INTRODUCTION
Intestinal tissues are innervated by a complex and extensive component known as the enteric nervous system (ENS)[1]. The ENS is characterized by the presence of neurons and enteric glial cells (EGCs) which are arranged into interconnected ganglia distributed between 2 major plexuses, and they control several gut functions[2,3]. During the course of time, the traditional view of EGCs has changed from being a mere mechanical support for surrounding neurons to that of a more articulate and complex nature, since they are actively involved in the regulation of homeostasis, motility and inflammatory processes within the gut[4,5].
Similar to the astrocytes in the central nervous system (CNS), the EGCs release several signaling molecules[4,5]. Among these, great importance has been given to the better comprehension of the specific glial-derived S100B protein[6-8]. This protein is a small, diffusible neurotrophin that is situated in the cytoplasm and/or the nucleus of both nervous and non-nervous tissues[9,10]. In the brain, S100B has been considered a “Janus face” neurotrophin[11,12] because it exerts opposite actions depending on its concentration in the extracellular milieu: it has a pro-proliferative and neurogenic effect on astroglia and on serotonergic neurons at nanomolar concentrations, as well as a neurodegenerative function[12] at micromolar concentrations, determining mysregulated glial cell proliferation and amplifying neuroinflammation. Similar to the brain, recent studies have suggested the involvement of S100B in inflammatory processes occurring in the gut, highlighting the importance of EGCs as key regulators of gut homeostasis[13-15].
In this review, we will focus on the role of EGCs and the S100B protein and we will take them into consideration by looking at both experimental animal models and some human diseases for which evidence exists, and in particular their involvement in inflammatory conditions of the human gut where the role of S100B appears to be prominent.
ENTERIC NERVOUS SYSTEM
The gut is characterized by a sequence, starting from the serosa, as follows: subserosa, longitudinal muscle, myenteric plexus, circular muscle, submucosal plexus, muscularis mucosae and mucosa[2]. The myenteric and submucosal plexuses are characterized by the presence of ganglia which, in turn, contain enteric neurons and EGCs in a ratio of 1:7[16]. In the ENS, the enteric ganglia are involved in basic gut functions, such as the regulation of peristalsis, secretion and blood flow and the modulation of the immune/inflammatory processes[17-19]. Several neurotransmitters are involved in the control of all these intestinal functions, such as vasoactive intestinal peptide and nitric oxide (NO)[20,21]. In particular, NO is produced by the biosynthetic enzyme neuronal NO synthase (NOS), which is expressed in myenteric neurons, and by the inducible form of NOS (iNOS), which is expressed in EGCs[15].
EGCs: both protective and destructive cells in the gut
EGCs are small cells with a “star-like” appearance[16] containing intracellular arrays of 10 nm filaments made up of glial fibrillary acidic protein (GFAP)[16,22-24]. This cell population was first described by Dogiel[25] using methylene blue staining on full thickness preparations. At present, the S100B protein and GFAP are commonly used as specific markers in order to identify EGCs[22,26]. More recently, other markers have been proposed for the identification of glial cells in the human gut, especially in whole-mount preparations: Sox8/9/10, a specific nuclear marker[16]. EGCs release a wide range of factors accounting for the development, survival and differentiation of peripheral neurons[27]. Traditionally, EGCs have been considered as a mechanical support for enteric neurons, but, in recent years, this restrictive view has changed to one of a more articulate and complex nature, since it has been described that EGCs are involved in the maintenance of intestinal homeostasis[28-30]. Indeed, EGCs control intestinal epithelial barrier (IEB) functions, as demonstrated in animal studies in which the ablation of enteroglial network enhances intestinal vascular permeability together with an increase in IEB paracellular permeability[31-34]. Furthermore, in vitro data has shown that EGCs partially decrease IEB permeability via the release of S-nitrosoglutathione and the regulation of zonulin-1 and occludin expression[35,36]. Although the function of glial mediators still have to be identified, it is conceivable that they could be actively involved in the EGCs-mediated effects on barrier functions.
Besides the well documented ‘protective role’, EGCs are activated by means of inflammatory insults and they may directly contribute to an inflammatory condition working as an antigen presenting cell-type promoting a variegate release of cytokine synthesis[13-15,35-37] in the gut milieu. Therefore, EGCs may act as “receptors” for cytokines and they themselves produce interleukin-6 (IL-6) and IL-1b[38,39]. Moreover, ECGs express iNOS and L-arginine, the machinery for the time-delayed and micromolar release of NO, one of the most important signaling molecules involved in host-immune defense against viruses and bacteria as well as a well-known pro-inflammatory mediator[15,40,41].
EGC-SELECTIVELY EXPRESSED PROTEINS
GFAP
Mature EGCs are rich in the intermediate filament protein, GFAP[42,43]. Its expression is modulated by glial cell differentiation, inflammation and injury[42], indicating that the level of GFAP accords with the functional state of glial cells.
In animals, two classes of glial cells can be distinguished, namely the GFAP positive (+) and GFAP negative (-) groups, as demonstrated by von Boyen et al[8]. In the same study, it was suggested that pro-inflammatory cytokines control GFAP+ enteric glia, which, in turn, are involved in the modulation of the integrity of the bowel during inflammation[8].
In humans, GFAP expression is altered in the mucosa of patients with inflammatory bowel diseases (IBD), as well as ulcerative colitis (UC) and Crohn’s disease[33].
S100B protein
S100B belongs to the S100 protein family that includes more than 20 EF-hand Ca2+-Zn2+ binding proteins[9,10,44-47]. S100B is the homodimer of β subunit[48]. In the brain, S100B in nanomolar concentrations promotes neuronal survival, neurite outgrowth[49] and it stimulates astrocytic proliferation[50], increasing the intracellular free Ca2+ levels in vitro[51]. On the other hand, micromolar amounts of S100B protein have been observed in several neuropathologies such as Alzheimer’s disease and Down’s syndrome[52,53].
In the human gut, among S100 proteins, only the S100B protein is specifically and physiologically expressed by EGCs[13-15], while other members, such as S100A8, S100A9 and S100A12 are found in phagocytes and in intestinal epithelial cells in patients affected by IBD[54,55].
Recent findings have demonstrated that aberrant expression and the release of S100B correlate with the gut inflammatory status[13,14]. Interestingly, the search for a specific S100B signaling receptor has demonstrated that this protein may accumulate at the RAGE (receptor for advanced glycation end products) site only in micromolar concentrations[14,56-59]. Such interaction leads to mitogen-activated protein kinase (MAPK) phosphorylation and consequent nuclear factor-κB (NF-κB) activation[13] which, in turn, leads to the transcription of different cytokines and iNOS protein. Thus, S100B can be considered as an easily diffusible pro-inflammatory cytokine which gains access to the extracellular space especially at immune-inflammatory reaction sites in the gut[13-15,60,61].
EGCs AND S100B IN GUT INFLAMMATION
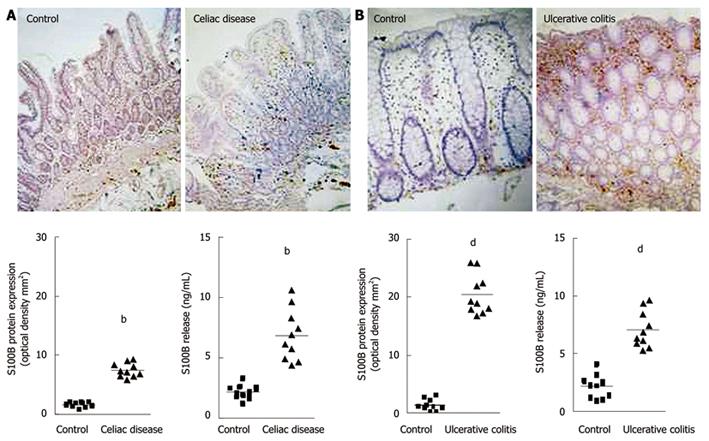
In humans, recent and increasing data has demonstrated that EGCs and S100B protein are directly involved in gut inflammatory diseases[13,14,33]. Previous investigations described abnormalities of the enteroglial network in patients with Crohn’s disease and UC[33]. More recently, glial abnormalities have been confirmed by 2 separate studies carried out by our group[13,14]. In particular, we demonstrated that, in patients with celiac disease (CD)[13] and UC[14], EGCs participate in the modulation of mucosal NO production via S100B overexpression and release. Indeed, in patients with CD, we demonstrated that S100B plays an active role in NO-dependent inflammation[13]. In particular, increased S100B protein expression and release were observed in the duodenal mucosa of patients with untreated CD, compared to healthy controls (Figure 1A)[13]. Very interestingly, S100B upregulation was accompanied by enhanced iNOS protein expression and consequent NO release, both representing crucial features in CD[62].
Figure 1 Changes in S100B protein expression during intestinal inflammation.
A: Celiac disease[13]. Immunohistochemistry shows stronger S100B immunopositivity in the duodenal mucosa of patients affected by celiac disease, compared with healthy controls (original magnification, × 100). The graphs represent S100B protein expression (left) and release (right) in healthy controls and patients with celiac disease (bP < 0.01); B: Ulcerative colitis[14]. Immunohistochemistry shows stronger S100B immunopositivity in the rectal submucosa of patients with ulcerative colitis, compared with healthy controls (original magnification, × 100). The graphs represent S100B protein expression (left) and release (right) in healthy controls and patients with ulcerative colitis (dP < 0.01).
The relationship between S100B and NO production was confirmed by the demonstration that the administration of exogenous S100B protein to non-inflamed duodenal biopsy specimens from healthy controls, resulted in both iNOS protein expression and NO release, indicating that micromolar concentrations of this protein are able to participate in the inflammatory response of even “healthy duodenum”[13]. Besides NO production, exogenous S100B mediates a significant increase in lipid peroxidation associated with a marked increase in phosphorylated-p38 MAPK protein expression and with the activation of NF-κB, in accordance with the previously mentioned studies[13].
Taken together, these observations represented the first data in humans suggesting that via S100B upregulation, EGCs directly participate in NO-dependent inflammation occurring in CD, and they paved the way by supposing that EGCs are part of complex immuno-regulatory effectors since they establish a strategic first defense line against foreign antigens. By means of EGC proliferation, changes in enteroglial architecture have been reported also in patients with IBD[33,63]. Several studies have shown that EGC markers are differentially altered in Crohn’s disease and UC with a decrease in EGC density in Crohn’s disease and a gliosis-like phenomenon in UC[33,63].
In support of these observations, it has recently been confirmed that EGCs directly participate in the chronic mucosal inflammation of patients with UC[14]. In fact, S100B immunoreactivity significantly increased in the rectal mucosa of these patients when compared to the mucosal S100B expression in healthy controls (Figure 1B)[14]. This upregulation was associated with the specific stimulation of iNOS and consequent abnormal mucosal NO production, both representing characteristic features of UC[64,65].
In addition, via iNOS expression, exogenous S100B induces a significant and concentration-dependent increase in NO production in the human rectal mucosa of healthy controls via RAGE interaction[14], confirming the ability of EGCs to modulate NO production and the specificity of S100B protein-mediated responses in the human gut.
Further confirming that EGCs are part of the complex system of immunoregulatory effectors in the gut, it has been shown that the addition of pro-inflammatory stimuli to rectal mucosal tissue led to EGC activation, again via RAGE involvement[14] as demonstrated by both S100B upregulation and enhanced NO production. These findings indicate that EGCs are able to recognize inflammatory stimuli and that once activated, they produce and release S100B up to micromolar concentrations, thereby contributing to NO production in the human gut.
CONCLUSION
ECGs as a target for new drugs aimed at inflammatory gut disorder management
In summary, emerging evidence now indicates that EGCs actively participate in the modulation of inflammatory responses in the human gut. Targeting their hyperactivation in the gut in inflammatory disorders may represent a novel approach to diminish tissue damage and to counteract the lack of long-term effectiveness of classical immunosuppressant agents.
Additional studies investigating the relationship between EGCs and immune cells are warranted in order to carry out an in-depth examination of the role of glial cells and glia-derived factors in the modulation of immune/inflammatory responses in the human gut. Preliminary data indicates that EGCs-derived S100B is able to affect peripheral blood and intestinal mucosal immune cell responses via RAGE[66].
The application of this approach may help the future evaluation of the relationships between EGCs and immune cells in order to better understand the pathophysiology of intestinal inflammation and to establish new therapeutic approaches towards the treatment of gut inflammatory disorders.