Published online Mar 7, 2010. doi: 10.3748/wjg.v16.i9.1104
Revised: January 6, 2010
Accepted: January 13, 2010
Published online: March 7, 2010
AIM: To study the individual effects of glucocorticoid (GC) therapy on the state of immune activation in patient serum.
METHODS: We developed a novel assay in which the effect of corticosteroid-treated patient serum on healthy donor peripheral blood mononuclear cells (target cells) was studied, with a panel of markers for effector [interferon (IFN)γ and interleukin (IL)-5] and regulatory T cells (FOXP3 and glucocorticoid-induced tumor necrosis factor receptor, GITR). The study group comprised 19 children with inflammatory bowel disease. The individual effect of patient serum on target cells was analyzed prior to GC therapy and 2 wk later.
RESULTS: The effect of GC therapy mediated by patient serum was seen as a decrease in the target cells expression of regulatory T-cell-related markers GITR (median suppression 24%, range of suppression 1%-63%, in 2 cases increase of 6% and 77%, P < 0.01 for mitogen-activated target cells) and FOXP3 (median suppression 33%, range of suppression 0%-79%, in one case an increase of 173%, P < 0.05 for resting cells), and secretion of IFNγ [from a mean of 87 700 pg/mL (SD 33 900 pg/mL) to 60 900 pg/mL (SD 44 200 pg/mL) in mitogen-activated target cells, 13 of the cases showed a decrease, P < 0.01]. The total or weight-related prednisolone dose did not correlate with the patient-serum-induced changes in the target cell markers.
CONCLUSION: GC response could be monitored at an individual level by studying the effect of patient serum on signaling pathways of target immune cells.
- Citation: Rintamäki H, Salo HM, Vaarala O, Kolho KL. New means to monitor the effect of glucocorticoid therapy in children. World J Gastroenterol 2010; 16(9): 1104-1109
- URL: https://www.wjgnet.com/1007-9327/full/v16/i9/1104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i9.1104
Since the description of the life-saving effect of glucocorticoids (GCs) in the treatment of inflammatory bowel disease (IBD), GCs have been the mainstay in the treatment of moderate and severe forms of the disease[1]. Although widely used, several patients show an inadequate response to GCs, a phenomenon that could not have been foreseen. In patients with GC resistance, therapeutic effect is lacking. Those considered to be steroid-dependent benefit from the treatment but their disease flares up immediately after lowering the GC dose or discontinuation of the therapy. Among IBD patients, up to 31%-45% seem to become steroid-dependent[2-4]. Other treatment options have not overcome the need for GCs in IBD, a disease that is constantly on the increase in many western countries[5,6].
The pathogenesis of IBD is multifactorial. An inappropriate response of a mucosal immune system to the indigenous flora and luminal antigens plays a key role that leads to activation of innate immune cells that direct the effector and regulatory T-cell responses of adaptive immunity[7].
GC action is mediated by a GC-receptor complex that is translocated into the nucleus. The genomic actions are classified as transrepression, that is, inhibition of the synthesis of the regulatory proteins, which results from the interaction between the activated GC-receptor complex and transcription factors, such as nuclear factor-κB or activator protein-1, and transactivation, that is, induction of the synthesis of regulatory proteins that leads to downregulation of inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-12 and interferon (IFN)γ, and upregulation of anti-inflammatory cytokines, such as IL-10, and transforming growth factor β[8-11]. GCs have also non-genomic interactions with cellular membranes[12].
Therapeutic response to GCs at an individual level is unpredictable. Here, we searched for new means to monitor the stage of immune activation during GC therapy. We developed a novel assay, in which the effect of patient serum on donor peripheral blood mononuclear cells (PBMCs) was studied using a panel of markers for effector (IFNγ and IL-5) and regulatory T cells (FOXP3 and glucocorticoid-induced tumor necrosis factor receptor, GITR). We found that attenuation of inflammation during GC therapy was reflected in these markers.
Nineteen consecutive pediatric patients with active IBD (Table 1) were prospectively introduced to oral prednisolone (Leiras, Finland), with a constant once daily dose of 1 mg/kg (range 15-60 mg) for 2 wk, as described in detail elsewhere[13,14]. The need for GCs was based on the physician’s global assessment of severe disease. After the 2-wk study protocol[13,14], the clinicians tapered the GC dose and adjusted the therapy. At the start of GC therapy, the maintenance therapies (Table 1) were unaltered. All patients had undergone gastrointestinal endoscopy to establish the diagnosis of IBD[15].
| Patient No. | Age (yr) | Sex | Diagnosis | Disease extension at diagnosis | Disease duration at inclusion (yr) | Maintenance medication at inclusion |
| 1 | 14 | Male | UC | Left-sided | 2.9 | 5-ASA |
| 2 | 14 | Female | UC | Pancolitis | 0.2 | 5-ASA |
| 3 | 5.6 | Female | UC | Distal | 0.8 | No |
| 4 | 18 | Male | UC | Pancolitis | 5.6 | 5-ASA |
| 5 | 17 | Male | UC | Pancolitis | 2.2 | 5-ASA |
| 6 | 15 | Female | UC | Pancolitis | 3 | 5-ASA |
| 7 | 15 | Male | UC | Pancolitis | 0.8 | 5-ASA |
| 8 | 14 | Male | UC | Pancolitis | 0.1 | 5-ASA |
| 9 | 14 | Male | UC | Pancolitis | 0 | 5-ASA |
| 10 | 13 | Female | UC | Pancolitis | 3.7 | 5-ASA, AZA |
| 11 | 12 | Male | UC | Pancolitis | 0 | 5-ASA |
| 12 | 12 | Male | UC | Pancolitis | 0 | 5-ASA |
| 13 | 12 | Female | UC | Pancolitis | 1.3 | 5-ASA |
| 14 | 9.4 | Male | UC | Pancolitis | 4.2 | 5-ASA |
| 15 | 7.4 | Male | UC | Pancolitis | 1.8 | 5-ASA |
| 16 | 7.2 | Male | UC | Pancolitis | 1.3 | 5-ASA |
| 17 | 15 | Male | CD | Pancolitis | 0 | 5-ASA |
| 18 | 12 | Female | CD | Pancolitis | 10 | 5-ASA |
| 19 | 16 | Male | IC | Pancolitis | 1.2 | 5-ASA |
The first blood sample was taken prior to the first GC dose on the day that the clinician instructed the start of GC therapy. The second sample was taken after 2 wk and the two samples were compared individually. Routine samples for blood count, hemoglobin, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were obtained at the start of GC therapy and after 2 wk.
PBMCs and the plasma from the blood of healthy donors and patients were separated by Ficoll-Paque (Amersham Biosciences, Freiburg, Germany) centrifugation according to the protocol of the manufacturer. PBMCs were suspended in 1 mL of medium (RPMI with HEPES buffer (Gibco, Carlsbad, CA, USA) with 0.002 mmol/L glutamine (Gibco), 25 μL/mL gentamicin (Gibco) and 5% serum), for counting. PBMCs were frozen at -136°C with DMSO (Sigma-Aldrich Corp, St. Louis, MO, USA). These cells from healthy donors were used as target cells. Target cells were diluted in cell culture medium (described above) without serum. Target cells, unactivated or activated with phytohemagglutin (PHA, 5 μg/mL) were cultured as duplicates in the presence of the IBD patients’ inactivated (56°C for 35 min) serum, at a concentration of 8%, for 72 h at 37°C in a humidified atmosphere with 50 mL/L CO2. After culture, the supernatants and target cells were collected by centrifugation at 400 g for 7 min. RT lysis buffer (Sigma-Aldrich) was added to the target cells, and the supernatants were stored at -70°C. The target cell assay was repeated using PBMCs from two different healthy donors (26-year-old woman and 31-year-old man) as target cells. Results represent one series of experiments.
IL-5 and IFNγ were measured with ELISA in duplicate from the supernatants collected from the target cell cultures incubated in the presence of patient serum. IFNγ and IL-5 were detected as described previously[16,17]. We subtracted the non-stimulated value from the stimulated value to obtain the Δ value for statistical analysis.
Total RNA was isolated from cell samples with GenElute Mammalian total RNA Miniprep kit (Sigma-Aldrich), and RNA concentration was measured by a spectrophotometer (ND-1000; NanoDrop Technologies Inc, Wilmington, DE, USA). Reverse transcription was performed by using TaqMan Reverse Transcription reagents (Applied Biosystems, Foster City, CA, USA), with additional treatment of total RNA at 10 ng/μL with DNAse I (0.01 U/μL) (Roche Diagnostics, Mannheim, Germany) to eliminate genomic DNA. Quantitative RT-PCR was performed using predesigned FAM-labeled TaqMan Gene Expression Assay reagents (Applied Biosystems) and the ABI Prism 7700 Sequence Detection System (Applied Biosystems) in triplicate wells. Assay reagents for FOXP3 (Hs00203958_m1), GATA3 (Hs00231122_m1), T-BET (Hs00203436_m1), GITR (Hs00188346_m1), IFNγ (Hs00174143_m1), IL-5 (Hs00542562_m1), IL-10 (Hs00174086_m1), STAT-5b (Hs00273500_m1), and 18s RNA (Hs99999901_s1) were used. The quantitative value obtained from the TaqMan run was the threshold cycle Ct, which indicated the number of PCR cycles at which the amount of amplified target molecule exceeded a predefined fluorescence threshold value. The difference value (ΔCt) was the normalized quantitative value of the expression level of the target gene, which was achieved by subtracting the Ct value of the housekeeping gene (18S) from that of the target gene. An exogenous cDNA pool calibrator was collected from PHA-stimulated PBMCs, and considered as an interassay standard to which normalized samples were compared. ΔΔCt was the difference between the ΔCt of the analyzed sample and that of the calibrator. Calculation of 2-ΔΔCt gave a relative amount of the target gene in analyzed sample compared with the calibrator, both normalized to an endogenous control (18S). For presentations, the relative amount of target genes was multiplied by 1000 and expressed as relative units. When fold change of gene expression following GC therapy was expressed, it was calculated by dividing the relative expression after therapy by that before therapy. For test reliability, those samples with mRNA expression under 10 ng/μL (n = 14 for resting and n = 1 for activated target cells) between triplicate wells were excluded from the statistical analysis.
We used the Wilcoxon rank test, two-tailed Mann-Whitney U test, two-tailed Spearman’s rho correlation test, and χ2 test when appropriate (SPSS version 13.0). In the few cases with IFNγ greater than the detection limit of 120 000 pg/mL, this high value was used in statistical analysis. P < 0.05 was set for statistical significance.
The study was approved by the Ethics Committee of Helsinki University Central Hospital and by the Institutional Review Board. The families attending the study signed an informed consent form.
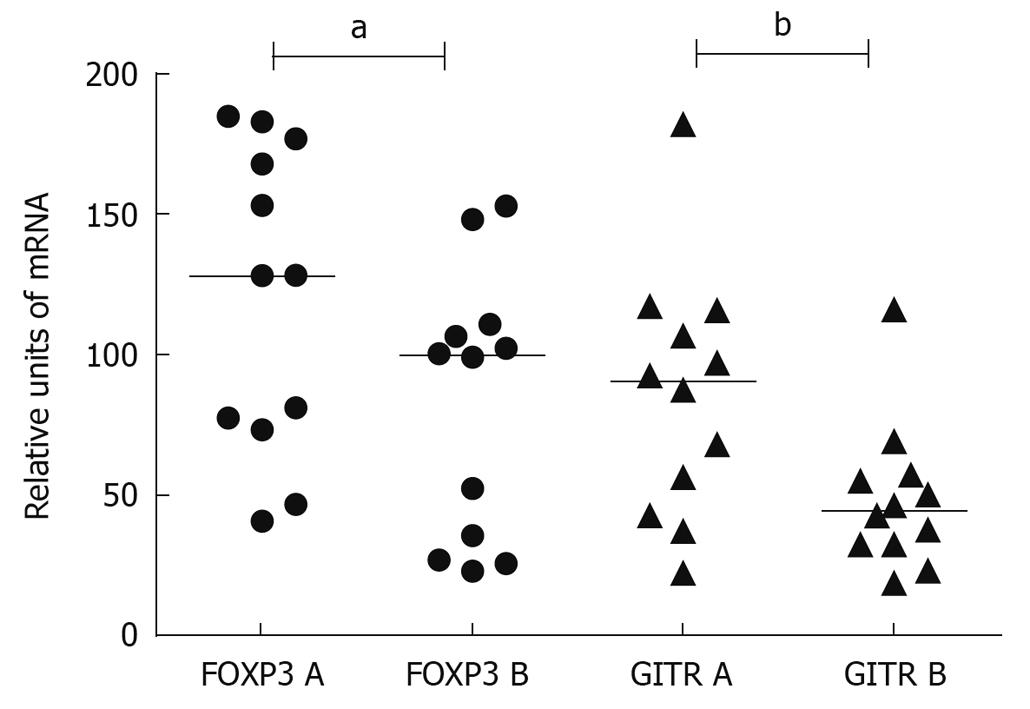
The median expression of FOXP3-specific mRNA in the resting target cells was suppressed by 33% (range of suppression 0%-79%, in one case, an increase of 173%, P < 0.05) and the median expression of GITR-specific mRNA by 46% (range 2%-78%, in three cases, an increase of 6%-124%, P = 0.050), when the effect of serum after GC was compared to that before therapy (Figure 1). In PHA-activated target cells, the median expression of GITR-specific mRNA was suppressed by 25% compared to the pre-treatment serum (range of suppression 1%-63%, in two cases, an increase of 6%-77%, P < 0.01).
The total or weight-related prednisolone dose did not correlate with the serum-induced GITR or FOXP3 gene expression of the target cells.
IFNγ secretion from target cells was lower when cultured with patient serum taken 2 wk after the start of GC therapy, compared to the culture with the serum sample taken before GC therapy. This decrease was seen in resting target cells [from a mean of 68 pg/mL (SD 110 pg/mL) to 1.5 pg/mL (SD 6.7 pg/mL); however, because of the low secretion of IFNγ in resting target cells, detectable levels were only seen in seven cases, six of whom showed a decrease, P < 0.05]. In activated target cells, 13 cases showed a decrease from a mean of 87 700 pg/mL (SD 33 900 pg/mL) to 60 900 pg/mL (SD 44 200 pg/mL) (P < 0.01, Table 2). The decrease in serum-induced IL-5 secretion from activated target cells decreased after GC therapy was close to the level of statistical significance [from a mean of 60 pg/mL (SD 50 pg/mL) to 32 pg/mL (SD 46 pg/mL); 12 cases showed a decrease, P = 0.055, Table 2]. Serum-induced IL-5 secretion from resting target cells was undetectable.
| Patient No. | IFNγ A PHA | IFNγ B PHA | IL-5 A PHA | IL-5 B PHA |
| 1 | 7200 | 19 000 | 11 | 28 |
| 2 | 99 000 | 8500 | 0.0 | 0.0 |
| 3 | 41 000 | 12 000 | 77 | 19 |
| 4 | 82 000 | 17 000 | 150 | 9.3 |
| 5 | 94 000 | 36 000 | 150 | 11 |
| 6 | > 120 000 | > 120 000 | 54 | 21 |
| 7 | > 120 000 | 100 000 | 140 | 38 |
| 8 | 63 000 | 28 000 | 1.2 | 1.2 |
| 9 | 59 000 | 34 000 | 99 | 34 |
| 10 | 110 000 | 120 000 | 75 | 89 |
| 11 | > 120 000 | 84 000 | 8.1 | 1.2 |
| 12 | > 120 000 | 85 000 | 12 | 3.5 |
| 13 | 91 000 | 19 000 | 84 | 17 |
| 14 | 40 000 | 62 000 | 73 | 130 |
| 15 | 91 000 | 6600 | 10 | 7.2 |
| 16 | > 120 000 | > 120 000 | 15 | 2.3 |
| 17 | 90 000 | 63 000 | 69 | 33 |
| 18 | > 120 000 | 97 000 | 33 | 0.0 |
| 19 | 68 000 | 120 000 | 89 | 160 |
The total or weight-related prednisolone dose did not correlate with IFNγ or IL-5 secretion from target cells.
There were no statistically significant changes in the mRNA levels of the cytokines IFNγ, IL-5 and IL-10, or the transcription factors FOXP3, GITR, GATA3, STAT-5b and T-bet, in PBMCs derived from patients with IBD before and after 2 wk of GC therapy.
ESR decreased from a median of 29 (range 0-64) mm/h to 12 (range 4-36) mm/h (P < 0.01), and CRP decreased from a median of 10 (range 5-75) mg/L to 5 (range 5-10) mg/L (P < 0.01) during 2 wk GC therapy. ESR or CRP did not correlate with the expression of GITR or FOXP3 from target cells, or with IFNγ or IL-5 secretion from target cells.
We found that serum from a GC-treated patient modulated in vitro the response of healthy donor-derived PBMCs and simulated the effects of GC treatment on the patient immune system. The attenuation of systemic inflammation during GC therapy was demonstrated with this assay at an individual level. The effect of GC treatment was mediated by patient serum, which caused a decrease in the target cell expression of regulatory T-cell-associated markers GITR[18] and FOXP3[19], and in the secretion of Th1 cytokine IFNγ.
The serum-induced decrease in cytokine and signaling molecule expression in the target cells was not attributed to the GC dose. This suggests that the observed changes in the levels of cell signaling markers were not solely due to GCs, but rather reflected the net systemic effect in patient serum. It has been reported previously that neither the level of GC in plasma or serum or serum GC bioactivity correlate with therapeutic responses[14,20,21]. To study whether serum-induced effects on target cells were mediated by GC receptors, we co-cultured the target cells with patient serum and GC receptor antagonist mifepristone[13,22]. Disappointingly, the results were uninformative as the GC receptor antagonist suppressed the target cell cytokine production and the treatment-mediated effects could not be assessed (data not shown).
It is highlighted that we did not see any direct effects of GC therapy on PBMCs derived from the patients. The PBMC population is heterogeneous and comprises naïve and memory T cells and effector and regulatory T cells. Thus, the net effect of GCs might be covered when studied in patient-derived PBMCs. Instead, we found that the effect of GC treatment was demonstrated as a patient-serum-induced decrease of FOXP3 and GITR in the target cells.
Earlier studies of the effect of GC therapy have focused on the direct changes induced in patient PBMC or lymphocyte subtypes such as regulatory T cells. The observation of GC-induced changes in regulatory T-cell-related FOXP3 is controversial, and there are no data from pediatric IBD. In an in vitro study on regulatory T cells of healthy donors, the administration of dexamethasone on purified CD4+ T cells induced a transient increase in the expression of FOXP3[23]. Accordingly, in asthmatic patients, an increase in FOXP3 mRNA expression has been observed in freshly isolated and purified CD4+ T cells after oral GC administration[23]. However, in another study, the counts of FOXP3-expressing CD4+CD25high T cells and mRNA expression of GITR in PBMCs were unaltered after 4 wk treatment with inhaled steroids[24]. Furthermore, in a study of patients with allergic rhinitis, FOXP3-positive cells from nasal mucosa biopsies decreased significantly after several weeks treatment with intranasal GCs[25]. These controversial findings of regulatory T-cell activation in different tissues and during different courses of GC therapy underline the importance of further studies to increase our understanding of the anti-inflammatory effects of GCs.
In line with our finding of a decrease in target cell IFNγ secretion, downregulation of pro-inflammatory cytokines, including IFNγ, has been reported in an in vitro study using PBMCs from healthy donors stimulated with dexamethasone and studied with DNA microarray chip analysis, online PCR and flow cytometry[26].
Recently, it has been shown that, in severe pediatric colitis, the response to intravenous GC could be predicted already on day 3, with the aid of clinical activity scores[27]. However, for oral GC therapy, there are no such data. We assessed the immunological responses of our patients at 2 wk, a time interval that was initially chosen for practical reasons. Therefore, it warrants further study to investigate if our findings could be detected during the first week of GC therapy.
To conclude, we developed a new cell culture assay to study the effect of GC therapy at an individual level, using serum samples taken from patients during GC therapy. We consider that the patient-serum-induced changes in the target cells reflect the net effect of the metabolism of GC and its immunological effects, and this mirrors the systemic inflammatory activity of the disease in the patient’s circulation during GC therapy.
Corticosteroids are widely used in the treatment of several diseases, for example, asthma, rheumatic disease and inflammatory bowel disease (IBD). Corticosteroids are effective in suppressing active inflammation but not all patients respond to treatment, and various side effects are common. To date, the therapeutic effect cannot be foreseen and there are no specific means to monitor the therapeutic response at an early phase of the treatment.
IBD is a chronic disorder with an increasing incidence in children. In pediatric patients, the disease course is often aggressive and the majority of patients need corticosteroids to suppress their symptoms. There is an urgent need for new means to monitor therapeutic responses to improve patient care.
The authors describe a novel assay that gives the possibility to monitor therapeutic responses to corticosteroids, using a serum sample from the patient. Disease activity is reflected in the serum that is used in an in vitro cell assay to stimulate human white blood cells. In cell culture, it is possible to measure how the effect of patient serum on specific white blood cell markers (T cells) varies according to the given therapy. With this novel assay, it is possible to study the individual effect of corticosteroid therapy.
The results suggest that the effect of corticosteroid therapy on disease activity can be measured from patient serum at an early phase of the therapy. The authors studied pediatric patients with IBD, and further study is warranted to establish whether this kind of method is suitable for other patients with corticosteroid treatment.
This is a well written and interesting manuscript.
Peer reviewer: Mr. Jamie Murphy, MRCS (Eng), MA, Lecturer in Colorectal Surgery, Centre for Academic Surgery, Royal London Hospital, 3rd Floor Alexandra Wing, London, E1 1BB, United Kingdom
S- Editor Wang JL L- Editor Kerr C E- Editor Lin YP
| 1. | Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. |
| 2. | Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360-362. |
| 3. | Tung J, Loftus EV Jr, Freese DK, El-Youssef M, Zinsmeister AR, Melton LJ 3rd, Harmsen WS, Sandborn WJ, Faubion WA Jr. A population-based study of the frequency of corticosteroid resistance and dependence in pediatric patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2006;12:1093-1100. |
| 4. | Hyams J, Markowitz J, Lerer T, Griffiths A, Mack D, Bousvaros A, Otley A, Evans J, Pfefferkorn M, Rosh J. The natural history of corticosteroid therapy for ulcerative colitis in children. Clin Gastroenterol Hepatol. 2006;4:1118-1123. |
| 5. | Hildebrand H, Finkel Y, Grahnquist L, Lindholm J, Ekbom A, Askling J. Changing pattern of paediatric inflammatory bowel disease in northern Stockholm 1990-2001. Gut. 2003;52:1432-1434. |
| 6. | Turunen P, Kolho KL, Auvinen A, Iltanen S, Huhtala H, Ashorn M. Incidence of inflammatory bowel disease in Finnish children, 1987-2003. Inflamm Bowel Dis. 2006;12:677-683. |
| 7. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. |
| 8. | Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124-137. |
| 9. | Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500:51-62. |
| 10. | Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711-1723. |
| 11. | Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006;27:413-426. |
| 12. | Pitzalis C, Pipitone N, Perretti M. Regulation of leukocyte-endothelial interactions by glucocorticoids. Ann N Y Acad Sci. 2002;966:108-118. |
| 13. | Vihinen MK, Kolho KL, Ashorn M, Verkasalo M, Raivio T. Bone turnover and metabolism in paediatric patients with inflammatory bowel disease treated with systemic glucocorticoids. Eur J Endocrinol. 2008;159:693-698. |
| 14. | Vihinen MK, Raivio T, Verkasalo M, Jänne OA, Kolho KL. Circulating glucocorticoid bioactivity during peroral glucocorticoid treatment in children and adolescents with inflammatory bowel disease. J Clin Gastroenterol. 2008;42:1017-1024. |
| 15. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-19. |
| 16. | Halminen M, Klemetti P, Vaarala O, Hurme M, Ilonen J. Interferon-gamma production in antigen specific T cell response: quantitation of specific mRNA and secreted protein. Scand J Immunol. 1997;46:388-392. |
| 17. | Honkanen J, Skarsvik S, Knip M, Vaarala O. Poor in vitro induction of FOXP3 and ICOS in type 1 cytokine environment activated T-cells from children with type 1 diabetes. Diabetes Metab Res Rev. 2008;24:635-641. |
| 18. | Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6216-6221. |
| 19. | Yi H, Zhen Y, Jiang L, Zheng J, Zhao Y. The phenotypic characterization of naturally occurring regulatory CD4+CD25+ T cells. Cell Mol Immunol. 2006;3:189-195. |
| 20. | Kaplan HP, Portnoy B, Binder HJ, Amatruda T, Spiro H. A controlled evaluation of intravenous adrenocorticotropic hormone and hydrocortisone in the treatment of acute colitis. Gastroenterology. 1975;69:91-95. |
| 21. | Powell-Tuck J, Buckell NA, Lennard-Jones JE. A controlled comparison of corticotropin and hydrocortisone in the treatment of severe proctocolitis. Scand J Gastroenterol. 1977;12:971-975. |
| 22. | Leonhardt SA, Edwards DP. Mechanism of action of progesterone antagonists. Exp Biol Med (Maywood). 2002;227:969-980. |
| 23. | Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Rückert B, Mantel PY, Menz G, Akdis CA, Blaser K. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114:1425-1433. |
| 24. | Zhang Q, Qian FH, Liu H, Zhou LF, Huang M, Zhang XL, Yin KS. Expression of surface markers on peripheral CD4+CD25high T cells in patients with atopic asthma: role of inhaled corticosteroid. Chin Med J (Engl). 2008;121:205-212. |
| 25. | Malmhäll C, Bossios A, Pullerits T, Lötvall J. Effects of pollen and nasal glucocorticoid on FOXP3+, GATA-3+ and T-bet+ cells in allergic rhinitis. Allergy. 2007;62:1007-1013. |