;92:643-650. |
| 6. | Meyer GW, Gerhardt DC, Castell DO. Human esophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol. 1981;241:G129-G136. |
| 7. | Dooley CP, Di Lorenzo C, Valenzuela JE. Esophageal function in humans. Effects of bolus consistency and temperature. Dig Dis Sci. 1990;35:167-172. |
| 8. | Dooley CP, Schlossmacher B, Valenzuela JE. Effects of alterations in bolus viscosity on esophageal peristalsis in humans. Am J Physiol. 1988;254:G8-G11. |
| 9. | Katzak DA, Metz DC. Esophagus and stomach. 1 ed. Mosby, 2003; ISBN:0323018866. . |
| 10. | Richter JE, Castell DO. The esophagus. Lippincott Williams and Wilkins, 2004; ISBN:0-7817-4199-8. . |
| 11. | Williams D, Thompson DG, Heggie L, Bancewicz J. Responses of the human esophagus to experimental intraluminal distension. Am J Physiol. 1993;265:G196-G203. |
| 12. | Russell CO, Bright N, Buthpitiya G, Alexander L, Walton C, Whelan G. Oesophageal propulsive force and its relation to manometric pressure. Gut. 1992;33:727-732. |
| 13. | Pope CE 2nd, Horton PF. Intraluminal force transducer measurements of human oesophageal peristalsis. Gut. 1972;13:464-470. |
| 14. | Winship DH, Zboralske FF. The esophageal propulsive force: esophageal response to acute obstruction. J Clin Invest. 1967;46:1391-1401. |
| 15. | Gravesen FH, McMahon BP, Drewes AM, Gregersen H. Measurement of the axial force during primary peristalsis in the oesophagus using a novel electrical impedance technology. Physiol Meas. 2008;29:389-399. |
| 16. | Gravesen FH, Gregersen H, Arendt-Nielsen L, Drewes AM. Reproducibility of axial force and manometric recordings in the oesophagus during wet and dry swallows. Neurogastroenterol Motil. 2010;22:142-149, e46-e47. |
| 17. | Gravesen FH, Funch-Jensen P, Gregersen H, Drewes AM. Axial force measurement for esophageal function testing. World J Gastroenterol. 2009;15:139-143. |
| 18. | Ren J, Massey BT, Dodds WJ, Kern MK, Brasseur JG, Shaker R, Harrington SS, Hogan WJ, Arndorfer RC. Determinants of intrabolus pressure during esophageal peristaltic bolus transport. Am J Physiol. 1993;264:G407-G413. |
| 19. | Kahrilas PJ, Dodds WJ, Hogan WJ, Kern M, Arndorfer RC, Reece A. Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology. 1986;91:897-904. |
| 20. | Weusten BL, Smout AJ. Analysis of 24-h oesophageal pH and pressure recordings. Eur J Gastroenterol Hepatol. 1995;7:1147-1151. |
| 21. | Smout AJ, Breedijk M, van der Zouw C, Akkermans LM. Physiological gastroesophageal reflux and esophageal motor activity studied with a new system for 24-hour recording and automated analysis. Dig Dis Sci. 1989;34:372-378. |
| 22. | Lazarus CL, Logemann JA, Rademaker AW, Kahrilas PJ, Pajak T, Lazar R, Halper A. Effects of bolus volume, viscosity, and repeated swallows in nonstroke subjects and stroke patients. Arch Phys Med Rehabil. 1993;74:1066-1070. |
| 23. | Williams D, Thompson DG, Marples M, Heggie L, O’Hanrahan T, Mani V, Bancewicz J. Identification of an abnormal esophageal clearance response to intraluminal distention in patients with esophagitis. Gastroenterology. 1992;103:943-953. |
| 24. | Williams D, Thompson DG, Marples M, Heggie L, O’Hanrahan T, Bancewicz J. Diminished oesophageal traction forces with swallowing in gastro-oesophageal reflux disease and in functional dysphagia. Gut. 1994;35:165-171. |
| 25. | Pouderoux P, Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of esophageal shortening during peristalsis. Gastroenterology. 1997;112:1147-1154. |
| 26. | Prinz JF, De Wijk RA, Huntjens L. Load dependency of the coefficient of friction of oral mucosa. Food Hydrocolloids. 2007;21:402-408. |
Peer reviewers: Satoshi Osawa, MD, First Department of Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Hamamatsu, 431-3192, Japan; Luigi Bonavina, Professor, Department of Surgery, Policlinico San Donato, University of Milano,
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH