Published online Feb 21, 2010. doi: 10.3748/wjg.v16.i7.881
Revised: December 19, 2009
Accepted: December 26, 2009
Published online: February 21, 2010
AIM: To investigate the prevalence, risk factors, and clinicopathologic characteristics of intrahepatic cholangiocarcinoma (ICC) in young patients.
METHODS: A retrospective analysis was performed in ICC patients referred to the Eastern Hepatobiliary Surgery Hospital in Shanghai, China. Among 317 consecutively enrolled patients, 40 patients were aged ≤ 40 years (12.61%). We compared the risk factors and clinicopathologic characteristics of these patients (group I: n = 40) with those aged > 40 years (group II: n = 277).
RESULTS: Group I had distinct features compared with group II, including a low frequency of hepatolithiasis (P = 0.000); a high positive rate of serum hepatitis B surface antigen (P = 0.000) and hepatitis B virus (HBV)-associated cirrhosis (P = 0.038); a high frequency of α-fetoprotein (> 400 μg/L) (P = 0.011); a low frequency of carbohydrate antigen 19-9 (> 37 U/mL) (P = 0.017); and a high frequency of liver histological inflammation (P = 0.002). Although there was no significant difference between the two groups in regards to hepatic schistosomiasis, alcohol-associated cirrhosis and cirrhosis due to other causes (P > 0.05), they only occurred in the elderly group.
CONCLUSION: The risk factors are significantly different between young and elderly ICC patients. HBV and HBV-associated cirrhosis are the most important risk factors for young ICC patients.
- Citation: Zhou HB, Wang H, Zhou DX, Wang H, Wang Q, Zou SS, Hu HP. Etiological and clinicopathologic characteristics of intrahepatic cholangiocarcinoma in young patients. World J Gastroenterol 2010; 16(7): 881-885
- URL: https://www.wjgnet.com/1007-9327/full/v16/i7/881.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i7.881
Intrahepatic cholangiocarcinoma (ICC) is a fatal cancer of the biliary epithelium, arising within the intrahepatic bile ducts. Globally, ICC is the most common primary hepatic malignancy after hepatocellular carcinoma (HCC). The incidence rates of ICC vary greatly among different areas of the world; this variation is related to the distribution of risk factors. Viral infection [hepatitis B virus (HBV) or hepatitis C virus (HCV)][1], primary sclerosingcholangitis (PSC)[2], liver fluke infestation (particularly the endemic Opisthorchis viverrini)[3,4], and hepatolithiasis are known as the risk factors for ICC[5,6]. ICC, similar to HCC, affects predominantly those individuals aged > 40 years[7]; however, younger patients have recently been diagnosed with ICC. The risk factors and clinicopathologic features of young ICC patients have not yet been studied.
The aim of the present study was to investigate the prevalence, risk factors, and the clinicopathologic characteristics of ICC in young patients, and to compare these findings with the characteristics and risk factors associated with elderly patients with ICC.
We performed a retrospective analysis using medical records of the patients initially diagnosed with ICC in the Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University, Shanghai, China from January 2003 to December 2006. The diagnosis of ICC was confirmed pathologically. We evaluated the age and sex distribution of the patients, and compared the risk factors and clinicopathologic characteristics of patients aged ≤ 40 years (group I) with those of patients aged > 40 years (group II).
To clarify a difference in the risk factors between the two groups, we analyzed HBV or HCV infection, liver cirrhosis, hepatolithiasis, and hepatic schistosomiasis (our previous study verified that these are the main risk factors for ICC patients from China). The presence of a seropositive HBsAg and/or anti-HCV (Abbott Laboratories, North Chicago, IL, USA) and HCV RNA (real time PCR; Abbott, IL, USA) was interpreted as an indication of chronic hepatitis infection. The diagnosis of ICC was confirmed by pathology. Liver function and serum tumor marker (carbohydrate antigen 19-9 and α-fetoprotein) concentrations were evaluated in all the patients.
To determine the pathological characteristics, the diameter of the largest tumor was measured directly in the surgical specimens from patients who had undergone hepatic resection. The WHO tumor classification was used for pathological grading of the tumor (well, moderate or poor differentiation). When histological diversity was observed in a tumor, the higher grade, according to the classification system, was taken as the overall grade. The tumor mass was estimated by computed tomography (CT), and enlarged lymph nodes were defined as lymph nodes 0.1 cm in diameter at the portal, celiac, retrocrural or retroperitoneal lymph node stations. Ultrasound, CT scan, or surgery was used to diagnose portal vein thrombosis.
Statistical analysis was performed using the χ2 test to compare discrete variables, and analysis of variance (ANOVA) was made to compare continuous variables. SPSS for Windows (version 16.0) was used. P < 0.05 was considered as significant difference.
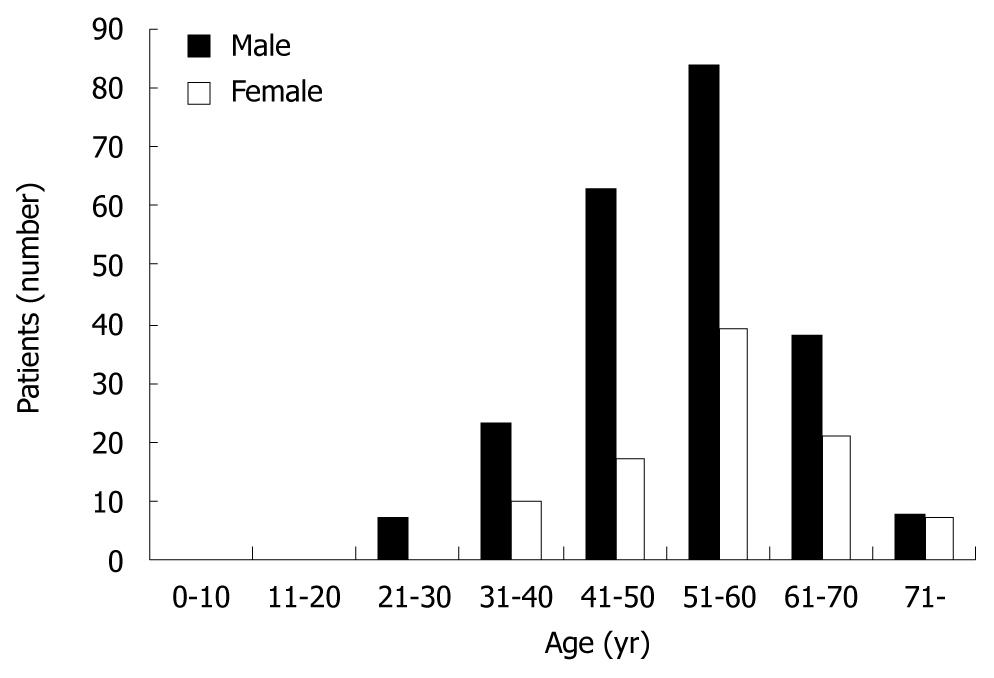
A total of 317 patients (223 men and 94 women, a male to female ratio, 2.37:1) initially diagnosed with ICC were enrolled in the present study. The mean age was 53.05 ± 10.53 years (range 21-78). Most of ICC developed during the 4th-7th decades, with a peak at 54 years of age. Forty (12.61%) patients were aged ≤ 40 years, including 30 men and 10 women (Figure 1).
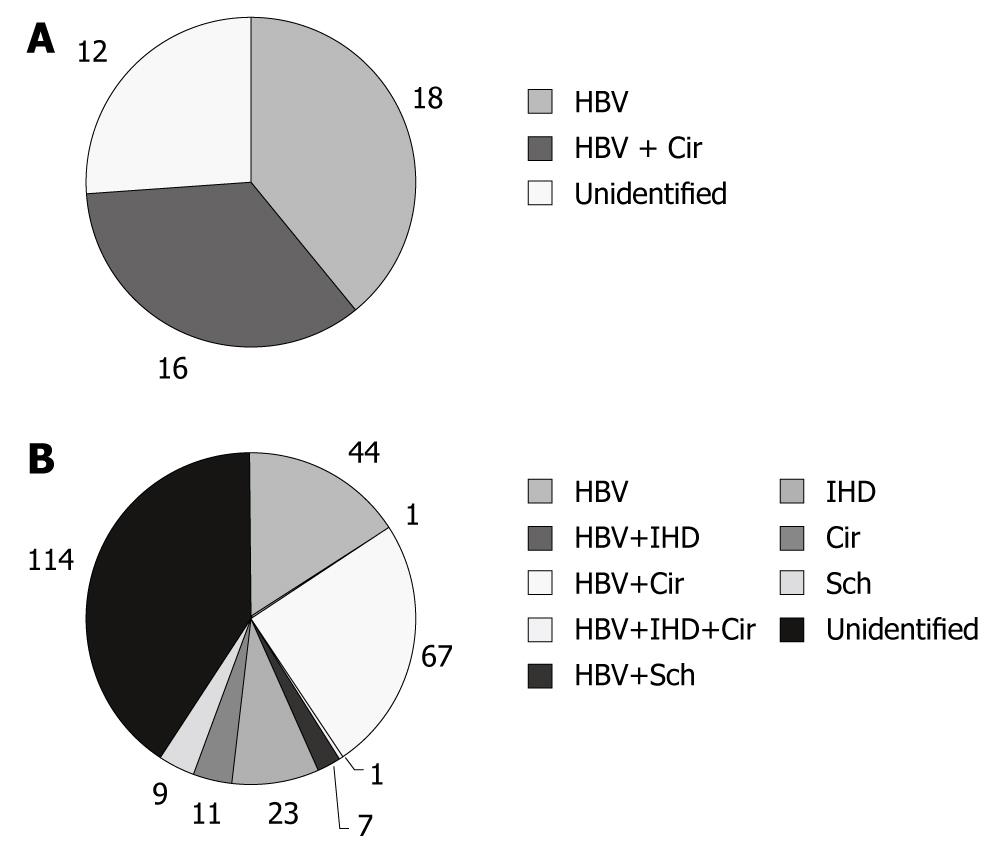
The risk factors for ICC are listed in Table 1. Of the 40 young ICC patients, 34 (85.0%) were seropositive for HBsAg, 16 (40.0%) had liver cirrhosis related to HBV, and none had intrahepatic-duct stones (IHD stones), liver schistosomiasis, or HCV (Figure 2A). In group II, 120 (43.3%) patients were seropositive for HBsAg, 1 (0.4%) was seropositive for anti-HCV and HCV RNA, 79 (28.5%) had liver cirrhosis, including 68 (24.5%) whose cirrhosis was related to HBV, 6 (2.2%) with liver cirrhosis related to alcohol, 5 (1.8%)with liver cirrhosis due to other causes (1 with liver cirrhosis related to HCV, 1 with nonalcoholic liver cirrhosis, and 3 with occult liver cirrhosis), 25 (9.0%) had IHD stones, and 16 (5.8%) had liver schistosomiasis (Figure 2B). It is worth mentioning that the HCV that is prevalent in Japan and some Western countries has been found to be the significant cause of ICC. In our series, only one case of ICC had HCV.
| Risk factors | Group I (n = 40) | Group II (n = 277) | P value |
| HBV infection | 34 (85.0) | 120 (43.3) | 0.000 |
| HCV infection | 0 (0.0) | 1 (0.4) | 0.703 |
| Liver cirrhosis | 0.038 | ||
| Related to HBV | 16 (40.0) | 68 (24.5) | 0.347 |
| Related to alcohol | 0 (0.0) | 6 (2.2) | 0.392 |
| Other causes | 0 (0.0) | 5 (1.8) | |
| Total | 16 (40.0) | 79 (28.52) | |
| Hepatolithiasis | 0 (0.0) | 25 (9.0) | 0.000 |
| Liver schistosomiasis | 0 (0.0) | 16 (5.8) | 0.119 |
For further comparison between the two groups, the following clinical variables were investigated: gender, total bilirubin (TBIL) (> 20 μmol/L vs≤ 20 μmol/L), albumin, alanine aminotransferase (ALT) (> 42 U/L vs≤ 42 U/L), aspartate aminotransferase (AST) (> 37 U/L vs≤ 37 U/L), r-glutamyl-transferase (r-GT) (> 64 U/L vs≤ 64 U/L), alkaline phosphatase (ALP) (> 119 U/L vs≤ 119 U/L), α-fetoprotein (AFP) (> 400 μg/L vs≤ 400 μg/L), and carbohydrate antigen 19-9 (CA19-9, > 37 ng/mL vs≤ 37 ng/mL). AFP (> 400 μg/L) and CA19-9 (> 37 U/mL) were significantly different between group I and group II by univariate analysis (Table 2).
| Group I (n = 40) | Group II (n = 277) | P value | |
| Gender (M/F) | 30/10 | 193/84 | 0.491 |
| ALT (> 42 U/L) | 15 (60.00) | 89 (32.13) | 0.499 |
| AST (> 37 U/L) | 12 (30.00) | 103 (37.18) | 0.377 |
| TBIL (> 20 μmol/L) | 7 (17.50) | 62 (22.38) | 0.484 |
| Albumin (g/L) | 43.82 ± 4.27 | 42.07 ± 5.12 | 0.236 |
| GGT (> 64 U/L) | 19 (47.50) | 176 (63.54) | 0.051 |
| ALP (> 119 U/L) | 15 (11.19) | 134 (48.38) | 0.198 |
| AFP (> 400 μg/L) | 7 (17.50) | 17 (6.14) | 0.011 |
| CA19-9 (> 37 U/mL) | 14 (35.00) | 153 (55.23) | 0.017 |
Table 3 reveals the pathological characteristics in the two groups. There was significantly more histological inflammation (present vs no hepatitis) in the younger patient group (P = 0.002). No difference was detected between the two groups with regard to the tumor number (< 2 vs≥ 2), location (right lobe, left lobe, or both lobes), tumor size (main tumor or the largest one), capsule (present vs absent), tumor differentiation (well, moderate or poor), portal vein invasion (invasion vs no invasion), microscopic satellite lesion (tiny nodule present around the main tumor vs no satellite), and immunohistochemical examination (cytokeratin 18 and cytokeratin 19).
| Group I (n = 40) | Group II (n = 277) | P value | |
| Tumor location | 0.827 | ||
| Right lobe | 25 (6.25) | 160 (57.76) | |
| Left lobe | 14 (35.00) | 111 (40.07) | |
| Both lobes | 1 (2.50) | 6 (15.00) | |
| Tumor size (cm) | 6.80 ± 3.92 | 6.67 ± 3.38 | 0.892 |
| Tumor number | 0.474 | ||
| 1 | 36 (90.00) | 258 (93.14) | |
| ≥ 2 | 4 (10.00) | 19 (6.86) | |
| Histological inflammation | 15 (37.50) | 47 (16.97) | 0.002 |
| Capsule | 5 (12.50) | 35 (12.64) | 0.981 |
| Tumor differentiation | 0.506 | ||
| Well | 0 (0.00) | 9 (3.25) | |
| Moderate | 29 (72.50) | 191 (68.95) | |
| Poor | 11 (27.50) | 71 (25.63) | |
| Microvascular invasion | 10 (25.00) | 41 (14.80) | 0.101 |
| Perineural infiltration | 0 (0.00) | 4 (1.44) | 0.444 |
| Portal vein invasion | 3 (7.50) | 30 (10.83) | 0.519 |
| Lymphatic metastasis | 8 (20.00) | 46 (16.61) | 0.594 |
| Microscopic satellite lesion | 7 (17.50) | 69 (24.91) | 0.305 |
| Immunohistochemical examinations | |||
| Ck18 positive | 38 (95.00) | 269 (97.11) | 0.475 |
| Ck19 positive | 40 (100.00) | 267 (93.39) | 0.222 |
Although several risk factors have been associated with ICC, such as viral infection (HBV and/or HCV), liver cirrhosis, IHD stones, and liver parasite infestation, the distribution characteristics of these risk factors in young and elderly ICC patients and the mechanism responsible for ICC remain unknown. Here, we not only demonstrated different etiological characteristics of ICC between young and elderly patients, but also the clinicopathologic features of young patients.
Our study focused on patients with ICC who were ≤ 40 years of age, and the “young patients” were defined as those ≤ 40 years of age at diagnosis. We chose the cut-off age of 40 years based on the recommended age for starting regular HCC screening for men in Asia[8] and according to Lam et al[9]. The age distribution of patients with ICC in China is described by a triangular-shaped curve (Figure 1). Only 12.61% of patients were ≤ 40 years of age.
HBV or HCV infection was strongly associated with ICC risk. Korean investigators performed a case-control study comparing 41 cases of ICC with 406 controls without cancer and found that 13.8% of cases and 3.5% of controls were anti-HCV positive and 12.5% of cases and 2.3% of controls were HBsAg positive[10]. In a Japanese hospital-based study, investigators found that 36% of 50 patients with ICC but only 3% of 205 controls (surgical patients who did not have primary liver cancer) were HCV seropositive [OR (odds ratio) = 16.87; 95% CI (confidence interval): 5.69-50.00][11]. Through a population based cohort study including 146 394 HCV-infected and 572 293 HCV-uninfected patients, El-Serag et al[12] showed a strong association of ICC with HCV infection (hazard ratio = 2.55, 95% CI: 1.31-4.95), but the association was not observed in extrahepatic cholangiocarcinoma. Our previous study also found that the incidence of HBV infection in ICC patients was significantly higher than that in non-cancer individuals (48.6% vs 6.6%), indicating that chronic HBV infection was independently the most important risk factor for ICC in Chinese population (OR = 9.669, 95% CI: 6.329-14.770). Our findings were consistent with that reported by Zhou et al[13] recently. In our study, 85.0% of young ICC patients had chronic HBV infection, which is significantly higher than that of elderly ICC patients (43.3%). It is interesting to note that HBV infection was also the only risk factor identified in young ICC patients. It is worth mentioning that the HCV that is prevalent in Japan and some Western countries has been proven to be the significant cause of ICC. In our series, only one case of ICC had HCV.
Cirrhosis, of any cause, has also been associated with intrahepatic cholangiocarcinoma[5]. A cohort study of over 11 000 patients with cirrhosis, followed up over 6 years, showed a 10-fold risk compared with the general population[14]. A prospective controlled study from Japan reported the risk of developing cholangiocarcinoma in patients with cirrhosis related to HCV as 3.5% at 10 years, 1000 times higher than in the general population[15]. The data obtained from our previous study also showed that cirrhosis, particularly HBV-associated cirrhosis, was an important risk factor for ICC in Chinese population. In the present study, the etiological distribution of cirrhosis was significantly different between young ICC patients and elderly ICC patients. HBV-associated cirrhosis had a higher incidence in young ICC patients than in the elderly group, while alcoholic cirrhosis and cirrhosis due to other causes only occurred in elderly ICC patients.
Hepatolithiasis is rare in Western countries, but relatively common in some parts of Asia, and is associated particularly with peripheral intrahepatic cholangiocarcinoma[4]. In Taiwan, up to 70% of patients with intrahepatic cholangiocarcinoma undergoing resection reportedly have intrahepatic biliary stones, and in Japan this figure is 6%-18%[16]. Biliary stones are thought to cause bile stasis, predisposing to recurrent bacterial infections and subsequent inflammation, a potential cofactor for cholangiocarcinogenesis. In our cohort, hepatolithiasis only occurred in elderly ICC patients. The result indicates hepatolithiasis may be not a risk factor of ICC development for young patients.
A large body of experimental and epidemiological data suggests a pathogenic association between liver parasite infection, especially Opisthorchis viverrini (and less definitively Clonorchis sinensis) and intrahepatic cholangiocarcinoma[1]. Our previous study showed that hepatic schistosomiasis had a higher incidence in ICC patients than in no-cancer individuals. The data suggests that hepatic schistosomiasis was a risk factor for ICC development (OR = 11.06, 95% CI: 3.368-36.337). Although there was no significant difference between the young ICC patents and elderly ICC patients in regards to hepatic schistosomiasis (P > 0.05), similar to alcohol-cirrhosis and cirrhosis due to other causes, hepatic schistosomiasis also only occurred in the elderly group.
α-fetoprotein (AFP), a 70-kDa glycoprotein, is normally produced during fetal development by the liver and yolk sac. The protein levels drop off rapidly after birth, and by the second year of life only trace amounts are detectable in the serum. AFP is increased in the majority of patients with HCC and is useful in the diagnosis and follow-up of cases. Studies suggest that, in patients with suspected HCC clinically, AFP levels > 400 ng/mL should strongly confirm the presence of HCC via a tissue diagnosis[17,18]. Some cancers may originate from cancer stem cells, which may form via the carcinogenesis of normal stem cells[19-21]. It has been suggested that hepatocytes and cholangiocytes arise from the same pool of hepatic precursor cells, also called oval cells. Carcinogenesis of such hepatic precursor cells may cause ICC[22]. Hepatic progenitor cells were also shown to strongly express AFP mRNA and produce AFP during differentiation[23]. Compared with the elderly ICC patients, young ICC patients exhibited a higher incidence of AFP > 400 μg/L (17.50% vs 6.14%). Our data indicated that the neoplastic transformation of oval cells may be one of the mechanisms for ICC development and that the oval cell precursor retains its ability to produce AFPin the process of malignant transformation.
In a recent prospective study, serum CA19-9 was found to be useful in diagnosing cholangiocarcinoma, in deciding whether the tumor had been radically resected and in monitoring the effect of treatment. Serum CA19-9 concentrations were significantly elevated in patients with cholangiocarcinoma compared with patients with HCC, benign biliary disease or healthy individuals. After curative resection, serum CA19-9 decreasesd to a preoperative level[24]. In the present study, young ICC patientsexhibited a lower serum CA19-9 level, similar to HCC.
In conclusion, the risk factors for ICC are different between young and elderly patients. HBV infection and HBV-associated cirrhosis may be the main risk factors for young ICC patients. Young ICC patients share etiological and many clinicopathologic similarities with HCC patients. These results indicated that ICC in young patients and HCC have a common process of carcinogenesis (through a similar long-term inflammatory carcinogenic process) and that both may arise from hepatic progenitor cells.
Although several risk factors have been associated with the development of Intrahepatic cholangiocarcinoma (ICC), such as hepatitis B virus (HBV), hepatitis C virus (HCV) or cirrhosis, the risk factors and clinicopathologic features of young ICC patients have not been fully studied.
ICC in young patients and hepatocellular carcinoma (HCC) shared a common process of carcinogenesis (through a similar long-term inflammatory carcinogenic process) and that both may arise from hepatic progenitor cells. However, this presumption awaits verification by more studies.
The authors clearly demonstrated that the risk factors for ICC differed significantly between young patients and elderly patients in a Chinese population. They also showed that HBV infection was closely associated with the development of ICC in young patients.
| 1. | Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016-1021. |
| 2. | Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7-11. |
| 4. | Hughes NR, Pairojkul C, Royce SG, Clouston A, Bhathal PS. Liver fluke-associated and sporadic cholangiocarcinoma: an immunohistochemical study of bile duct, peribiliary gland and tumour cell phenotypes. J Clin Pathol. 2006;59:1073-1078. |
| 5. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. |
| 6. | Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620-626. |
| 7. | Kim JH, Choi MS, Lee H, Kim do Y, Lee JH, Koh KC, Yoo BC, Paik SW, Rhee JC. Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol. 2006;21:588-594. |
| 8. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. |
| 9. | Lam CM, Chan AO, Ho P, Ng IO, Lo CM, Liu CL, Poon RT, Fan ST. Different presentation of hepatitis B-related hepatocellular carcinoma in a cohort of 1863 young and old patients - implications for screening. Aliment Pharmacol Ther. 2004;19:771-777. |
| 10. | Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, Ahn YO, Shigemastu T. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933-940. |
| 11. | Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95:592-595. |
| 12. | El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116-123. |
| 13. | Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632-635. |
| 14. | Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921-925. |
| 15. | Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471-2477. |
| 16. | Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 1: epidemiology and etiology. J Gastroenterol Hepatol. 2002;17:1049-1055. |
| 17. | Bruce MG, Bruden D, McMahon BJ, Christensen C, Homan C, Sullivan D, Deubner H, Williams J, Livingston SE, Gretch D. Clinical significance of elevated alpha-fetoprotein in Alaskan Native patients with chronic hepatitis C. J Viral Hepat. 2008;15:179-187. |
| 18. | Murugavel KG, Mathews S, Jayanthi V, Shankar EM, Hari R, Surendran R, Vengatesan A, Raghuram K, Rajasambandam P, Murali A. Alpha-fetoprotein as a tumor marker in hepatocellular carcinoma: investigations in south Indian subjects with hepatotropic virus and aflatoxin etiologies. Int J Infect Dis. 2008;12:e71-e76. |
| 19. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. |
| 20. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. |
| 21. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. |
| 22. | Wilkens L, Bredt M, Flemming P, Klempnauer J, Heinrich Kreipe H. Differentiation of multicentric origin from intra-organ metastatic spread of hepatocellular carcinomas by comparative genomic hybridization. J Pathol. 2000;192:43-51. |
| 23. | Ishikawa K, Sasaki A, Haraguchi N, Yoshikawa Y, Mori M. A case of an alpha-fetoprotein-producing intrahepatic cholangiocarcinoma suggests probable cancer stem cell origin. Oncologist. 2007;12:320-324. |
| 24. | Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10:427-432. |
Peer reviewer: Yoshio Shirai, Associate Professor, Division of Digestive and General Surgery, Niigata University Graduate School of Medical and Dental Sciences, 1-757 Asahimachi-dori, Niigata City 951-8510, Japan
S- Editor Wang YR L- Editor Ma JY E- Editor Ma WH