INTRODUCTION
Although the optimal treatment for hepatocellular carcinoma (HCC) is surgical resection, there are only a small number of patients who meet resectability criteria for HCC[1]. Percutaneous radiofrequency thermal ablation (RFA) is one of the emerging therapeutic modalities used for the minimally invasive treatment in the management of liver malignancies, particularly in patients who cannot undergo surgery[2,3]. It has been reported that the morbidity and mortality rates are low, and there are few complications associated with RFA[4,5]. Although RFA is relatively well-tolerated, severe and potentially fatal complications, such as liver failure, colon perforation and portal vein thrombosis can arise[6,7]. In addition, there are non-fatal serious complications, such as liver abscess, pleural effusion, skin burns, hypoxemia during treatment, pneumothorax, subcapsular hematoma, acute renal insufficiency, hemoperitoneum, needle tract seeding, and self-limited post-ablation syndrome[8]. However, acute gouty arthritis attacks following RFA for HCC have not been reported. We describe a case of gouty arthritis in a 71-year-old man who was treated with RFA for a large HCC in segment IV of the liver adjacent to the gallbladder bed.
CASE REPORT
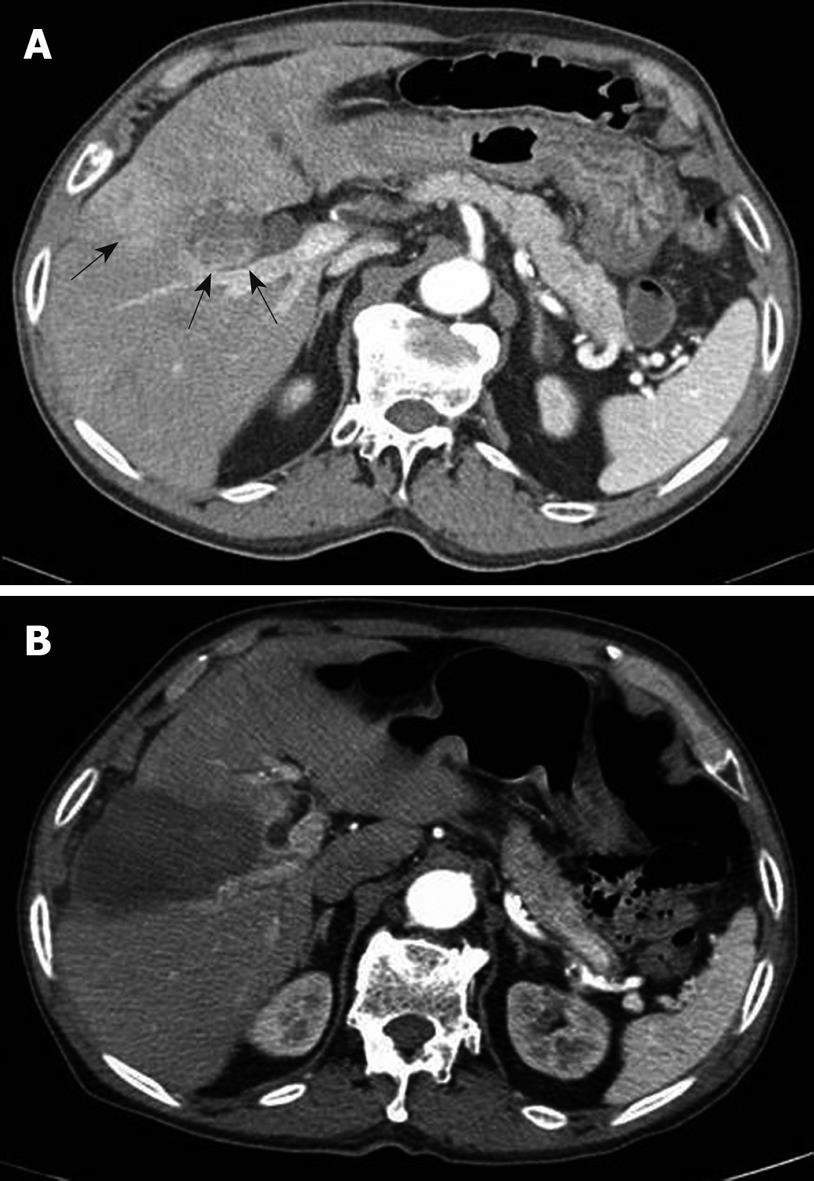
A 71-year-old male with hepatitis B virus-associated cirrhosis had undergone a previous percutaneous ethanol injection for a small HCC. Regular surveillance detected a 3.5 cm HCC lesion at segment IV of the liver adjacent to the gallbladder, posterior to the lesion where a previous percutaneous ethanol injection had been performed (Figure 1A). The baseline laboratory tests showed thrombocytopenia (platelet count, 101 × 103/mm3; normal range, 150-440 × 103/mm3), an elevated blood urea nitrogen level (44 mg/dL; normal range, 8-20 mg/dL), an increased creatinine level (1.8 mg/dL; normal range, 0.4-1.0 mg/dL), a normal uric acid level (5.5 mg/dL; normal range, 2.6-8.0 mg/dL), and a normal potassium level (3.7 mmol/L; normal range, 3.6-5.0 mmol/dL). The liver function tests revealed a borderline low albumin level (3.2 g/dL; normal range, 3.2-4.9 g/dL), a normal total bilirubin level (1.1 mg/dL; normal range, 0.3-1.2 mg/dL) and a normal prothrombin time (international normalized ratio, 0.93). The other laboratory tests, including the total calcium, phosphorus, lactate dehydrogenase (LDH), and aspartate transaminase levels, were within normal limits. Among several curative treatment options available, RFA was chosen for further therapy because the patient had declined surgery. Written informed consent was obtained from the patient before RFA.
Figure 1 Computed tomography (CT) scan of the patient.
A: CT scan in an arterial phase demonstrates a 3.5 cm hepatocellular carcinoma lesion (arrows) at segment IV of the liver adjacent to the gallbladder, posterior to the lesion where a previous percutaneous ethanol injection has been performed; B: CT scan obtained after radiofrequency thermal ablation reveals complete ablation for the hepatocellular carcinoma without apparent complications such as a gallbladder injury on the immediate follow-up.
RFA was performed under sonographic guidance using a 3.5 MHz convex probe (Sequoia, Siemens Medical Solutions). The treatment was performed under local anesthesia using 100 μg of fentanyl citrate (Myengmun) to control pain. The vital signs were monitored continuously during the procedure. Once proper positioning of the electrode in the tumor area had been confirmed by sonography, the electrodes were connected to a 500 KHz monopolar radiofrequency generator (CC-1, Valleylab) capable of producing 200 W. Four internally cooled 17-gauge electrodes (Cool-Tip, Valleylab) with 3.0 cm exposed tips delivered radiofrequency energy to the tumors. During withdrawal of the electrode, the entire electrode track was heated briefly to a temperature of 80°C by application of radiofrequency. The procedure lasted 23 min with complete ablation of the HCC without apparent complications, such as injury to the gallbladder, on the immediate follow-up computed tomography scan (Figure 1B).
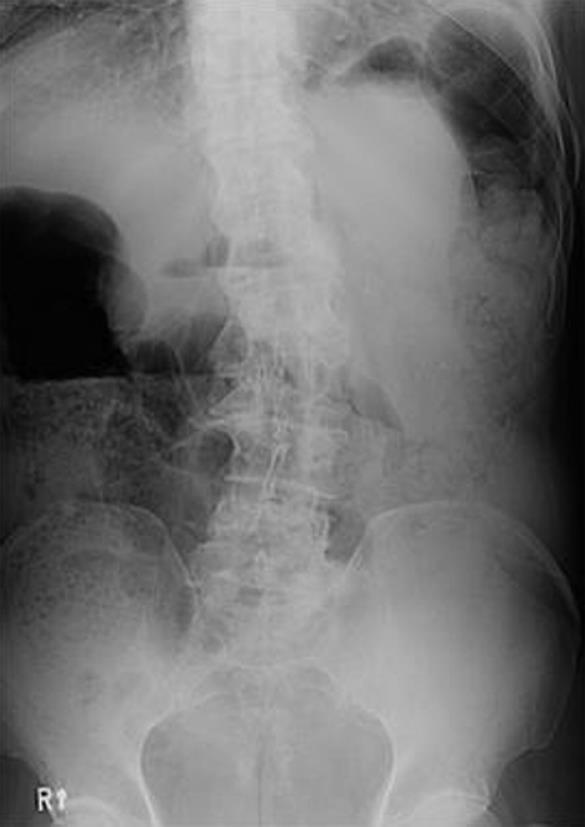
The morning after the procedure, the patient complained of abdominal distension. A simple abdominal radiograph revealed no evidence of bowel perforation, although the large intestine was distended (Figure 2). The laboratory results were normal, except an elevation of the potassium level (5.0 mmol/L) and a sustained elevated creatinine level (1.7 mg/dL). On the third post-procedural day, although the patient’s symptoms had not changed, repeat laboratory results showed that his uric acid level had increased to 8.7 mg/dL, and the potassium level was maintained at 5.1 mmol/L, which were compatible with a tumor lysis syndrome. The urine output was adequate, and the acid-base gas analysis revealed no disturbances. The patient was hydrated with crystalloids and subjected to close observation. On the 6th post-procedural day, the patient complained of new right knee pain. The physical examination showed swelling and tenderness on the medial side of the right knee that was warm to touch. Joint aspiration revealed a yellow serous fluid that was confirmed, by polarizing microscopy, to be monosodium urate monohydrate crystals (Figure 3). On the same day, the laboratory data revealed that the serum uric acid and creatinine levels increased to 9.8 mg/dL and 3.2 mg/dL, respectively, which were both higher than the baseline levels before the RFA procedure. We made the diagnosis of acute gouty arthritis arising not only from tumor lysis caused by HCC ablation, but also from liver infarction adjacent to the tumor by broad ablation, which was aggravated by acute renal insufficiency in chronic renal failure. We treated the patient with an adequate intravenous crystalloid infusion and oral colchicine (0.6 mg/d). As a result, the patient’s right knee pain subsided. On the 11th post-procedural day, the uric acid level was within normal limits (7.2 mg/dL) and the levels of other electrolytes and creatinine had returned to baseline values.
Figure 2 A simple abdominal radiograph reveals no evidence of bowel perforation, except a distension of the large intestine.
Figure 3 Strongly negative birefringent, needle-shaped monosodium urate crystals (arrow) in synovial fluid from a patient under compensated polarized light.
DISCUSSION
Tumor lysis syndrome has been reported for many different types of poorly differentiated lymphomas, such as high grade non-Hodgkin’s lymphoma, Burkitt’s lymphoma, and acute lymphocytic leukemia[9]. Because this syndrome is mostly frequently related to the cytotoxic treatment of poorly differentiated lymphomas or combination chemotherapy, it has rarely been reported for solid organ tumors, including lung, breast, and advanced gastric cancers[10-12]. However, several recent reports have demonstrated that tumor lysis syndrome may occur after various treatments of HCC, including RFA and transarterial chemoembolization (TACE)[13-15]. Shiba et al[14] reported a case of TACE-induced tumor lysis syndrome resulting from necrosis of a large HCC. Moreover, they suggested that factors predisposing a patient to tumor lysis include large tumor size, tumors with high sensitivity to treatment, renal insufficiency, dehydration, and hyperuricemia before treatment[14].
Tumor lysis syndrome causes hyperuricemia, hyperkalemia, hyperphosphatemia and hypocalcemia, and may lead to acute renal failure or metabolic acidosis resulting from deposition of uric acid in the kidney, and calcium phosphate complex in renal tubules[16]. In our patient, although metabolic acidosis did not develop, tumor lysis syndrome, including hyperuricemia, hyperkalemia, and acute renal failure did develop. Therefore, we believe that acute tumor lysis syndrome occurred in our patient as a result of tumor necrosis following RFA.
Lehner et al[15] reported a death caused by acute tumor lysis syndrome after ablation of a 3.2 cm HCC. They indicated that patients with pre-existing azotemia, along with elevated baseline LDH which exists in most cases of cirrhosis, and elevated uric acid level are at increased risk of tumor lysis syndrome[15]. Therefore, we suggest that the predisposing factors in our case included underlying cirrhosis with pre-existing azotemia. Moreover, because we observed that the ablated field was large compared to the target lesion, we believe hyperuricemia could be caused by liver infarction induced by ablation.
We describe, for the first time, a case of acute gouty arthritis as a possible complication following thermal ablation for HCC. A definite diagnosis of gout was made by identification of monosodium urate crystals within phagocytes in the synovial fluid using compensated polarized microscopy[17]. It is well known that numerous circumstances, such as surgery, dietary overindulgence, and ingestion of drugs affecting serum uric acid concentrations are associated with acute attacks of gouty arthritis[18]. The likely explanation for the development of acute gouty arthritis in this elderly man was the abrupt increase in the serum uric acid level from tumor cell necrosis after HCC ablation. Therefore, in this case, the acute gouty attack on the 6th post-procedural day was likely caused not only by chronic renal insufficiency, but also by hyperuricemia following tumor cell lysis and liver infarction.
After the diagnosis of acute gouty arthritis was established, the patient received colchicine immediately. Although colchicine is not a urate-lowering agent, adequate hydration and colchicine administration resulted in lowering of the serum level of uric acid to the baseline value and resolution of the patient’s right knee pain 2 d after drug administration.
To date, RFA is considered an effective procedure for providing local control in HCC patients. RFA is associated with the possibility of effecting long-term, disease-free survival in selected patients compared to other local techniques, such as cryoablation and percutaneous ethanol injection[19,20]. Although RFA is a generally well-tolerated and relatively safe localized-regional therapy, complications can develop[4-8]. A recent report showed that RFA was associated with a 4% rate of major complications, a 4.8% rate of minor complications, and a negligible risk of death[4,5]. As shown in this case, acute gouty arthritis is not a life-threatening condition, but it affects the quality of life of the patient. Therefore, during therapy, patients who are at risk should be monitored closely for this possible complication. To avoid hyperuricemia and acute gouty arthritis after RFA therapy for HCC, early identification of patients at risk is warranted, such as those with a large tumor, rapid tumor growth, and renal insufficiency, and preventative measures should be considered.
In conclusion, this is the first described case of acute gouty arthritis after RFA for a HCC lesion in a patient with underlying chronic renal insufficiency. This complication should be considered in patients at risk. Appropriate screening, management, and treatment may reduce the complications associated with RFA.