Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5773
Revised: August 27, 2010
Accepted: September 4, 2010
Published online: December 7, 2010
AIM: To detect the expression of mitochondrial uncoupling protein 2 (UCP2) in colon cancer and analyze the relation between UCP2 expression and clinical pathological features of colon cancer.
METHODS: Fifteen colon tissue samples and 15 its adjacent tissue samples were obtained from colon cancer patients during surgical interventions. UCP2 expression was detected with immunohistochemical method in 10 normal controls, 10 hyperplastic polyp patients, 20 tubular adenoma patients and 78 colon cancer patients. Patients with rectal cancer were excluded. Quantitative reverse transcription polymerase chain reaction and Western blotting were used to detect UCP2 expressions in colon cancer tissue samples and its adjacent tissue samples. Relation between UCP2 expression and clinical pathological features of colon cancer was also analyzed.
RESULTS: The UCP2 mRNA expression level was four-fold higher in colon cancer tissue samples than in its adjacent tissue samples. The UCP2 protein expression level was three-fold higher in colon cancer tissue samples than in its adjacent normal tissue samples. The UCP2 was mainly expressed in cytoplasm. The UCP2 was not expressed in normal colon mucosa. Strong positive staining for UCP2 with a diffuse distribution pattern was identified throughout the mucosa in colon cancer tissue samples with a positive expression rate of 85.9%. The UCP2 expression level was higher in colon cancer tissue samples at clinical stages III and IV than in those at stage I + II. Univariate analysis showed that the high UCP2 expression level was significantly correlated to colon cancer metastasis (hazard ratio = 4.321, confidence interval = 0.035-0.682, P = 0.046).
CONCLUSION: UCP2 is highly expressed in human colon cancer tissue and may be involved in colon cancer metastasis.
- Citation: Kuai XY, Ji ZY, Zhang HJ. Mitochondrial uncoupling protein 2 expression in colon cancer and its clinical significance. World J Gastroenterol 2010; 16(45): 5773-5778
- URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5773.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5773
Colon cancer is one of the malignant tumors threatening to human health, and the mortality of colon cancer patients ranks second among all malignant tumors in developed countries. In recent years, its incidence has been increasing in China, particularly in the economically developed coastal cities[1,2]. Its etiology and pathogenesis remain unclear. Great achievements have been made in studies on changes in tumor suppressor gene or proto-oncogene, but some problems cannot be explained. Recent studies showed that mitochondrial dysfunction is involved in the occurrence and development of tumor[3-5]. Uncoupling protein-2 (UCP2) is a mitochondrial membrane protein, which negatively regulates the production of reactive oxygen species (ROS)[6-8]. Adaptive mechanisms of cancer cells include resistance to tumor growth inhibition and evasion of apoptosis, and cellular events that are appreciably affected by oxidative stress[9,10]. The UCP2 expression level is significantly higher in colon cancer tissue than in its adjacent tissue and UCP2 may play a role in intestinal epithelial cells from benign to malignant transformation[11]. However, the role of UCP2 in development of colon cancer is unclear. In this study, the expression of UCP2 in normal human colon tissue and colon cancer tissue was detected, and the relation between UCP2 expression in colon cancer tissue and clinical pathological features of colon cancer was also analyzed.
Fifteen colon caner tissue samples and 15 its adjacent tissue samples were obtained from the First Affiliated Hospital of Nanjing Medical University, snap-frozen and stored at -70°C. UCP2 expression was detected with immunohistochemical method in 10 normal controls, 10 hyperplastic polyp patients, 20 tubular adenoma patients, and 78 patients (45 males and 33 females) with colon cancer at different stages. Rectal cancer patients were excluded. Clinical pathological characteristics of the 78 colon cancer patients are listed in Table 1.
| Characteristics | n (%) |
| Gender | |
| Female | 33 (42.3) |
| Male | 45 (57.7) |
| Age (yr): median 60.7, range 31-78 | |
| < 60 | 36 (46.2) |
| ≥ 60 | 42 (53.8) |
| Primary sites | |
| Left colon | 44 (56.4) |
| Right colon | 34 (43.6) |
| Clinical stage | |
| I(T1N0M0) | 10 |
| II (T2N0M0) | 17 |
| III | 37 |
| IV | 14 |
| Tumor differentiation | |
| Well | 16 |
| Moderately | 38 |
| Poorly | 24 |
Tissue sections were stained with rabbit polyclonal antibody against human UCP2 (LS-C41270, LifeSpan BioSciences, Seattle, WA, USA), horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Inc, USA) and visualized using peroxidase. Negative control sections were treated in PBS instead of primary antibodies. Intensity of UCP2 staining was scored as negative (0), weak (+1), moderate (+2), and strong (+3).
After extraction of total RNA from snap-frozen colonic surgical samples with TRIzol reagent (Invitrogen, Carlsbad, CA) and removal of contaminating genomic DNA with DNase I and RNasefree (Roche Diagnostics Corp., Indianapolis, IN), reverse transcription polymerase chain reaction (RT-PCR) was performed using a first-strand cDNA synthesis kit (Roche Diagnostics Corp, Indianapolis, IN) following its manufacturer’s instructions. Quantitative RT-PCR was performed using an ABI Prism 7300 real-time PCR detection system (Bio-Rad, Hercules, CA) following its manufacturer’s instructions. The sequences of UCP2 and internal control GAPDH primers used in this study are as follows: Ucp2 gene: R: 5'-TCAGAATGGTGCCCATCACA-3', F: 5'-CCGGTTACAGATCCAAGGAGAA-3', GAPDH: R: 5'-ACCCTGTTGCTGTAGCCA-3', F: 5'-CCACTCCTCCACCTTTGAC-3'. The PCR amplification conditions were 95°C for 5 min, followed by 40 cycles at 95°C for 30 s, at 60°C for 1 min, and a final extension at 72°C for 3 min. The relative amount of gene expression = 2 Δct × 100 (Δct = ct target gene-ct internal reference).
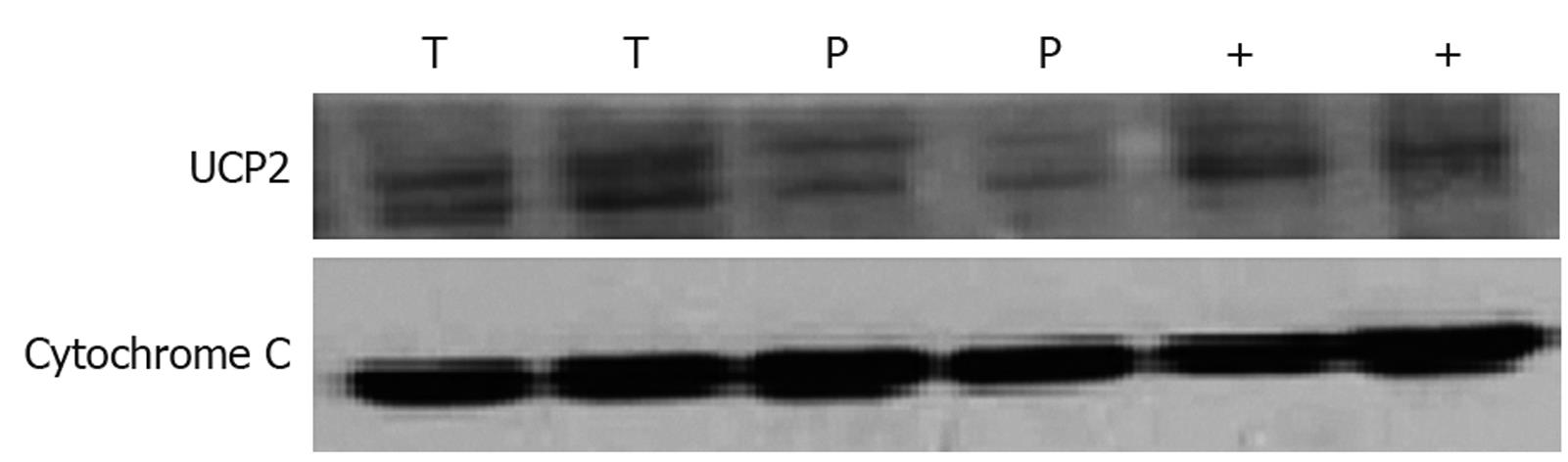
Sample was prepared for Western blotting using a mitochondria isolation kit following its manufacturer’s instructions (AR0156, Wuhan Boster Biological Technology, LTD, Wuhan, China). An equal amount of protein was size-fractionated with 12.5% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Perkin-Elmer Life Sciences, Boston, MA). Immunoblots were developed using anti-UCP2 antibody (C-20; Santa Cruz Biotechnology, Inc, USA). Mitochondrial protein obtained from wild-type mouse spleen was used as a positive control. An equal loading was confirmed using cytochrome C (sc-13561, Santa Cruz Biotechnology, Inc, USA). Membranes were incubated with secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc, USA), detected with enhanced chemiluminescence (ECL detection system, NEN, Boston, MA, USA), and used for visualization.
Data are expressed as mean ± SE. Correlation between categorical groups was evaluated by χ2 test or Fisher’s exact test, when appropriate. Univariate analysis of hazard model was employed to detect independent predictors of colon cancer metastasis. When appropriate, linear regression and Pearson r correlation were calculated. Two-tailed P value of ≤ 0.05 was considered statistically significant.
The expression of UCP2 mRNA in 15 colon cancer tissue samples and 15 its adjacent tissue samples was detected by quantitative RT-PCR. The expression level of UCP2 mRNA was about four-fold higher in colon cancer tissue samples than in its adjacent tissue samples (Table 2).
| Patient No. | Tissue | UCP2 mRNA relative abundance | T/P |
| 1 | T | 8.12 | 3.45 |
| P | 2.35 | ||
| 2 | T | 19.68 | 5.70 |
| P | 3.45 | ||
| 3 | T | 13.6 | 2.96 |
| P | 4.6 | ||
| 4 | T | 21.4 | 2.32 |
| P | 9.21 | ||
| 5 | T | 26.2 | 3.67 |
| P | 12.7 | ||
| 6 | T | 78.6 | 2.51 |
| P | 31.2 | ||
| 7 | T | 86.5 | 3.59 |
| P | 24.1 | ||
| 8 | T | 12.31 | 6.00 |
| P | 20.5 | ||
| 9 | T | 45.2 | 8.85 |
| P | 5.1 | ||
| 10 | T | 36.1 | 0.84 |
| P | 42.6 | ||
| 11 | T | 9.12 | 2.37 |
| P | 3.85 | ||
| 12 | T | 3.67 | 1.78 |
| P | 2.05 | ||
| 13 | T | 10.34 | 4.40 |
| P | 2.35 | ||
| 14 | T | 17.63 | 5.86 |
| P | 3.01 | ||
| 15 | T | 6.54 | 4.47 |
| P | 1.46 | ||
| Average T/P ratio | 3.92 | ||
The expression of UCP2 protein in 15 colon cancer tissue samples and 15 its adjacent tissue samples was detected by Western blotting. The expression level of UCP2 protein was three-fold higher in colon cancer tissue samples than in its adjacent tissue samples (Figure 1, Table 3).
| Patient No. | Tissue | UCP2 protein (total gray) | T/P |
| 1 | T | 2.10 | 2.14 |
| P | 0.98 | ||
| 2 | T | 4.16 | 5.33 |
| P | 0.78 | ||
| 3 | T | 2.45 | 2.82 |
| P | 0.87 | ||
| 4 | T | 5.14 | 4.25 |
| P | 1.21 | ||
| 5 | T | 3.02 | 3.70 |
| P | 0.82 | ||
| 6 | T | 6.24 | 2.70 |
| P | 2.31 | ||
| 7 | T | 5.55 | 5.00 |
| P | 1.09 | ||
| 8 | T | 8.27 | 3.93 |
| P | 2.1 | ||
| 9 | T | 9.43 | 8.65 |
| P | 1.08 | ||
| 10 | T | 1.96 | 0.78 |
| P | 2.51 | ||
| 11 | T | 3.15 | 3.80 |
| P | 0.83 | ||
| 12 | T | 7.12 | 2.13 |
| P | 3.34 | ||
| 13 | T | 4.51 | 3.72 |
| P | 1.21 | ||
| 14 | T | 7.12 | 5.45 |
| P | 1.31 | ||
| 15 | T | 2.54 | 4.17 |
| P | 0.62 | ||
| Average T/P | 3.27 | ||
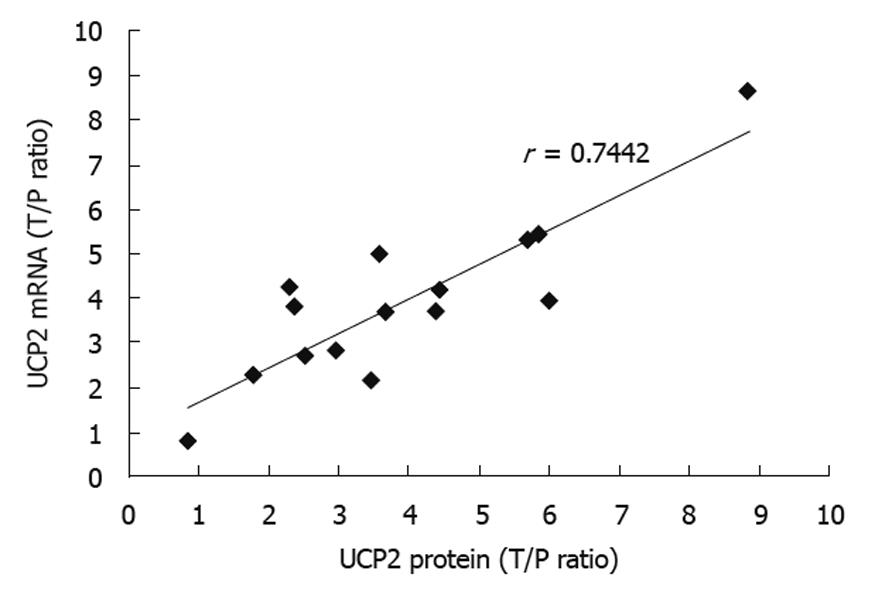
The correlation between UCP2 protein and UCP2 mRNA expressions in human colon cancer tissue samples was observed. A strong linear correlation was found between the T/P ratio of UCP2 mRNA and protein expression (r = 0.7442, P < 0.05), suggesting that increased UCP2 expression in colon cancer tissue is largely determined at the transcriptional level (Figure 2).
The expression of UCP2 was detected by immunohistochemistry in colon tissue samples from 78 colon cancer patients, 20 adenoma patients, 10 hyperplastic polyp patients and 10 normal controls. UCP2 was mainly expressed in cytoplasm but not expressed in epithelium of normal colon. In contrast, strongly positive staining for UCP2 with a diffuse distribution pattern was identified throughout the mucosa in most tubular adenomas and adenocarcinomas. The positive staining rate was 85.9% and 55% for colon cancer tissue and colon adenoma tissue, respectively (Figure 3, Table 4).
| Tissue | n | Negative | Mild | Moderate | Strong | Rate of positive (%) |
| Normal colon | 10 | 10 | 0 | 0 | 0 | 0 |
| Hyperplastic polyps | 10 | 9 | 2 | 0 | 0 | 20 |
| adenoma | 20 | 9 | 3 | 6 | 2 | 55 |
| Colon cancer | 78 | 10 | 15 | 30 | 22 | 85.9 |
The correlation between UCP2 expression and clinical pathological features of colon cancer (including tumor stage and cell differentiation) was analyzed. The expression level of UCP2 was significantly higher in patients with colon cancer at clinical stages III and IV than in those at stage I + II. However, the UCP2 expression seemed to be irrelevant to cell differentiation status, indicating that further study is needed (Table 5).
The relation of colon cancer metastasis with sex and age of patients, tumor location, UCP2 expression and other features was assessed by univariate analysis, showing that cell differentiation and UCP2 expression were involved in colon cancer metastasis. However, colon cancer metastasis seemed to be irrelevant to age and sex of patients and tumor location (Table 6).
| Variable | Hazard ratio | 95% CI | P-value |
| Age (≥ 60 yr) | 0.098 | 0.081-33.058 | 0.915 |
| Gender | 0.895 | 0.078-41.221 | 0.389 |
| Primary site | 2.135 | 0.610-18.281 | 0.078 |
| Tumor differentiation | 1.270 | 0.031-1.672 | 0.049 |
| UCP2 high expression | 4.321 | 0.035-0.682 | 0.046 |
In this study, different UCP2 expressions in colon cancer tissue and normal peritumoral tissue samples were observed. The expression of UCP2 mRNA in colonic mucosa samples from patients undergoing colon cancer resection was detected by quantitative PCR. As shown in Table 2, the UCP2 mRNA expression level was 4-fold higher in colon cancer mucosal tissue samples than in grossly normal peritumoral colonic mucosal tissue samples (3.92 ± 0.84, n = 15). The expression level of UCP2 protein in colon cancer tissue samples was measured by Western blotting and densitometry, respectively (3.27 ± 0.78, n = 15). A strong linear correlation was found between the T/P ratio of UCP2 mRNA and protein expressions (r = 0.744, P < 0.05), suggesting that increased UCP2 expression in colon cancer tissue is largely determined at the transcriptional level, which is consistent with the reported findings[11].
To characterize the UCP2 protein expression in different human colonic lesions, immunohistochemistry staining was performed for colon tissue sections (including normal colon tissue, non-neoplastic hyperplastic polyp tissue, tubular adenoma tissue, and colon cancer tissue). UCP2 in epithelium of normal colon was not stained. In contrast, strongly positive staining for UCP2 with a diffuse distribution pattern was identified throughout the mucosa from most tubular adenomas and adenocarcinomas. These findings indicate that colonic epithelium is the primary source of increased UCP2 expression in colon cancer. The positive UCP2 expression rate was 20% in hyperplastic polyp tissue samples, and over 50% in tubular adenoma samples, and 85.9% in colon cancer tissue samples, respectively, suggesting that UCP2 expression may intensify along the adenoma-carcinoma sequence.
The relation between UCP2 expression and clinical pathological features of colon cancer was analyzed. The expression level of UCP2 was significantly higher in patients with colon cancer at clinical stages III and IV than in those at stage I + II. However, the UCP2 expression seemed to be irrelevant to cell differentiation status, indicating that further study is needed (Table 5). Furthermore, the relation of colon cancer metastasis with sex and age of patients, tumor location, UCP2 expression and other features was also assessed by univariate analysis, showing that highly expressed UCP2 was significantly correlated to colon cancer metastasis (hazard ratio = 4.321, confidence interval = 0.035-0.682, P = 0.046).
Very few studies have reported the potential role of UCP2 in carcinogenesis[12]. Limited literature showed that experimental data are somewhat controversial[13,14]. It has been shown that the UCP2 expression level is moderately higher in human colon cancer cells (LoVo) treated with ionized radiation than in controls not treated with ionized radiation[12,15], and the amount of UCP2 transcripts is greater in apoptosis-sensitive lymphoma cell line after radiation than in apoptosis-resistant cell line[16], suggesting that the decreased mitochondrial membrane potential mediated by UCP2 may activate the cell death pathways[17,18]. Recent reports from multiple laboratories offer a different conclusion[14]. It was reported that drug-resistant tumor cell sub-lines increase the UCP2 expression with a lower mitochondrial membrane potential and a diminished susceptibility to oxidative stress[19-21], indicating that tumor cells may use UCP2 in their metabolic adaptation to avoid ROS-mediated apoptosis.
Over-expression of UCP2 reduces neuronal cell death in transgenic mice and in cell culture exposed to hypoxia and glucose deprivation, coinciding with a decrease in mitochondrial ROS formation[21], and over-expression of UCP2 in cultured cardiomyocytes limits mitochondrial ROS production and suppresses loss of mitochondrial membrane potential elicited by H2O2 treatment[22], suggesting that UCP2 over-expression may protect different normal cells against apoptosis, and the cyto-protective role of UCP2 is likely involved in reduction of mitochondrial ROS production.
It has been shown that glycolysis is the preferred energy-producing pathway in rapidly growing cancer cells, while their mitochondrial respiration is diminished[23]. Changes in cancer cell bioenergetics are often associated with more aggressive tumor growth and drug resistance, resulting in worse prognosis[24]. This metabolic switch in cancer cells is to steer away reducing equivalents from the mitochondria to limit ROS generation.
In conclusion, UCP2 is over-expressed in human colon cancer in vivo. Increased UCP2 expression in colon cancer is largely determined at the transcriptional level. Highly expressed UCP2 is associated with colon cancer metastasis. Over-expression of UCP2 may be involved in tumor aggressiveness and UCP2 may be a novel target therapy for colon cancer.
Colon cancer is one of the malignant tumors threatening to human health, and the mortality of colon cancer patients ranks second among all malignant tumors in developed countries. Its etiology and pathogenesis remains unclear. Adaptive mechanisms in cancer cells include resistance to tumor growth inhibition and evasion of apoptosis, cellular events that are appreciably affected by oxidative stress. Uncoupling protein-2 (UCP2) negatively regulates the production of reactive oxygen species (ROS).
The etiology and pathogenesis of colon cancer remain unclear. Recent studies showed that mitochondrial dysfunction is involved in the occurrence and development of tumor. UCP2 is a mitochondrial membrane protein, which negatively regulates the production of ROS. The role of UCP2 in development of colon cancer is unclear. In this study, the expression of UCP2 in normal human colon tissue samples and colon cancer tissue samples was detected and the relation between UCP2 expression and clinic-pathological features of colon cancer was analyzed.
Recent studies showed that mitochondrial dysfunction is involved in the occurrence and development of tumor. UCP2 is a member of the inner mitochondrial membrane anion-carrier protein super family and negatively regulates the production of ROS. This is the first study to report the relation between high expression of UCP2 and clinical pathological features of colon cancer.
By understanding how the expression of UCP2 in colon cancer and analyzing the relation between UCP2 expression and clinical pathological features of colon cancer, we showed that UCP2 might be a novel target therapy for colon cancer.
UCP2 is a member of the inner mitochondrial membrane anion-carrier protein super family and negatively regulates the production of ROS. Cancer cells acquire drug resistance as a result of selection pressure dictated by unfavorable microenvironments. This survival process is facilitated through efficient control of oxidative stress originating from mitochondria that typically initiates programmed cell death. This critical adaptive response in cancer cells is linked to UCP2, a mitochondrial suppressor of reactive oxygen species.
The authors detected the expression of UCP2 and the relation between UCP2 expression and clinical pathological features of colon cancer. The authors showed that UCP2 expression was increased in colon cancer, and UCP2 might be involved in colon cancer metastasis.
| 1. | Tischoff I, Tannapfel A. [Epigenetic alterations in colorectal carcinomas and precancerous lesions]. Z Gastroenterol. 2008;46:1202-1206. |
| 2. | Zhang ZY, Zhao ZZ. Epidemiology of colorectal cancer and future prospects. Zhongliu Fangzhi Yanjiu. 2000;27:154-156. |
| 3. | Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37-51. |
| 4. | Wolvetang EJ, Johnson KL, Krauer K, Ralph SJ, Linnane AW. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994;339:40-44. |
| 5. | Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ. Molecular mechanism of 'mitocan'-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett. 2006;580:5125-5129. |
| 6. | Boss O, Hagen T, Lowell BB. Uncoupling proteins 2 and 3: potential regulators of mitochondrial energy metabolism. Diabetes. 2000;49:143-156. |
| 7. | Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755-767. |
| 8. | Casteilla L, Rigoulet M, Pénicaud L. Mitochondrial ROS metabolism: modulation by uncoupling proteins. IUBMB Life. 2001;52:181-188. |
| 9. | Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420-425. |
| 10. | Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159-164. |
| 11. | Horimoto M, Resnick MB, Konkin TA, Routhier J, Wands JR, Baffy G. Expression of uncoupling protein-2 in human colon cancer. Clin Cancer Res. 2004;10:6203-6207. |
| 12. | Derdák Z, Fülöp P, Sabo E, Tavares R, Berthiaume EP, Resnick MB, Paragh G, Wands JR, Baffy G. Enhanced colon tumor induction in uncoupling protein-2 deficient mice is associated with NF-kappaB activation and oxidative stress. Carcinogenesis. 2006;27:956-961. |
| 13. | Sreekumar A, Nyati MK, Varambally S, Barrette TR, Ghosh D, Lawrence TS, Chinnaiyan AM. Profiling of cancer cells using protein microarrays: discovery of novel radiation-regulated proteins. Cancer Res. 2001;61:7585-7593. |
| 14. | Harper ME, Antoniou A, Villalobos-Menuey E, Russo A, Trauger R, Vendemelio M, George A, Bartholomew R, Carlo D, Shaikh A. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. FASEB J. 2002;16:1550-1557. |
| 15. | Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag 2002; 127-129. |
| 16. | Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, Herzenberg LA, Steinman L, Herzenberg LA. Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. Proc Natl Acad Sci USA. 2000;97:2680-2685. |
| 17. | Mehta SL, Li PA. Neuroprotective role of mitochondrial uncoupling protein 2 in cerebral stroke. J Cereb Blood Flow Metab. 2009;29:1069-1078. |
| 18. | Li L, Prabhakaran K, Mills EM, Borowitz JL, Isom GE. Enhancement of cyanide-induced mitochondrial dysfunction and cortical cell necrosis by uncoupling protein-2. Toxicol Sci. 2005;86:116-124. |
| 19. | Collins P, Jones C, Choudhury S, Damelin L, Hodgson H. Increased expression of uncoupling protein 2 in HepG2 cells attenuates oxidative damage and apoptosis. Liver Int. 2005;25:880-887. |
| 20. | Derdak Z, Mark NM, Beldi G, Robson SC, Wands JR, Baffy G. The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res. 2008;68:2813-2819. |
| 21. | Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062-1068. |
| 22. | Teshima Y, Akao M, Jones SP, Marbán E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res. 2003;93:192-200. |
| 23. | Warburg O. The metabolism of tumours. London: Arnold Constable 1930; 254-270. |
| 24. | Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaría G, Kim H, Zapata JM, Marusawa H, Chamorro M, Reed JC. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002;62:6674-6681. |
Peer reviewer: Guangcun Huang, MD, PhD, Research Institute at Nationwide Children’s Hospital, Center for Clinical and Translational Research, Columbus, OH 43205, United States
S- Editor Sun H L- Editor Wang XL E- Editor Lin YP