Published online Mar 14, 2011. doi: 10.3748/wjg.v17.i10.1362
Revised: November 24, 2010
Accepted: December 1, 2010
Published online: March 14, 2011
AIM: To evaluate the association between of the interleukin-10 (IL-10) promoter polymorphisms and survival of advanced gastric cancer (GC) patients.
METHODS: The IL-10 (-1082, rs1800896; -819, rs1800871; and-592, rs1800896) genotypes in 234 patients with advanced gastric cancer and in 243 healthy controls were determined by polymerase chain reaction-restriction fragment length polymorphism assay. Odds ratios (OR) and 95% confidence intervals (CI) were calculated by unconditional logistic regression for the associations between IL-10 genotypes and the risk of GC. The Kaplan-Meier method with log-rank testing was used to evaluate the association between genotype and survival of the patients.
RESULTS: The IL-10 -1082 G allele and GCC (-1082, -819 and -592) haplotype were associated with increased gastric cancer risks (OR 1.2, 95% CI 0.6-3.2, P = 0.007, for -1082 G allele, OR = 2.3, 95% CI, 1.2-4.1, P = 0.005, for GCC haplotype, respectively). However, none of the three IL-10 gene polymorphisms (-1082, -819 and -592) was correlated with gastric cancer survival (P > 0.05), and none of the genotypes of the three IL-10 sites was found as independent prognostic risk factors in the multivariate test.
CONCLUSION: IL-10 gene promoter polymorphisms may not be associated with the prognosis of advanced gastric cancer.
- Citation: Liu J, Song B, Wang JL, Li ZJ, Li WH, Wang ZH. Polymorphisms of interleukin-10 promoter are not associated with prognosis of advanced gastric cancer. World J Gastroenterol 2011; 17(10): 1362-1367
- URL: https://www.wjgnet.com/1007-9327/full/v17/i10/1362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i10.1362
Deregulated components of the immune system, such as cytokines, may be linked to the incidence and clinical course of malignant diseases with development of acute or chronic inflammatory reactions at tumor sites. Deregulated expression of defined subsets of cytokines was found to be associated with the transformation of lymphatic cells either as autocrine growth factors for the transformed cells or as factors in rebuilding the tumor microenvironment, likely affecting tumor progression and dissemination[1].
The mechanisms underlying differences in immune response between individuals are complex, but include inherited genetic variation. Some reports suggest that functional polymorphisms in the genes regulating the immune and inflammatory response may contribute to the susceptibility to and clinical outcome of gastric cancer[2-4]. Interleukin-10 (IL-10) is a pleiotropic cytokine produced by macrophages, T-helper 2 cells and B lymphocytes and can suppress and stimulate the immune response[5-7]. IL-10 has been shown to inhibit various immune functions, such as antigen presentation, cytokine production, macrophage activation, and antigen-specific T-cell proliferation[8,9]. By interfering with antigen-presenting cells, IL-10 reduces antigen-specific T-cell proliferation. It has been postulated that IL-10 plays a key role in the oncogenetic and metastatic ability of neoplasms[10,11]. Increased levels of serum IL-10 were found in patients with solid and hematopoietic tumors[12]. However, a large body of evidence in different animal tumor models showed that IL-10 can favor immune-mediated cancer rejection[13,14].
The gene encoding IL-10 is located on chromosome 1 (1q31-1q32). Several SNPs have been identified in the IL-10 gene promoter region. These include IL-10-1082 A/G, -819T/C and -592A/C, which influence the transcription of IL-10 messenger RNA and the expression of IL-10 in vitro[15,16]. Although several studies have shown the possible involvement of IL-10 in the pathogenesis of gastric cancer[17-21], its association with prognosis of gastric cancer was not extensively studied. The only study by Deans et al[22] found that GG for IL-10-1082 was associated with reduced survival of gastric cancer patients, but it was not an independent prognosis factor for gastric cancer. The aim of this study was, therefore, to explore the relationship between polymorphisms of IL-10 -1082, -819 and -592 and the prognosis of patients with advanced gastric cancer in the northern area of China.
A total of 234 patients (162 men and 72 women) with histologically or cytologically confirmed advanced gastric cancer registered from July 2005 to July 2008 at the Department of Oncology, Shandong Cancer Hospital and Institute were included in this study. The patients should have a histological diagnosis of gastric carcinoma with an unresectable primary tumor and/or metastases that were measurable or assessable by means of clinical examination, X-ray, computed tomography (CT) or ultrasound. The median age of the patients was 61.2 years (range, 27-79 years). There were 147 cases of antrum gastric cancer, 35 gastric cardia cancer, and 52 cases of other types, including 150 cases at stage IIIB and 84 cases at stage IV. Pathologically, 10 patients had well-differentiated adenocarcinoma, 45 had moderately differentiated adenocarcinoma, 161 poorly differentiated adenocarcinoma, and 18 signet ring cell carcinoma. Two hundred and six patients were treated with conventional and 5-fluorouracil (5-FU)-based chemotherapy. Among them, 96 cases were treated with capecitabine/fluorouracil + cisplatin/oxaliplatin regimen and 89 cases were treated with docetaxel + oxaliplatin + fluorouracil regimen. The median chemotherapy courses consisted of 5 (1-11) cycles. Follow-up time was calculated from the initiation of diagnosis to July 2009, with a median of 13.3 mo (range, 3.5-34.4 mo). Thirty-three patients were lost to follow-up and 188 patients died, including 3 patients who died from causes other than gastrointestinal carcinoma during the follow-up.
We also selected 243 control individuals (aged 26-79 years) who visited the Shandong Cancer Hospital and Institute between July 2005 and July 2008 for general physical exams. The control individuals were screened to ensure that none had ever been diagnosed with cancer or other serious diseases. The selected controls were age matched to the cases (± 5 years). All the subjects were unrelated ethnic Han Chinese, and written informed consent was obtained from each participant. The study was approved by the Review Board of Shandong Cancer Hospital and Institute. A 2-mL peripheral blood sample was collected from each study participant.
Genomic DNA was extracted from peripheral blood using a Genomic DNA Extraction Kit (Fastagen, Shanghai, China) according to the manufacturer’s protocol. IL-10 promoter polymorphisms were identified by PCR amplification and restriction analysis (PCR-RFLP), (Table 1). Each PCR reaction was performed in a GeneAmp PCR System 9600 thermocycler (Applied Biosystems, Foster, CA) at a final volume of 25 μL (containing 5 pmol for each primer, 50 ng genomic DNA, 1.5 mmol/L MgCl2, 5 mol/L dNTPs and 1 U of Taq DNA polymerase in PCR buffer containing 10 mmol/L Tris). PCR cycles used were as follows: 95°C for 5 min, 35 cycles of denaturing at 95°C for 40 s, annealing at the indicated temperature for 1 min, extension at 72°C for 40 s, and a single final extension at 72°C for 10 min. The amplified products were digested with corresponding restriction endonucleases (New England Biolabs, MA, USA), and separated by electrophoresis on a 10% polyacrylamide gel stained with sliver nitrate for visualization.
| Polymorphism | Primer sequence | Annealing temperature | Restriction enzyme | Allele size |
| IL-10-1082G/A | 5′-CTCGCTGCA ACCCAACTGGC-3′ | 58°C | MnlI | A: 139 bp |
| 5′-TCTTACCTATCCCTACTTCC-3′ | G: 106, 33 bp | |||
| IL-10-819C/T | 5′-TCATTCTATGTGCTGGAGATGG-3′ | 59°C | Mae III | C: 125, 84 bp |
| 5′-TGGGGGAAGTGGGTAAGAGT-3′ | T: 209 bp | |||
| IL-10-592C/A | 5′-GTGAGCACTACCTGACTAGC-3′ | 58°C | RsaI | C: 412 bp |
| 5′-CCTAGGTCACAGTGACGTGG-3′ | A: 175, 237 bp |
Statistical analysis was performed using SPSS 13.0 software (SPSS, Florida, USA). Demographic data between the study groups were compared using the Chi-square test and Student’s t test. Each polymorphism was tested for deviation from the Hardy-Weinberg equilibrium by comparing the observed and expected genotype frequencies using the χ2 test. Genotype frequencies of IL-10 were compared between groups using the χ2 test, and odds ratios (OR) and 95% confidence intervals (CIs) were calculated using unconditional logistic regression with adjustment for age and sex. The haplotypes of IL-10 (-1082, -819 and -592) were analyzed using the SHEsis software (Bio-X Inc., Shanghai, China), which uses a full-precise-iteration (FPI) algorithm to reconstruct haplotypes. The Kaplan-Meier method with a log-rank test was used to evaluate the association between genotype and survival. Multivariate analysis was performed using Cox proportional hazards regression. Statistical significance was interpreted as P < 0.05.
The genotype and allele frequencies of the IL-10 SNP in 234 gastric cancer patients and 243 healthy controls are shown in Table 2. All genotype frequencies in both patient and control groups were in the Hardy-Weinberg equilibrium (P < 0.05). There were significant differences in the genotype and allele frequencies of the IL-10 promoter -1082 A/G polymorphism between gastric cancer and control groups. The -1082 AG genotypes were associated with a significantly increased risk of gastric cancer as compared with the -1082 AA genotypes (OR 1.9, 95% CI 1.1-3.4, P = 0.019). The -1082 G allele was associated with a significantly increased risk of gastric cancer as compared with the -1082 A allele (OR 1.2, 95% CI 0.6-3.2, P = 0.007). However, genotype and allele frequencies of the IL-10 -819 T/C and -592 A/C polymorphisms in gastric cancer patients were not significantly different compared with those in healthy controls (P > 0.05). The estimated haplotype frequencies of IL-10 polymorphisms in gastric cancer patients and controls are also shown in Table 2. Complete linkage disequilibrium was observed between locus -819T/C and locus -592A/C. Four possible haplotypes were demonstrated in our population. The most frequent haplotype in both patients (60.3%) and controls (64.6%) was the ATA (-1082A, -819T and -592A) haplotype. By haplotype analyses, we found that the GCC (-1082G, -819C and -592C) haplotype was associated with a significantly increased risk of gastric cancer as compared with the ATA haplotype (OR = 2.3, 95% CI 1.2-4.1, P = 0.005).
| Genotypes/allele | Casesn (%) | Controlsn (%) | Adjusted OR (95% CI)1 | P |
| IL-10-1082 | 234 | 243 | ||
| AA | 189 (80.4) | 217 (89.3) | 1 | |
| AG | 39 (17.2) | 23 (9.7) | 1.9 (1.1-3.4) | 0.019a |
| GG | 6 (2.4) | 3 (1.0) | 2.4 (0. 6-9.4) | 0.244 |
| A | 417 (89.1) | 457 (94.0) | 1 | |
| G | 51 (10.9) | 29 (6.0) | 1.2 (0.6-3.2) | 0.007a |
| IL-10-819 | ||||
| TT | 99 (42.3) | 109 (44.9) | 1 | |
| TC | 96 (41.0) | 106 (43.6) | 0.9 (0.7-1.6) | 0.975 |
| CC | 39 (16.8) | 28 (11.5) | 1.6 (0.9-2.8) | 0.145 |
| T | 294 (62.8) | 324 (66.7) | 1 | |
| C | 174 (37.2) | 162 (33.3) | 1.2 (0.9-1.6) | 0.221 |
| IL-10-592 | ||||
| AA | 99 (42.3) | 109 (44.9) | 1 | |
| AC | 96 (41.0) | 106 (43.6) | 0.9 (0.7-1.6) | 0.975 |
| CC | 39 (16.8) | 28 (11.5) | 1.6 (0.9-2.8) | 0.145 |
| A | 294 (62.8) | 324 (66.7) | 1 | |
| C | 174 (37.2) | 162 (33.3) | 1.2 (0.9-1.6) | 0.221 |
| Haplotype | ||||
| ATA | 282 (60.3) | 314 (64.6) | 1 | |
| ACC | 135 (28.9) | 143 (29.4) | 1.1 (0.8-1.5) | 0.731 |
| GCC | 39 (8.3) | 19 (3.9) | 2.3 (1.2-4.1) | 0.005a |
| GTA | 12 (2.5) | 10 (2.1) | 1.3 (0.6-3.2) | 0.504 |
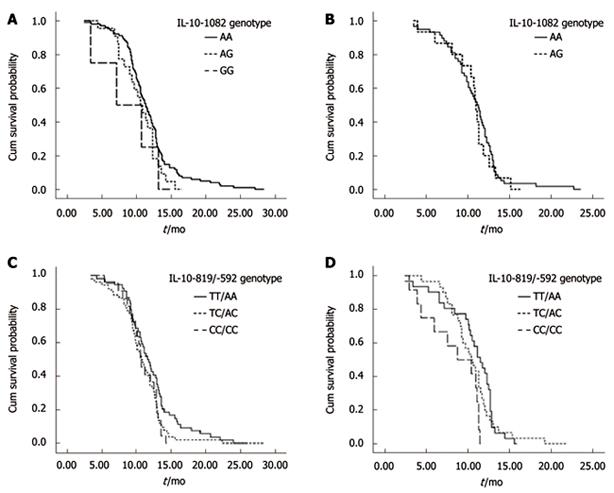
Of the 234 patients, 33 were lost to follow-up. We compared the IL-10 genotype with survival time in 201 patients with advanced gastric cancer. The median follow-up time was 13.3 mo (range, 3.0-30.3 mo), and the median survival time was 11.2 mo. The median survival time was 11.4 mo for patients with the IL-10 -1082AA genotype, 10.8 mo for patients with the GA genotype, and 11.0 mo for patients with the GG genotype. The IL-10 -1082 genotypes were not associated with the prognosis of gastric cancer (P =0.709, log-rank test). Similarly, the median survival time was 11.5 mo for patients with the IL-10 -819TT/-592AA genotype, 10.8 mo for patients with the -819TC/-592AC genotype, and 10.2 mo for patients with the -819CC/-592CC genotype (P = 0.090, log-rank test). When stratified by the clinical stage, the association between the IL-10 gene polymorphisms (-1082, -819 and -592) and the gastric cancer survival was not statistically significant either in stage IIIB or stage IV (Figure 1). Multivariate Cox regression was used to analyze the effect of different risk factors (IL-10-1082A/G, -819T/C and -592A/C genotype, chemotherapy regimen, age, gender, disease stage, and histology) on survival time. However, none of the genotypes of the three IL-10 sites was recognized as an independent prognostic indicator (Table 3).
| Variable | P | Hazard ratio | 95% CI for hazard ratio | |
| Lower | Upper | |||
| IL-10-1082 genotype | 0.815 | 1.2 | 0.8 | 1.7 |
| IL-10-819/-592 genotype | 0.671 | 1.1 | 0.5 | 1.9 |
| Treatment method | 0.426 | 1.4 | 0.9 | 2.3 |
| Age | 0.595 | 0.9 | 0.7 | 1.3 |
| Stage | 0.234 | 1.2 | 0.9 | 1.5 |
| Histology | 0.519 | 1.2 | 0.7 | 2.1 |
Cytokines play a crucial role in the regulation of key pathways of immunity, the balance between cell-mediated (Th1) and humoral (Th2) responsiveness. IL-10, which is produced mainly by macrophages and T lymphocytes, is an important anti-inflammatory and immunosuppressive cytokine, it inhibits the Th1-type pathway activation, prevents antigen-presenting cells (APC) from obtaining access to tumor antigens, and down-regulates surface expression of costimulatory molecules CD80 or CD86 on tumor cells[5-7]. Due to its immunosuppressive and anti-inflammatory properties, it has been hypothesized that IL-10 may contribute to the escape of tumor cells from immune surveillance and favor tumor growth. On the other hand, several studies showed that IL-10 may regulate angiogenesis in various cancers and is believed to play a protective and preventive role against tumors[13,14].
Although the genetic control of IL-10 expression is not clearly understood yet, polymorphisms in promoter regions have been reported to determine inter-individual differences in IL-10 production. Previous studies indicated that three common-1082 A/G, -819 T/C and -592 A/C polymorphisms of the IL-10 promoter may influence production and expression of IL-10. IL-10 promoter-1082G allele or GCC haplotype (defined by three SNPs at positions of -1082, -819 and -592) is associated with increased IL-10 production and ATA haplotype is generally assumed to be a lower IL-10 responder[15,16].
In this study we evaluated the association between the polymorphisms of the IL-10 promoter and advanced gastric cancer in a Chinese population. Our data showed significant differences in allele, genotype and haplotype frequencies between gastric cancer patients and healthy controls. In concordance with our study, Lee et al[20], Sugimoto et al[21] and several studies from China[18,19] reported that the IL-10 gene promoter polymorphisms were associated with the risk of gastric cancer in Korean, Japanese and Chinese populations. However, these results are not consistent with studies previously conducted in American and Spain, in which the IL-10 promoter genotype was not associated with gastric cancer risk[23,24]. Although it is difficult to determine the reasons behind the contradictory results in these studies, the different genetic background of study populations may be one of the main factors.
Although the IL-10 promoter polymorphisms are associated with increased risk of advanced gastric, few studies have investigated the relationship between IL-10 promoter polymorphisms and gastric cancer prognosis. In this study, we investigated whether there are any associations between the three IL-10 promoter SNPs and survival time of advanced gastric cancer patients. Our results showed that the three IL-10 gene polymorphisms (-1082, -819 and -592) did not correlate with the advanced gastric cancer survival. Multivariate testing also found no genotype of the three IL-10 sites as independent prognostic risk factors. Several studies have observed that serum IL-10 is of independent prognostic utility in patients with advanced gastrointestinal carcinoma, and the reasonable interpretation is that the higher serum IL-10 level is secreted by the tumor itself rather than the inflammatory infiltration[25,26]. However, only one study focused on the polymorphisms of cytokine genes and gastric cancer prognosis and found GG in IL-10-1082 to be associated with reduced survival of gastric cancer patients, but not an independent prognosis factor for gastric cancer[22]. The reason why our study did not show the association between IL-10 genotype and gastric cancer prognosis may be the relatively small series of patients examined (the number of gastric cancer patients with GG genotype at position -1082 in our study population is small, only six cases). It remains to be confirmed whether the relationship is reproducible in larger Chinese samples.
In conclusion, our results suggested that IL-10 promoter polymorphisms were associated with an increased risk of gastric cancer, but these polymorphisms did not influence the prognosis of the Chinese patients with advanced gastric cancer. As this is the first report of the association between IL-10 promoter polymorphism and prognosis of the Chinese patients with gastric cancer, the results of the present study should be viewed cautiously. Since the dual biological effects of IL-10 and the functional significance of IL-10 promoter polymorphisms in determining IL-10 expression still need to be elucidated, further investigations are required to explore the relationship of the polymorphisms of IL-10 promoter and clinical outcome of gastric cancer.
The immune dysfunction may be linked to the incidence and clinical course of malignant cancers. Interleukin-10 (IL-10) is a pleiotropic immunoregulatory cytokine which is involved in inflammatory reaction and immune regulation and is considered to exert effects in malignant transformation. IL-10 promoter polymorphisms have been reported to determine inter-individual differences in IL-10 production and are associated with the risk and pathogenesis of several cancers.
Several studies have shown the possible involvement of IL-10 polymorphisms in the pathogenesis of gastric cancer, although its association with prognosis of gastric cancer was not well explored. The aim of this study was to explore the association between polymorphisms of IL-10 promoter and the prognosis of patients with advanced gastric cancer.
This study indicated that IL-10 promoter polymorphisms were associated with an increased risk of gastric cancer, but these polymorphisms did not influence the prognosis of the patients with advanced gastric cancer.
This is the first report of the association between IL-10 promoter polymorphism and prognosis of the Chinese patients with gastric cancer. The results of this study will help understand the genetic background of the immune-related gene and gastric cancer incidence and prognosis.
IL-10 is an important anti-inflammatory and immunosuppressive cytokine, which inhibits the Th1-type pathway activation, prevents antigen-presenting cells from obtaining access to tumor antigens, and down-regulates surface expression of costimulatory molecules CD80 or CD86 on tumor cells.
| 1. | Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175-1183. |
| 2. | Seno H, Satoh K, Tsuji S, Shiratsuchi T, Harada Y, Hamajima N, Sugano K, Kawano S, Chiba T. Novel interleukin-4 and interleukin-1 receptor antagonist gene variations associated with non-cardia gastric cancer in Japan: comprehensive analysis of 207 polymorphisms of 11 cytokine genes. J Gastroenterol Hepatol. 2007;22:729-737. |
| 3. | Rosenstiel P, Hellmig S, Hampe J, Ott S, Till A, Fischbach W, Sahly H, Lucius R, Fölsch UR, Philpott D. Influence of polymorphisms in the NOD1/CARD4 and NOD2/CARD15 genes on the clinical outcome of Helicobacter pylori infection. Cell Microbiol. 2006;8:1188-1198. |
| 4. | Peek RM Jr. Pathogenesis of Helicobacter pylori infection. Springer Semin Immunopathol. 2005;27:197-215. |
| 5. | Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. |
| 6. | Cervenak L, Morbidelli L, Donati D, Donnini S, Kambayashi T, Wilson JL, Axelson H, Castaños-Velez E, Ljunggren HG, Malefyt RD. Abolished angiogenicity and tumorigenicity of Burkitt lymphoma by interleukin-10. Blood. 2000;96:2568-2573. |
| 7. | Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043-1051. |
| 8. | de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915-924. |
| 9. | Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964-2971. |
| 10. | Avradopoulos K, Mehta S, Blackinton D, Wanebo HJ. Interleukin-10 as a possible mediator of immunosuppressive effect in patients with squamous cell carcinoma of the head and neck. Ann Surg Oncol. 1997;4:184-190. |
| 11. | Wojciechowska-Lacka A, Matecka-Nowak M, Adamiak E, Lacki JK, Cerkaska-Gluszak B. Serum levels of interleukin-10 and interleukin-6 in patients with lung cancer. Neoplasma. 1996;43:155-158. |
| 12. | Fortis C, Foppoli M, Gianotti L, Galli L, Citterio G, Consogno G, Gentilini O, Braga M. Increased interleukin-10 serum levels in patients with solid tumours. Cancer Lett. 1996;104:1-5. |
| 13. | Huang S, Ullrich SE, Bar-Eli M. Regulation of tumor growth and metastasis by interleukin-10: the melanoma experience. J Interferon Cytokine Res. 1999;19:697-703. |
| 14. | Kundu N, Beaty TL, Jackson MJ, Fulton AM. Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. J Natl Cancer Inst. 1996;88:536-541. |
| 15. | Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1-8. |
| 16. | Gibson AW, Edberg JC, Wu J, Westendorp RG, Huizinga TW, Kimberly RP. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol 2001; 166: 3915-3922. . |
| 17. | Chen KF, Li B, Wei YG, Peng CJ. Interleukin-10 -819 promoter polymorphism associated with gastric cancer among Asians. J Int Med Res. 2010;38:1-8. |
| 18. | Xiao H, Jiang Y, Li R, Xia B. [Association of IL-10 gene polymorphisms with gastroduodenal diseases in Hubei Han population]. Zhonghua Yixue Yichuanxue Zazhi. 2009;26:423-426. |
| 19. | Bai XL, Sun LP, Liu J, Chen W, Zhang Y, Yuan Y. [Correlation of interleukin-10-1082G/a single nucleotide polymorphism to the risk of gastric cancer in north China: a case-control study]. Ai Zheng. 2008;27:35-40. |
| 20. | Lee JY, Kim HY, Kim KH, Kim SM, Jang MK, Park JY, Lee JH, Kim JH, Yoo JY. Association of polymorphism of IL-10 and TNF-A genes with gastric cancer in Korea. Cancer Lett. 2005;225:207-214. |
| 21. | Sugimoto M, Furuta T, Shirai N, Nakamura A, Kajimura M, Sugimura H, Hishida A. Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol. 2007;22:1443-1449. |
| 22. | Deans C, Rose-Zerilli M, Wigmore S, Ross J, Howell M, Jackson A, Grimble R, Fearon K. Host cytokine genotype is related to adverse prognosis and systemic inflammation in gastro-oesophageal cancer. Ann Surg Oncol. 2007;14:329-339. |
| 23. | Murphy G, Thornton J, McManus R, Swan N, Ryan B, Hughes DJ, O’Morain CA, O’Sullivan M. Association of gastric disease with polymorphisms in the inflammatory-related genes IL-1B, IL-1RN, IL-10, TNF and TLR4. Eur J Gastroenterol Hepatol. 2009;21:630-635. |
| 24. | García-González MA, Lanas A, Quintero E, Nicolás D, Parra-Blanco A, Strunk M, Benito R, Angel Simón M, Santolaria S, Sopeña F. Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am J Gastroenterol. 2007;102:1878-1892. |
| 25. | De Vita F, Orditura M, Galizia G, Romano C, Infusino S, Auriemma A, Lieto E, Catalano G. Serum interleukin-10 levels in patients with advanced gastrointestinal malignancies. Cancer. 1999;86:1936-1943. |
| 26. | Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer. 2009;12:95-100. |
Peer reviewer: Dr. Fahd Al-Mulla, Associate Professor and Head of Molecular Pathology, Kuwait University, Faculty of Medicine,Department of Pathology, PO Box 24923, Safat 13110, Kuwait
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH