INTRODUCTION
Optical coherence tomography (OCT) is a relatively new method in medical imaging, with first attempts being recorded at the beginning of the last decade[1]. Based on the analyses of light interference properties generated by a low-coherence source (low-coherence interference), OCT is a noninvasive, high-resolution method. With a resolution in the range of 10-15 μm for standard OCT and up to 1-5 μm if special sources are used[2,3], this method offers cross-section imaging of the sample with a penetration depth of 2-3 mm. Depending on the OCT configuration, Doppler capabilities are also available in order to provide both structural and functional analyses. With the first application in the field of ophthalmology[4], promising OCT systems have recently been developed for other fields of medicine including cardiology[5-7], dermatology[8] and gastroenterology[9].
Angiogenesis describes the formation of new vessels from preexisting vasculature under physiological and pathological conditions. Tumor neo-angiogenesis is a fundamental step for solid tumor growth and for metastasis, because it results in spread and development of the network of capillaries from existing blood vessels that are capable of providing the necessary oxygen and nutrients. The process is mediated by various stimulatory and inhibitory factors[10], including cytokines (hormone-like peptides and low-molecular-weight albumins) and growth factors. These factors activate different enzymes (proteases, collagenases, gelatinases and heparinases) that degrade the extracellular matrix and proteins of the basal membrane, thus generating the proliferation and migration of endothelial cells. One of the main triggers of neo-angiogenesis is the secretion of the vascular endothelial growth factor (VEGF)/vascular permeability family of factors[11,12]. VEGFs are cytokines that have many effects on vascular endothelium, and these multifunctional effects can contribute to angiogenic responses[11].
The influence of these angiogenic factors is included in different mathematical models, using different living tissue or artificial tissue models such as chick embryo chorioallantoic membrane (CAM) or porous biomaterials[13-15], in order to describe both the vascular network growth and flow modeling. The purpose of these studies was either to develop understanding of the angiogenesis mechanism or to describe the possibility of direct drug delivery and monitoring of the effects at the level of tumor blood vessels.
Evaluation of angiogenesis thus represents an important step for tumor assessment, especially because of the advent of angiogenesis inhibitors that are already established as anticancer agents. Consequently, to study such a complex process requires multiple correlated investigations that can assess and quantify low-velocity, low-volume blood flow. High-resolution functional imaging methods like 3D-OCT and/or Doppler-OCT could prove to be of considerable importance, especially for the real-time assessment of tumor microvascular flow.
OCT
For the sake of clarity and to associate the physics of the method with its medical applications, a short description of the OCT and Doppler-OCT principles, together with specific characteristics of the experimental system, are provided. Detailed description of the physical principles and mathematical equations that govern the method have been provided by several other papers[16-20].
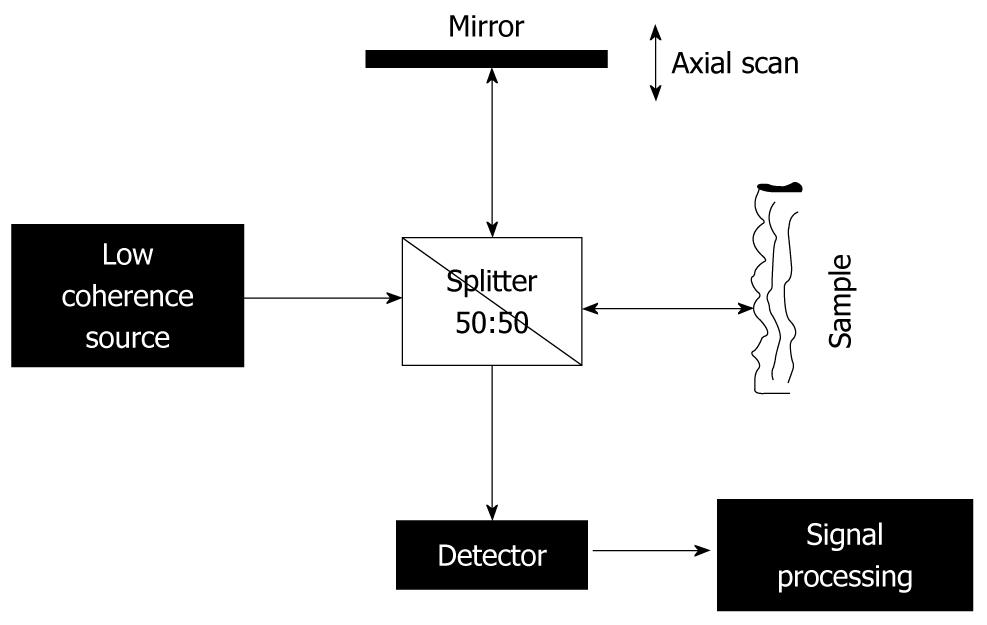
Basically, an OCT device provides an analysis similar to B-mode ultrasound investigation[18]. The main difference is that, instead of using an ultrasound beam, OCT uses low-coherence light, which is provided by a source with emission in the infrared range, typically between 700 and 1500 nm, depending on the optical properties of the target[21]. In the case of OCT measurements, direct analyses of the reflected intensity is not possible, because of using light, contrary to the case of ultrasound investigation, for which the image obtained is a direct mapping process of the reflected intensity from the target. This is due to the large difference between the velocity of the ultrasound beam and that of the light. Instead, in a standard OCT experiment (Figure 1), the infrared radiation is directed towards an interferometric set-up, where it is split into two beams: one used as reference and directed to a moving mirror, and the other is sent towards the sample. After reflection, the sample and reference beams are mixed together, which produces the physical process of interference. Interference occurs only as long as they remain coherent, and the properties of the interference pattern are used as input signal for the imaging process. It should be emphasized that the characteristics of the OCT method are directly related to the properties of the light source used, with the optical characteristics of the sample and with the set-up type.
Figure 1 Scheme of a standard optical coherence tomography set-up based on a Michelson interferometer.
Based on the above-mentioned factors, advantages and disadvantages of OCT analysis in comparison with ultrasound investigation can be summarized as follows. (1) The main advantage of OCT analysis is higher resolution; 3-15 μm is possible[2,3]. This is explained by the conditions for the interference process; to produce interference, two beams should be coherent. The light source is partially coherent, which means that coherence of the source is finite, and consequently, the two waves emitted by the source are coherent as long as they are not apart by more than a coherence length of the source. This means that the (optical) length of the reference and sample arm should not differ by more than the coherence length of the source; otherwise the two waves travelling back and forth along these arms do not interfere with each other. It also means that, by controlling the moving mirror in the reference arm (i.e. controlling the length of the reference arm), one has the possibility to control the depth in the sample, from where the reflected signal is received and analyzed (axial resolution); (2) Special mention should be made about the fact that, in an OCT experiment, no connection between the lateral and axial resolution of the measurements exists (in lateral resolution, both control and shape of the beam are playing a major role)[18,20]. Axial resolution can be estimated using the formula[16,18]: z = [(2 ln 2)/π][λ02/∆λ] (eq. 1), where λ0 is the central wavelength and ∆λ is the bandwidth, with the assumption that the incoming beam has a Gaussian shape and it travels through the air; nevertheless, in many practical set-ups, the radiation is travelling through optical fibers; (3) Infrared radiation (low-energy photons) is used and the source also has low power (mW range), therefore, this method is completely noninvasive; and (4) A major disadvantage compared to ultrasound assessment is the low penetration depth (2-3 mm, depending on the wavelength and optical properties of the analyzed sample[21,22]), due to the high dispersion and absorption coefficient for the visible-infrared light in biological tissues. Nevertheless, in order to perform analyses that require only millimeter-range penetration depth (different types of mucosa investigation, surface blood microvessels), OCT investigations provide a valuable tool.
As mentioned above, a few parameters (properties of the light source, optical characteristics of the sample, and set-up type) determine the OCT experiments. There are several different set-up configurations for an OCT device used for medical investigations[16-20]. These include time domain OCT (TD-OCT), Fourier domain OCT (FD-OCT), and swept source OCT (SS-OCT). FD-OCT and SS-OCT are usually grouped together under the name of spectral domain OCT.
TD-OCT was one of the first methods used and it involves a set-up similar to the well-known Michelson interferometer (Figure 1), which has the moving mirror in one arm and the sample in the other. It uses a broad-band light source[23] and it works similar to the above description. The produced interferogram is measured by a single detector as a function of time delay between the light travelling back and forth along the two arms[17]. The axial resolution depends on the coherence length of the source[16,17], and can be given by the equation above (eq. 1). The main disadvantage of this OCT set-up is that it involves mechanical moving parts (moving mirror) that make it difficult to achieve the high scanning rate necessary for in vivo 2D and 3D investigations.
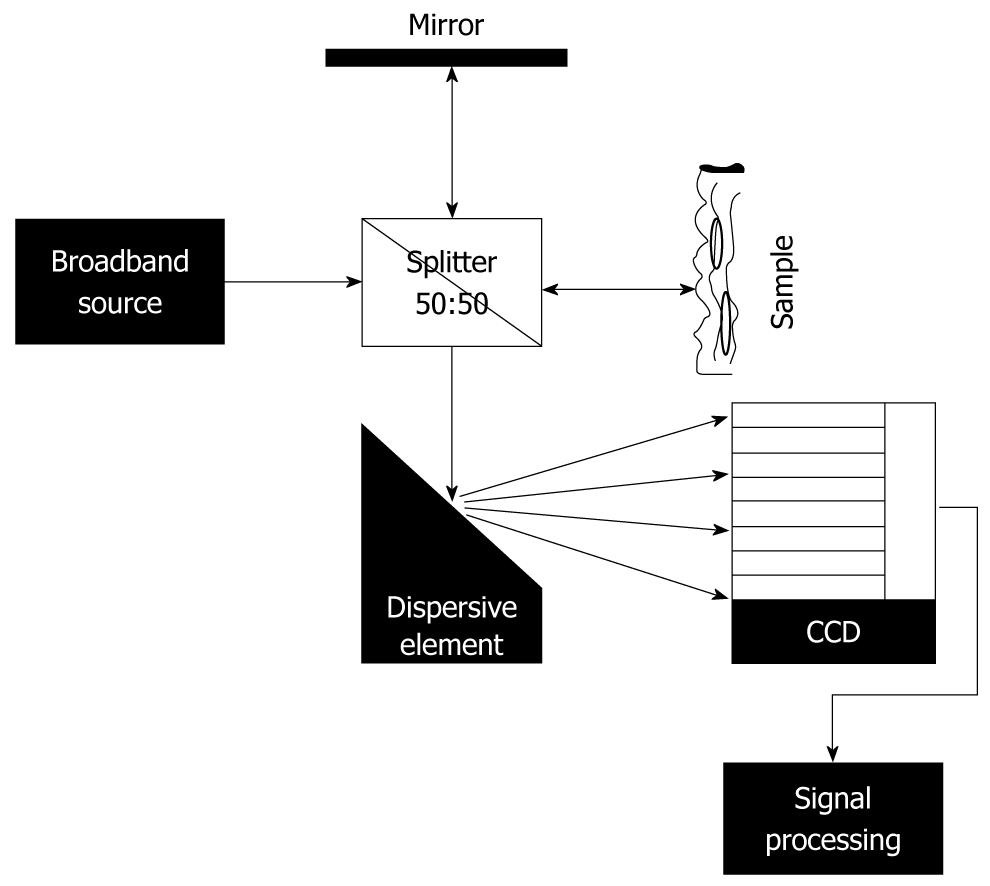
FD-OCT uses a charge coupled device or an array photodetector for registering the signal from the interfering light, instead of using a moving mirror and a single detector[17,18]. A dispersive element (grating or spectrometer) is introduced in the set-up (Figure 2), which projects on to the detector a distribution of the intensity as a function of wavelength. A Fourier transform of the registered signal provides the back-scattering signal from the sample as a function of time (practically as a function of penetration depth[16-18]). The main disadvantage in this case is the presence of motion artefacts[16,19,24].
Figure 2 Scheme of fourier domain-optical coherence tomography set-up.
SS-OCT is similar to FD-OCT but, instead of using a dispersive element to select different wavelengths and an array detector for analyses of the signal, it has a swept source (tunable laser) and a single detector[17,18,25].
Both FD-OCT and SS-OCT allow direct access to the whole spectrum within one measurement, which offers high sensitivity and imaging resolution, together with high scan rates, which make possible 2D and 3D investigations or fast Doppler measurements[18,22].
Of these three basic schemes, polarization sensitive detection or phase-sensitive detection can be added in order to improve the imaging process[16,17].
Doppler-OCT basically estimates the shift in frequency of the laser beam (laser Doppler velocimetry) when the process of the scattering takes place on a moving element[18,26,27]. As a result of the fact that OCT does not measure directly the reflected intensity, but an interference signal, special mathematical methods are required in order to analyze the received signal; methods which depend on the OCT set-up type[18]. An important detail is represented by the fact that, if laminar flow is assumed[20,28], then transversal measurements (in the range of ± 15°) are possible in order to visualize the blood vessels under the sample surface.
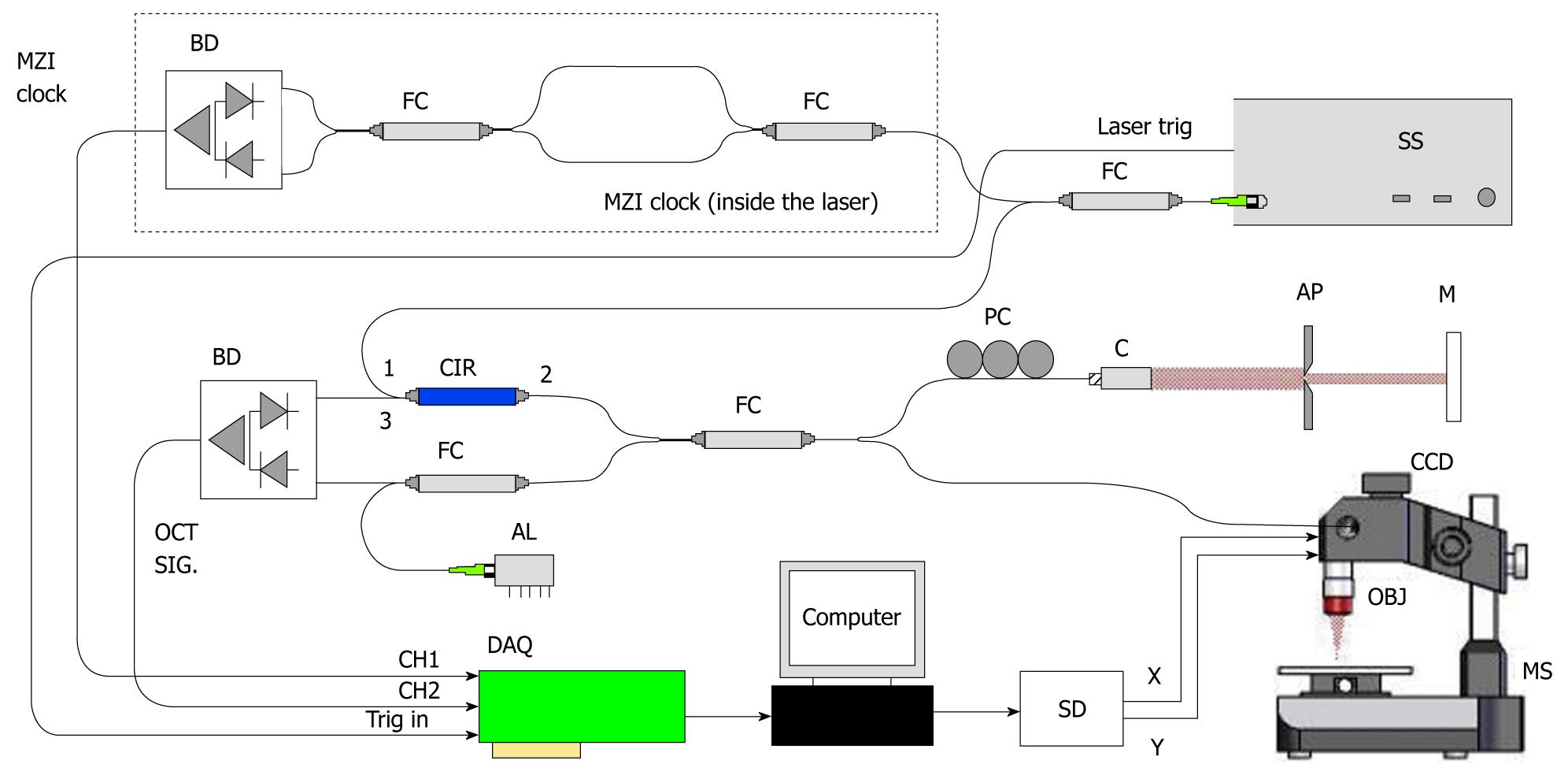
The system we have used in our measurements is an SS-OCT from THORLABS (OCS1300SS; Munich, Germany) (Figure 3). The source is a swept laser (55 kHz) with a central wavelength of 1325 nm and an average power of 12 mW. The system is capable of 2D and 3D scans (with an A-scan rate of 55 KHz), with an axial resolution of 12 μm and a lateral resolution of 15 μm. Optical power on the sample is 5 mW. A Doppler module is also available. The system has an image acquisition rate of 50 frames/s.
Figure 3 Set-up for OCS1300SS (courtesy of Thorlabs).
OCT: Optical coherence tomography; SS: Swept source.
CLINICAL APPLICATIONS
The possibility offered by the OCT system to deliver near histopathological resolution images makes this method a valuable tool for assessing the gastrointestinal tract. The possibility to investigate tumor structures with high resolution, without performing a classical biopsy, has become very attractive, especially in situations when harvesting biopsy is difficult to realize, or is very hazardous[9]. Several authors have investigated gastrointestinal cancers by OCT but, due to the late development of Doppler capabilities, few have performed investigations of the angiogenesis process. Generally, to date, most of the studies have evaluated the structural (architectural) changes in the digestive mucosa under pathological conditions, and have identified the correct OCT layer structure inside normal/pathological tissue.
Early studies using OCT techniques have focused on the structure and characteristics of the gastrointestinal tract layers, in order to distinguish between different conditions[29-32] and patterns, in an attempt to develop computer-assisted diagnostic methods. Also, a lot of interest has been directed towards the development of the OCT systems (e.g. new sources, fast acquisition rates, different types of catheters, and modeling of the involved physical processes) for the purpose of management during in vivo investigations[29,31,33]. For example, it has been shown that the structure of the esophageal wall (i.e. squamous epithelium, lamina propria, muscularis mucosa, submucosa, and muscularis propria), as well as the stomach layers (i.e. glandular epithelium, muscularis mucosa, submucosa, and muscularis propria) can be assessed by OCT in order to diagnose pathological aspects[32].
Recently[34], ultra-high resolution investigations have been performed in gastric tissues. Based on a higher resolution than standard OCT (10 μm), these studies have demonstrated that computer-aided image analysis is possible, even for detection of different grades of dysplasia[34,35] . Furthermore, it has been shown that OCT imaging using a standard resolution OCT system with a catheter, which acquires a linear longitudinal plane at a rate of 2/s, can identify specialized intestinal metaplasia at the squamo-columnar junction, with an accuracy close to that of endoscopy[36]. A pertinent review of OCT in the gastrointestinal tract (esophagus, stomach, small intestine and colon) has been carried out[37] for normal and pathological (pre-neoplastic tissue, dysplasia and neoplasia) tissues, specifying the detected characteristics. For bile and pancreatic ducts, OCT has also been shown to be a valid tool[38,39] that allows analysis of near histopathological resolution. The lower gastrointestinal tract has been subjected to OCT investigations[40] in correlation with histology. Colon cancer was initially studied on mouse models, which have shown that OCT is capable of distinguishing between characteristics of the mucosa (i.e. thickening of the mucosal layer and loss of visibility of tissue boundary lines[41]), even before adenoma arises. Recently, 3D-OCT investigation of the colon has been performed[42], in order to illustrate the differences in pathology. A pilot study on six volunteers was performed, which made possible the observation of clear differences between normal glandular epithelium, normal squamous epithelium and chronic inflammation from ulcerative colitis.
From the point of view of gastrointestinal tract mapping, most of the studies have involved the upper tract (esophagus, with emphasis on Barrett’s esophagus), where studies and imaging analysis are the most advanced[9,20,43,44] and can assist classic endoscopy. A more challenging task is the stomach, where low resolution and penetration depths are observed[9,20]. In contrast, the colon is a region where images with good investigative potential have been recorded[9,18,31].
The Doppler-OCT technique was developed later than standard OCT, and only a few studies have been published[27,45-48]. Most of the expectations for this method were initially to detect and analyze mucosal and submucosal microvascularization. Position (e.g. lamina propria and submucosa.) and size (small vessel ≤ 100 μm, medium vessels ≤ 400 μm, and large vessels ≥ 400 μm) of the blood vessels could be detected[48] in different parts of the gastrointestinal tract. Nevertheless, for the clinical translation of Doppler-OCT into a standard medical investigation, patterns and different characteristics of the microvascularization should be identified and used to produce computer-aided diagnostic procedures. There are not many sources that provide values for physical parameters of biological tissues, e.g. (refractive index, dispersion coefficient); values which are necessary for physical and mathematical modelling, and research should be directed towards such measurements.
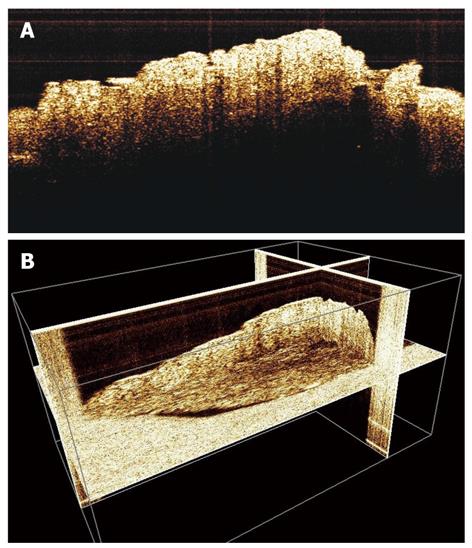
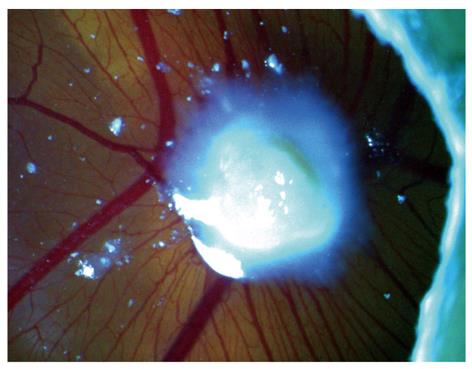
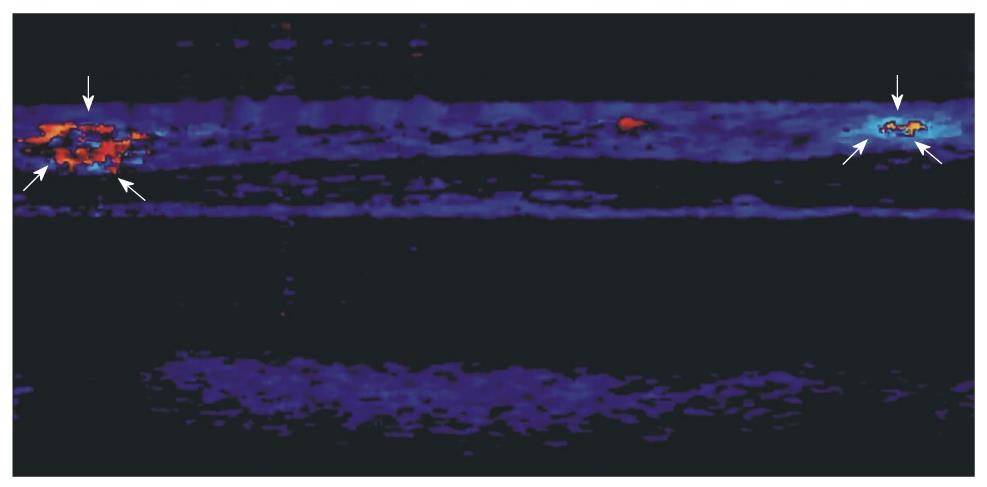
We have conducted our early experiments on biopsies (Figure 4A and B) and in a chick embryo CAM model (Figures 5 and 6), where human biopsy implants (xenografts) were placed. The images clearly showed the possibility to identify the microvascularization around the implant zone and the detection of blood flow. It seems that parameters that characterize the capillary network, such as density, flux and diameter, are accessible to the imaging process, and in addition with other characteristics provided by OCT (i.e. architectural distortion, presence of submucosal glands, and alteration of the layer structure[9,20]), they can be useful to describe the angiogenesis process.
Figure 4 2-D (A) and 3-D (B) optical coherence tomography images of gastric tissue biopsy with visualization of normal components of the parietal layers.
Figure 5 Macroscopic view of a chick embryo chorioallantoic membrane with human gastric tumor implant.
Figure 6 Doppler-optical coherence tomography imaging of a chick embryo chorioallantoic membrane (2 mm × 2 mm) showing capillary activity (arrows) near a human gastric tumor implant.
CONCLUSION
Even though, at present, OCT investigations in gastroenterology are not able alone to provide adequate results (most of the published studies report accuracy and positive predictive values lower than endoscopy), it is worth developing combination of classical endoscopy and OCT techniques to harness the advantages of both. Also, given the large number of medical studies that use these types of devices, as well as recent technical improvements to the systems, it is clear that OCT methods are very promising. At the same time, acquisition of Doppler 3-D images could prove very valuable for the quantitative description of the angiogenesis process, due to the high resolution of this method and the fact that the results can be correlated with structural and functional properties of the samples.
Peer reviewer: Xiao-Peng Zhang, Professor, Department of Radiology, Peking University School of Oncology, Beijing Cancer Hospital and Institute, No. 52 Haidian District, Beijing 100142, China
S- Editor Wang JL L- Editor Kerr C E- Editor Zheng XM