Published online Nov 21, 2010. doi: 10.3748/wjg.v16.i43.5424
Revised: September 13, 2010
Accepted: September 20, 2010
Published online: November 21, 2010
AIM: To evaluate the role of leptin in the internal disorders during hepatic ischemia/reperfusion injury.
METHODS: A rat model of 70% hepatic ischemia/reperfusion injury was established, with groups of sham-operation (Sham), 60 min ischemia/60 min reperfusion (I60’R60’), I60’R150’, I60’R240’ and I60’R360’. Serum leptin was detected by a self-produced radioimmunoassay; serum glucose, total anti-oxidation capacity, myeloperoxidase, alanine transaminase and diamine oxidase were determined by relevant kits, while histological alterations and protein levels of leptin in the lung, liver and duodenum were examined by hematoxylin-eosin staining and immunohistochemistry. Spearman’s rank correlation between leptin and other variables or grading of tissue impairment were analyzed simultaneously.
RESULTS: Serum leptin in I60’R360’ was significantly higher than in Sham and I60’R240’ groups (both P < 0.05), serum glucose in I60’R360’ was higher than in Sham and I60’R150’ (both P < 0.05), and serum total anti-oxidation capacity in I60’R240’ and I60’R360’ were higher than in Sham (both P < 0.05) and I60’R150’groups (both P < 0.01). Serum myeloperoxidase in groups of I60’R240’ and I60’R360’ were lower than in I60’R150’group (both P < 0.05), serum alanine transaminase in the four reperfusion groups were higher than in the Sham group (all P < 0.05), while serum DAO in I60’R360’ was lower than in I60’R60’ (P < 0.05). Histological impairment in the lung, liver and duodenum at the early phase of this injury was more serious, but the impairment at the later phase was lessened gradually. Protein levels of leptin in the lung in the four reperfusion groups were significantly lower than in the Sham group (all P < 0.01), decreasing in the order of I60’R150’, I60’R60’, I60’R360’ and I60’R240’; the levels in the liver in I60’R60’ and I60’R240’ were higher than in the Sham group (both P < 0.01), while the levels in I60’R240’ and I60’R360’ were lower than in I60’R60’ (both P < 0.01); the levels in duodenum in I60’R240’ and I60’R360’ were higher than in Sham, I60’R60’ and I60’R150’ (all P < 0.01), while the level in I60’R150’ was lower than in I60’R60’ (P < 0.05). There was a significantly positive correlation between serum leptin and alanine transaminase (ρ = 0.344, P = 0.021), a significantly negative correlation between the protein level of leptin in the lung and its damage scores (ρ = -0.313, P = 0.036), and a significantly positive correlation between the protein level of leptin in the liver and its damage scores (ρ = 0.297, P = 0.047).
CONCLUSION: Endogenous leptin fluctuates in hepatic ischemia/reperfusion injury, exerts a potency to rehabilitate the internal disorders and represents a potential target for supportive therapy.
- Citation: Lin J, Gao XN, Yan GT, Xue H, Hao XH, Wang LH. Endogenous leptin fluctuates in hepatic ischemia/reperfusion injury and represents a potential therapeutic target. World J Gastroenterol 2010; 16(43): 5424-5434
- URL: https://www.wjgnet.com/1007-9327/full/v16/i43/5424.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i43.5424
Hepatic ischemia/reperfusion injury is a complication of liver resection, transplantation and hypovolemic shock, which leads to local and systemic cellular damage as well as organ dysfunction[1]. Several lines of evidence suggest that the first consequence of hepatic ischemia/reperfusion injury is tissue hypoxia, which disturbs the intracellular energy metabolism and enzyme functions, resulting in depletion of adenosine triphosphate, accumulation of free radicals, and lesions in vital organs[2]. This injury is largely a result of an acute inflammatory cascade, and a certain endogenous network may exist to modulate the internal disorders. However, the conceivable mechanism has not been clarified.
Leptin is an ob gene-expressed protein mainly secreted by adipose tissues, with a primary role of inhibiting food intake, modulating weight balance and promoting energy metabolism[3]. Previous researches have revealed that leptin is a stress mediator after injuries, and it proceeds to maintain homeostasis by accelerating oxidation of glucose and fatty acids, alleviating reactive oxygen species-induced apoptosis, and ameliorating post-septic multiple organ dysfunction[4-6]. All these results suggest an important role for leptin in the recovery of hepatic ischemia/reperfusion injury, but there is no available report, which prompts us to study the fluctuation of leptin levels and its association with metabolic disorders as well as the functional and structural impairment in vital organs during this injury.
We established a rat model of 70% hepatic ischemia/reperfusion injury, checked serum leptin levels at different reperfusion time points, and detected serum glucose and total anti-oxidation capacity (TAC) for representing metabolic disorders. Serum myeloperoxidase (MPO), alanine transaminase (ALT), diamine oxidase (DAO) and histological alterations were evaluated in the functional and structural impairment in the lung, liver and duodenum. Protein levels of leptin were also examined in the distal, local and proximal vital organs mentioned above. Correlation analysis between serum leptin and other variables in serum, and the same analysis between tissue leptin and histological alterations in each organ were performed simultaneously. We aimed to find out the potential role for leptin in the recovery of internal disorders after hepatic ischemia/reperfusion injury.
Three male New Zealand white rabbits (weight 1.5 ± 0.1 kg) and 45 male Sprague-Dawley rats (weight 250 ± 6.0 g) were supplied by the experimental animal center of our hospital. Animals were maintained in a room at 22-25°C under a constant day/night rhythm and given food and water ad libitum. All animal experiments were carried out in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at our hospital.
Recombined murine leptin was purchased from PeproTech Inc. (London, UK). Complete and incomplete Freund’s adjuvant was purchased from Gibco/BRL (Gaithersburg, USA). Sodium iodide (Na125I) was purchased from Amersham Biosciences (Piscataway, USA). Immunoturbidimetric kits for serum glucose were purchased from Biosino Biotechnology and Science Inc. (Beijing, China). Colorimetric kits for serum TAC, MPO and ALT were purchased from Jiancheng Bioengineering Institute (Nanjing, China). Spectrophotometric assay for serum DAO was established and kindly provided by Professor Jun-You Li from Burns Institute, the First Affiliated Hospital of Chinese PLA General Hospital. Biotinylated anti-rabbit IgG, peroxidase-conjugated streptavidin and diaminobenzidine chromogenic kits were purchased from Zhongshan Golden Bridge Biotechnology Co. Ltd. (Beijing, China). Other reagents were purchased locally and of analytically pure grade.
New Zealand white rabbits were immunized subcutaneously with an emulsion of 120 μg leptin in 3 mL complete Freund’s adjuvant for the first injection, then with another emulsion in incomplete Freund’s adjuvant for four times to attain antibody. Iodination of leptin was performed according to our previous report[7]. The 125I-labeled leptin with a high specific binding rate and a low fault binding rate was taken as successful iodinated antigen. They were mixed with an equal volume of 1.5% bovine serum albumin and stored at -20°C.
Forty-five rats were divided randomly into five groups, including sham-operation (Sham), hepatic ischemia for 60 min/reperfusion for 60 min (I60’R60’), I60’R150’, I60’R240’ and I60’R360’, and each group contained 9 rats. Rats were deprived of food 12 h prior to the start of experiment, with free access to drinking water ad libitum.
Rats were anesthetized with pentobarbital sodium (60 mg/kg, i.p.), and their abdominal skin was sterilized. After a 2-cm midline incision was made in abdomen, the portal vein, hepatic artery and bile duct of the left and middle lobes were occluded with a microvessel clip for 60 min, and then the clip was released for reperfusion. The three remaining caudal lobes retained an intact portal/arterial blood supply and venous outflow, thus preventing intestinal venous hypertension and possible leakage of bacteria or bacterial products into the circulation that would be found in 100% hepatic ischemia/reperfusion injury[8].
The portal vein, hepatic artery and bile duct of the left and middle lobes were not occluded in the rats of Sham group. The incision was closed in layers, and resuscitation with isotonic saline (30 mL/kg, i.p.) was supplied. Then, rats were sterilized with iodophor on their suture sites and released back to cages, with free access to drinking water ad libitum.
Blood was collected from the heart of rats at different time points under anesthesia with a syringe, and serum was separated from whole blood through incubating samples in 37°C water for 20 min and centrifuged by 3000 ×g for 10 min at 4°C. Lung, liver and duodenum samples of each rat were harvested immediately after attaining blood samples. Each tissue sample was fixed in 40 g/L neutral phosphate-buffered formaldehyde solution and embedded in paraffin, and then serial sections (4 μm thick) were made.
Serum leptin was measured by a self-produced radioimmunoassay according to our previous report[7], using the leptin antibody and iodinated leptin mentioned above. Serum glucose, TAC, MPO, ALT and DAO were measured by the kits or assay according to the provided protocol of the manufacturers.
Tissue sections were stained with hematoxylin and eosin, and observed under microscope to investigate histological alterations in the lung, liver and duodenum. Nine microscopic fields of each organ in each group were randomly selected, and grading of the structural impairment in each organ was evaluated according to the criteria outlined in the reference[9].
Protein levels of leptin in the lung, liver and duodenum were detected by immunohistochemistry. Briefly, tissue sections were collected on 0.1% poly-lysine coated slides, deparaffinized by the xylene-ethanol sequence, rehydrated in a graded ethanol scale and in phosphate buffered saline (PBS). After prior antigen retrieval by heating in a pressure cooker in citrate buffer, the slides were incubated sequentially with the self-produced leptin antibody, biotinylated anti-rabbit IgG and peroxidase-conjugated streptavidin at 37°C for 1 h, using a dilution of 1:50, 1:200 and 1:200 in PBS. Diaminobenzidine chromogenic kits were used to develop the color.
Negative controls were set up simultaneously by replacing the leptin antibody with PBS. The images were visualized with Leica DC300F Digital Camera Systems, and 9 microscopic fields of each organ from each group were randomly selected. Image-Pro Plus 6.0 software was used to analyze the ratio of accumulated optical density of specific positive staining/specific tissue area in each image.
Stata 7.0 software (Stata Corp, USA) was used to analyze the data. One-way analysis of variance and Student’s t test were applied for the data including serum variables and tissue leptin. Wilcoxon signed-rank test was used for the data about grading of tissue impairment. Spearman’s rank correlation was selected for correlation analysis. A P value of less than 0.05 was chosen as a threshold for statistical significance, and all data were shown as mean ± SD.
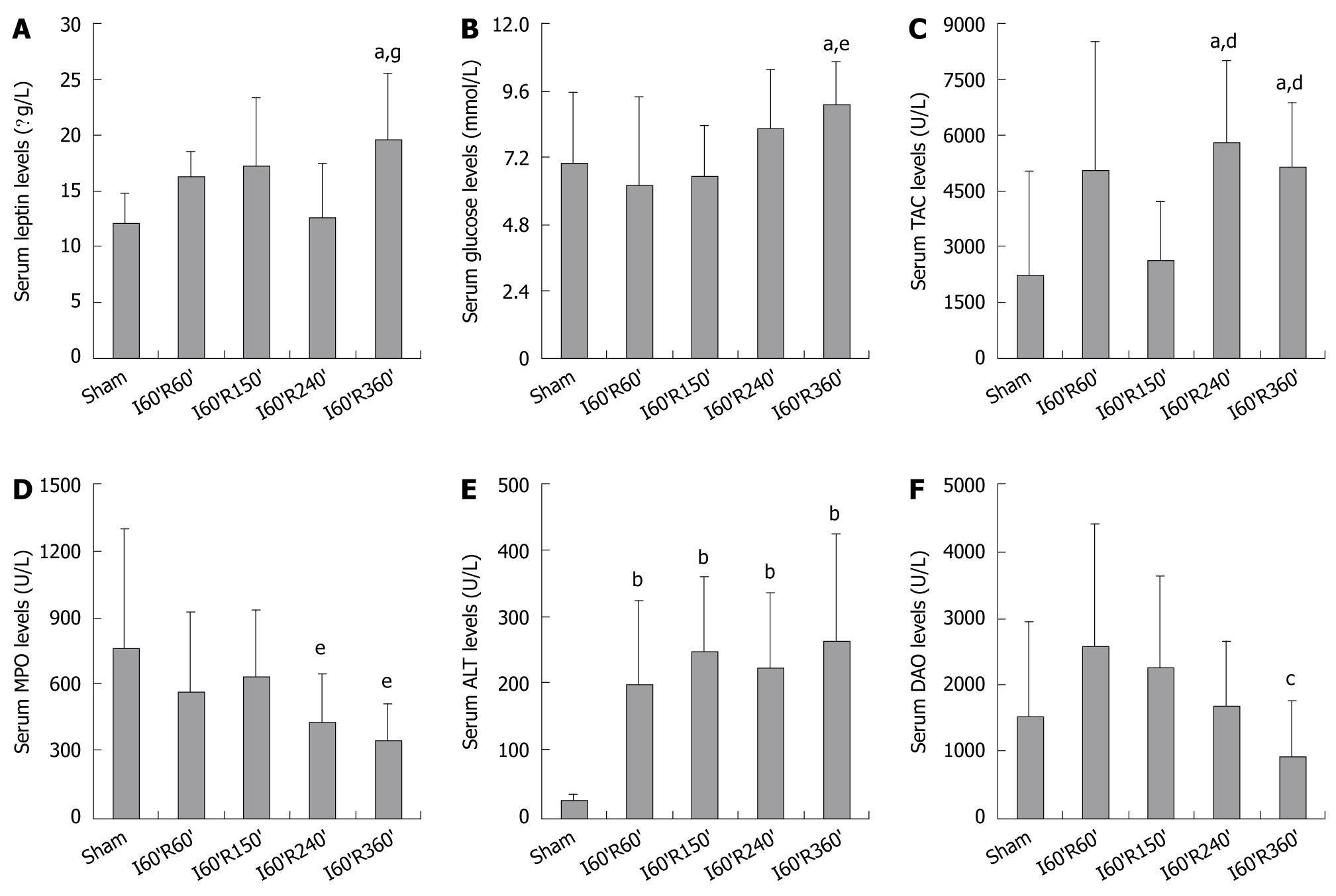
Serum leptin in I60’R360’ was significantly higher than in Sham and I60’R240’ (t = 3.410 and 2.659, both P < 0.05), while there was no significant difference in the levels among the Sham, I60’R60’, I60’R150’ and I60’R240’groups, as shown in Figure 1A.
Serum glucose in I60’R360’ was significantly higher than in Sham and I60’R150’ (t = 2.190 and 3.286, both P < 0.05), and it was inclined to be higher than in I60’R60’ (P = 0.051). No difference was observed in the levels among Sham, I60’R60’, I60’R150’ and I60’R240’ (Figure 1B).Serum TAC in I60’R240’ and I60’R360’ were significantly higher than in Sham (t = 3.050 and 2.693, both P < 0.05) and I60’R150’ (t = 3.566 and 3.270, both P < 0.01), while there was no difference either between the levels in I60’R240’ and I60’R360’ or in those among Sham, I60’R60’ and I60’R150’ (Figure 1C).
Serum MPO in the four reperfusion groups was not different from that in Sham, but the levels in I60’R240’ and I60’R360’ were significantly lower than in I60’R150’ (t = -2.259 and -2.489, both P < 0.05), while no difference was found in the levels among I60’R60’, I60’R240’ and I60’R360’ (Figure 1D).
Serum ALT in I60’R60’, I60’R150’, I60’R240’ and I60’R360’ were significantly higher than in Sham (t = 4.132, 5.888, 5.201 and 4.359, all P < 0.01), while there was no difference in the levels among the four reperfusion groups, as shown in Figure 1E. Serum DAO in the four reperfusion groups was not different from that in Sham, but the level in I60’R360’ was significantly lower than in I60’R60’ (t = -2.411, P < 0.05), demonstrating a decreasing tendency as compared with that in I60’R150’ (P = 0.086). No difference was observed in the levels among I60’R60’, I60’R150’ and I60’R240’ (Figure 1F).
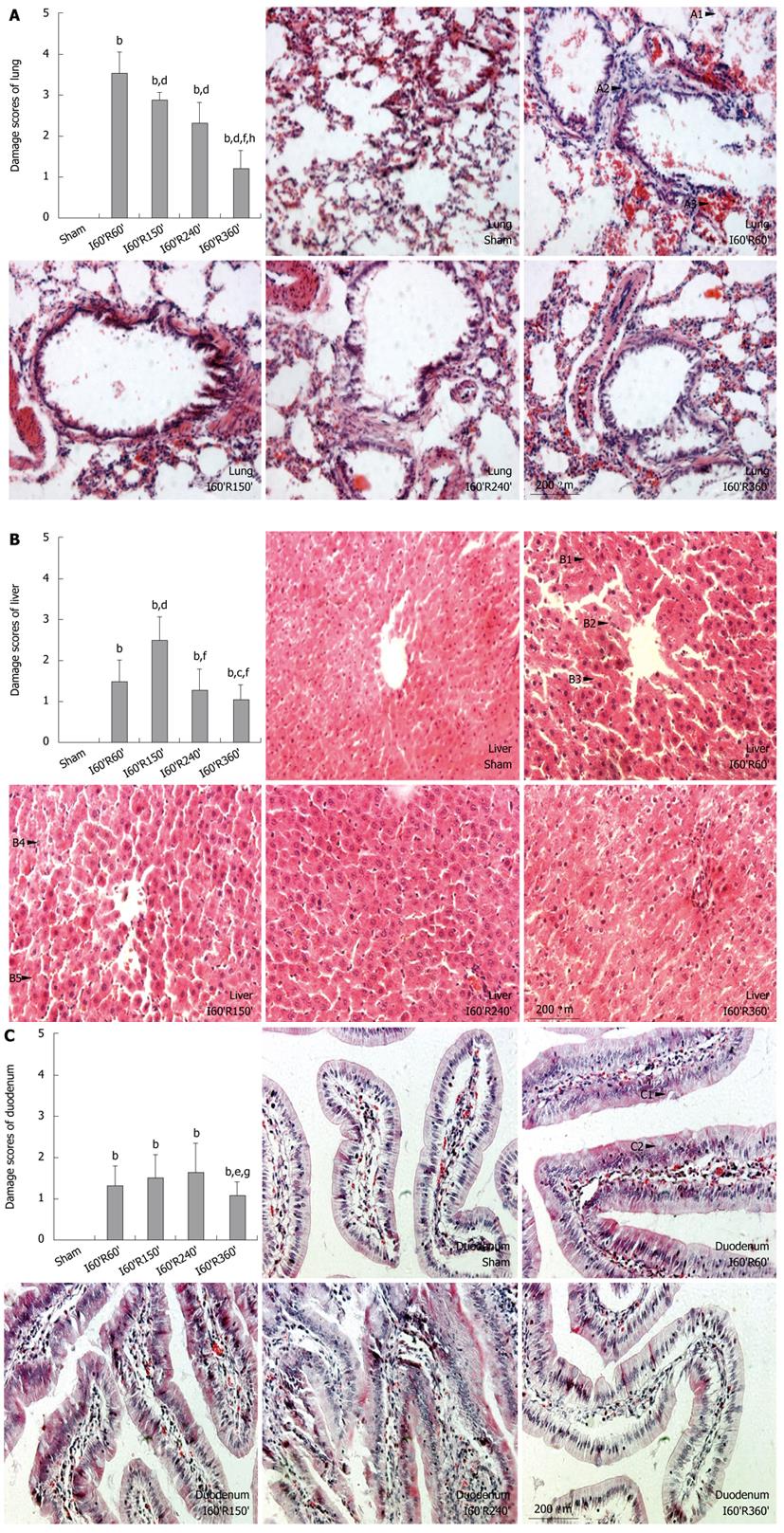
Compared with pulmonary alterations in the Sham group after injury, alveolar walls in I60’R60’ were disrupted (Figure 2, A1) and infiltrated by numerous inflammatory cells (A2), and the interstitial tissue was fulfilled with erythrocytes (A3). Similar but significantly alleviated alterations were observed in I60’R150’ and I60’R240’, while apparently minor disruption of alveolar structure, hypercellularity and vascular congestion became evident in I60’R360’. Damage scores of the lung in the four reperfusion groups were significantly higher than in the Sham (all P < 0.01), while the scores in I60’R150’, I60’R240’ and I60’R360’ were significantly lower than in I60’R60’ (z = -3.000, -2.810 and -2.762, all P < 0.01). The scores in I60’R150’ and I60’R240’ were significantly higher than in I60’R360’ (z = 2.762 and 2.887, both P < 0.01), but without difference (Figure 2A).
Compared with hepatic alterations in the Sham group after injury, hepatic cords in I60’R60’ were widened and arranged disorderly, hepatic sinusoids were narrowed or vanished (Figure 2, B1), hepatocytes were found swollen with loosened cytoplasm (B2) and a few Councilman bodies were scattered (B3). Apparently worsened vacuolization and lytic necrosis of hepatocytes (B4), increased Councilman bodies as well as vascular congestion (B5) were observed in I60’R150’, while minor alterations similar with that in I60’R60’ were found in I60’R240’, and the lesions in I60’R360’ were obviously attenuated. Damage scores of the liver in the four reperfusion groups were significantly higher than in the Sham (all P < 0.01), while the scores in I60’R60’, I60’R240’ and I60’R360’ were significantly lower than in I60’R150’ (z = -3.000, -2.810 and -2.739, all P < 0.01). The score in I60’R60’ was significantly higher than in I60’R360’ (z = 2.000, P = 0.0455), while no difference was found in the scores between I60’R240’ and I60’R360’, as shown in Figure 2B.
Compared with duodenal alterations in the Sham after injury, the ciliated epithelial cells in I60’R60’ were impaired and arranged irregularly (Figure 2, C1), the quantity of Goblet cells (C2) and mucus secretion were increased, and mild congestion in the mucosal lamina propria was observed (C3). Distinctly aggravated impairment of the ciliated epithelial cells, further increased the quantity of Goblet cells but minor congestions were found in I60’R150’ and I60’R240’, while significantly alleviated alterations were observed in I60’R360’. Damage scores of duodenum in the four reperfusion groups were significantly higher than in the Sham group (all P < 0.01), while the score in I60’R360’ was significantly lower than in I60’R150’ and I60’R240’ (z = -2.000 and -1.987, both P < 0.05). No difference was found in the scores among I60’R60’, I60’R150’ and I60’R240’ (Figure 2C).
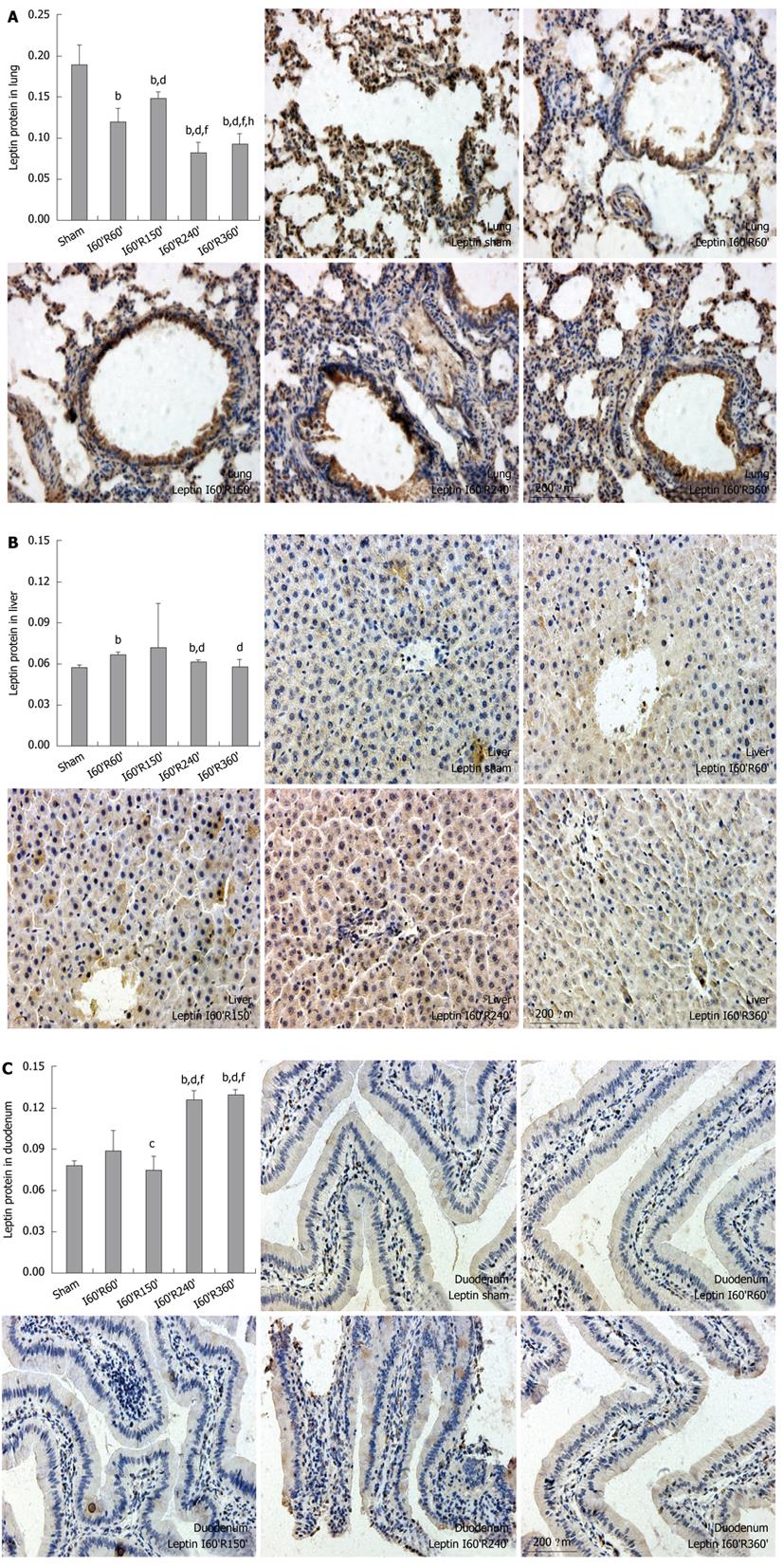
In the lung, the protein levels of leptin in I60’R60’, I60’R150’, I60’R240’ and I60’R360 were significantly lower than in the Sham (t = -7.241, -4.962, -11.970 and -10.821, all P < 0.01), while the level in I60’R150’ was significantly higher than in I60’R60’, I60’R240’ and I60’R360’ (t = 4.633, 13.096 and 11.086, all P < 0.01). The levels in I60’R240’ and I60’R360’ were significantly lower than in I60’R60’ (t = -5.166 and -3.708, both P < 0.01), and the level in I60’R360’ was significantly higher than in I60’R240’ (t = 3.046, P < 0.01) (Figure 3A).
In the liver, the protein levels of leptin in I60’R60’ and I60’R240’ were significantly higher than in the Sham (t = 13.769 and 6.952, both P < 0.01), while the levels in I60’R150’ and I60’R360’ showed no difference as compared with that in the Sham. The levels in I60’R240’ and I60’R360’ were significantly lower than in I60’R60’ (t = -13.754 and -4.683, both P < 0.01), while no difference was observed in the levels among I60’R150’, I60’R240’ and I60’R360’, as shown in Figure 3B.
In the duodenum, the protein levels of leptin in I60’R240’ and I60’R360’ were significantly higher than in the Sham (t = 22.847 and 35.411, both P < 0.01), I60’R60’ (t = 6.890 and 7.760, both P < 0.01) and I60’R150’ (t = 12.538 and 14.237, both P < 0.01), while the levels in I60’R60’ and I60’R150’ were not different from that in the Sham. The level in I60’R150’ was significantly lower than in I60’R60’ (t = -2.431, P < 0.05), and there was no difference in the levels between I60’R240’ and I60’R360’ (Figure 3C).
There was no significant correlation between serum leptin and glucose, TAC, MPO or DAO, while a significantly positive correlation was found between serum leptin and ALT (ρ = 0.344, P = 0.021).The protein level of leptin in the lung had significantly negative correlation with its damage scores (ρ = -0.313, P = 0.036), but the level in the liver showed a significantly positive correlation with its damage scores (ρ = 0.297, P = 0.047), and no significant correlation was observed between the levels in the duodenum and its damage scores (Table 1).
| Analyzed pairs | ρ | P |
| Serum leptin + glucose | -0.061 | 0.693 |
| Serum leptin + total anti-oxidation capacity | 0.254 | 0.092 |
| Serum leptin + myeloperoxidase | -0.083 | 0.586 |
| Serum leptin + alanine transaminase | 0.344 | 0.021 |
| Serum leptin + diamine oxidase | -0.098 | 0.523 |
| Tissue leptin + damage scores of lung | -0.313 | 0.036 |
| Tissue leptin + damage scores of liver | 0.297 | 0.047 |
| Tissue leptin + damage scores of duodenum | 0.180 | 0.237 |
Despite advances in supportive therapy, hepatic ischemia/reperfusion injury continues to negatively affect patient mortality and morbidity[10]. As the liver is the most important organ participating in energy metabolism and nutrient absorption, this injury will inevitably lead to variation of endogenous metabolic factors such as leptin. Recent researches have suggested a protective role for leptin in trauma/sepsis-induced organ dysfunction[11,12], but there is no report mentioning the affirmatory linkage between leptin and hepatic ischemia/reperfusion injury, thus our study was the first to investigate whether leptin is a novel strategy aimed at minimizing the internal disorders after this injury. In the early stage after reperfusion, the high metabolic status changed the body into a starvation-like situation[13]. As leptin is down-regulated by the neuroendocrine system during starvation[14] and there exists a possible destructive effect of free radicals on circulating proteins, serum leptin from I60’R60’ to I60’R240’ remained significantly unchanged though their mean values seemed to increase. In the later stage, the starvation-like status and the negative effect of free radicals recovered gradually, therefore serum leptin in I60’R360’ increased significantly and this elevation fluctuation indicated a potential benefit of leptin during this injury.
As serum glucose and TAC represent the energy reserves and the overall ability to eliminate reactive oxygen species, respectively, we chose them as variables reflecting metabolic disorders after hepatic ischemia/reperfusion injury. Although a decrease in serum glucose was observed during starvation[14], the stimulating effect by catecholamines release in the early stage after stress[15] reversed the descent, and no significant change of serum glucose was observed in I60’R60’ to I60’R240’. As time went on, the starvation-like status and catecholamines release underwent synergic recovery, but serum glucose in I60’R360’ was distinctly higher due to a possible feedback provocation of resumptive energy reserve. Hepatic ischemia/reperfusion injury is characterized by Kupffer cell-induced oxidant stress[16]; our results showed that the anti-oxidation system responded promptly and exerted a valid capacity to eliminate free radicals from I60’R240’ to I60’R360’, suggesting that a competent endogenous regulation might exist to accelerate the recovery of this injury. The synchronous increase of serum leptin, glucose and TAC in the later stage also indicated a potentiality of leptin to protect the body by promoting energy metabolism and anti-oxidation capacity. Unexpectedly, no significant correlation was found either between serum leptin and glucose or between serum leptin and TAC, which might be attributed to the non-unique and complex regulation on glucose and TAC by other metabolic factors.
In liver transplantation, prolonged intensive care unit stay or lack of an adequate nutritional support results in starvation of the donor, leading to increased incidence of hepatocellular injury and primary dysfunction[17,18]. Given the fact that leptin decreases under malnutrition and starvation[14,19], it is attractive to speculate that leptin may associate with the liver impairment (even in other organs) during hepatic ischemia/reperfusion injury, and its elevation may proceed to maintain homeostasis. MPO is mainly expressed by neutrophils with a similar amount of nearly 5% of net weight in each cell[20], and it may indirectly reflect the severity of lung injury evoked by neutrophil infiltration. ALT and DAO are sensitive indicators for liver and duodenal injuries, thus we selected these three variables for representing the functional impairment in the lung, liver and duodenum (the distal, local and proximal vital organs), respectively. Our results showed that both serum MPO and DAO in the later stage after reperfusion were significantly lower than in the early stage, while serum ALT in the four reperfusion groups were higher than in Sham but were not aggravated as reperfusion time was elongated. These changes demonstrated that the functional impairment in the vital organs during 70% hepatic ischemia/reperfusion injury were relatively minor compared with 100% injury, and there should be an endogenous regulation to restrict deterioration of this injury and accelerate its recovery, which also indicated a potential benefit of leptin since it elevated synchronously in the later stage. Interestingly, no significant correlation was observed either between serum leptin and MPO or between serum leptin and DAO, while there was a significantly positive correlation between serum leptin and ALT, suggesting a direct protective role for leptin in the local vital organ but an indirect regulatory role in the distal and proximal vital organs during this injury.
A research has revealed that serum leptin is apparently higher in patients after liver, heart or kidney transplantation[21], which provides a clinical probability of leptin as a beneficial cytokine for ischemia/reperfusion-induced organ injuries. However, no histological alterations and their association with leptin were reported. Our results showed that although hepatic ischemia/reperfusion injury initiated distinct and diverse structural impairment, the severity in the later stage after reperfusion were alleviated compared with the early stage, suggesting once again that an endogenous regulation did exist to stimulate the recovery of this injury. Protein levels of leptin in the lung, liver and duodenum underwent different fluctuations due to disparate grades of structural impairment, with a timely response in the lung and liver in the early stage but a delayed response in the duodenum in the late stage, which also indicated a regulatory role for tissue leptin during the injury. Perplexingly, a significantly negative correlation between tissue leptin in the lung and its damage scores was observed, suggesting a conflicting role for leptin in the lung injury. Two critical researches have found that lung injury is moderately attenuated in leptin-deficient mice and intratracheal pretreatment with a leptin receptor inhibitor mitigates leptin-induced lung edema[22,23]. Our investigation also suggested that endogenous leptin might be detrimental to the recovery of lung injury despite that exogenous leptin has been proved beneficial for lung and other organs[24-26]. The significantly positive correlation between tissue leptin in the liver and its damage scores indicated once again a direct protective role for leptin in the local vital organ, while the indistinctive correlation between tissue leptin in duodenum and its damage scores, but a contrary variation in the later stage, still provided a rehabilitative potency of leptin in the proximal vital organ by inhibiting its structural impairment.
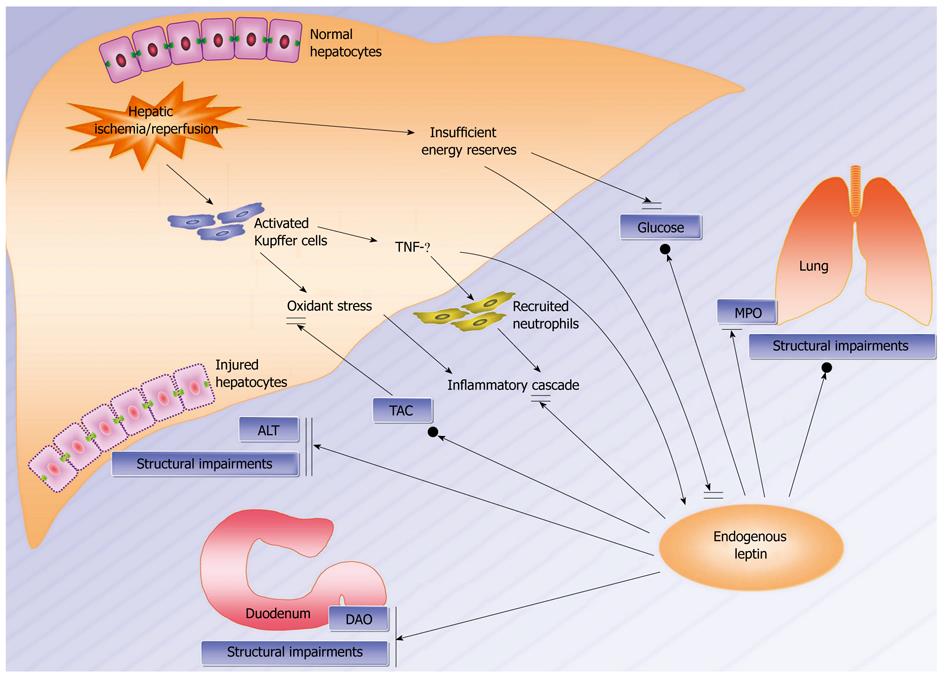
Although many studies have been conducted regarding the inflammatory mechanisms during hepatic ischemia/reperfusion injury[16,27,28], little is known about the endogenous network involving leptin that modulates the internal disorders in this injury. Ischemia/reperfusion-evoked activation of Kupffer cells results in production and release of reactive oxygen species and pro-inflammatory mediators such as tumor necrosis factor-α (TNF-α), which further activate and recruit neutrophils into target organs especially in the lung, and they contribute to the inflammatory cascade and have powerful effects on the local environment and remote organ functions[13,29]. Since a rise in TNF-α activity parallels an increase in leptin gene and peripheral expression, it is proposed that leptin participates in a protective mechanism against the inflammatory cascade downstream to TNF-α[30,31]. Despite a decrease in serum glucose and leptin observed in the starvation-like status which augmented susceptibility to inflammatory damage, the synchronous upregulation of serum leptin, glucose and TAC in the late stage still declared a potential rehabilitative role of leptin in metabolic disorders during this injury. Although an ambiguous induction by leptin in the structural impairment in the lung was presented, leptin directly inhibited the functional and structural impairment in the liver, while it potentially suppressed the functional impairment in the lung as well as the functional and structural impairment in the duodenum. Therefore, leptin evolves to harmonize an endogenous network maintaining homeostasis in the distal, local and proximal vital organs during this injury (Figure 4).
In our experimental model, different doses of exogenous leptin can be administered at different time points during the injury, and relevant inflammatory cytokines or growth factors can be measured to elucidate the rehabilitative characteristics of leptin. Signal pathway downstream to leptin can be investigated simultaneously to further clarify the association between leptin and the internal disorders. Moreover, liver cell lines may be used to establish a hypoxia/reoxygenation model, and applied to determine whether endogenous regulation or exogenous supplement of a functional leptin network will represent a beneficial component for the host response to the injury.
In conclusion, endogenous leptin fluctuates in serum and vital organs during hepatic ischemia/reperfusion injury, and exerts a potency to rehabilitate metabolic disorders as well as the functional and structural impairment in the lung, liver and duodenum, especially in the local vital organ. The association between leptin and the internal disorders has suggested that an artificial nutritional support, adequate content of antioxidants and exogenous leptin recruitment aimed to upregulate endogenous leptin may represent a new supportive therapy for hepatic ischemia/reperfusion injury.
Recent researches have revealed that leptin participates in the modulation of energy metabolism, neuroendocrine, angiogenesis, reproduction and immune responses, suggesting an important role of leptin in the recovery of functions. To study the fluctuation of leptin and its association with the internal disorders after hepatic ischemia/reperfusion injury may provide a potential target for the treatment of hepatic ischemia/reperfusion injury.
Liver is the vital organ for nutrition absorption and energy metabolism, its ischemia/reperfusion injury inevitably leads to local and remote dysfunction. As leptin is a stress mediator proceeding to maintain homeostasis and there is no available report about leptin and this injury, to explore the variation and roles of leptin will clarify whether it is a beneficial component for this injury.
This is the first study to report that endogenous leptin fluctuates in serum and vital organs in a rat model of 70% hepatic ischemia/reperfusion injury, and exerts a potency to rehabilitate metabolic disorders, the functional and structural impairment in the lung, liver and duodenum, especially in the local vital organ.
This study suggests that endogenous leptin may represent a potential target for minimizing the internal disorders after hepatic ischemia/reperfusion injury, and an artificial nutritional support, adequate content of antioxidants and exogenous leptin recruitment aimed to upregulate endogenous leptin may provide a new supportive therapy for this injury and relevant diseases.
Hepatic ischemia/reperfusion injury is a complication of liver resection, transplantation and hypovolemic shock, resulting in depletion of adenosine triphosphate, accumulation of free radicals, and lesion in vital organs. Leptin is an ob gene-expressed protein with a primary role of inhibiting food intake, modulating weight balance and promoting energy metabolism, but its potential role in this injury has not been reported.
The authors illustrated a well designed experiment to evaluate the role for leptin in the internal disorders during hepatic ischemia/reperfusion injury. Furthermore, relevant background information was given in order to interpret the importance of the trial. The manuscript is interesting and comprehensive. The part of discussion is compactly constructed and brilliant.
| 1. | Husted TL, Lentsch AB. The role of cytokines in pharmacological modulation of hepatic ischemia/reperfusion injury. Curr Pharm Des. 2006;12:2867-2873. |
| 2. | Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37:1653-1656. |
| 4. | Eguchi M, Liu Y, Shin EJ, Sweeney G. Leptin protects H9c2 rat cardiomyocytes from H2O2-induced apoptosis. FEBS J. 2008;275:3136-3144. |
| 5. | Lin J, Yan GT, Xue H, Hao XH, Zhang K, Wang LH. Leptin protects vital organ functions after sepsis through recovering tissue myeloperoxidase activity: an anti-inflammatory role resonating with indomethacin. Peptides. 2007;28:1553-1560. |
| 6. | Palacio A, Lopez M, Perez-Bravo F, Monkeberg F, Schlesinger L. Leptin levels are associated with immune response in malnourished infants. J Clin Endocrinol Metab. 2002;87:3040-3046. |
| 7. | Lin J, Yan GT, Hao XH, Wang LH, Zhang K, Xue H. Effect of intestinal ischemia-reperfusion injury on protein levels of leptin and orexin-A in peripheral blood and central secretory tissues. World J Gastroenterol. 2005;11:1000-1004. |
| 8. | Matsui N, Kasajima K, Hada M, Nagata T, Senga N, Yasui Y, Fukuishi N, Akagi M. Inhibiton of NF-kappaB activation during ischemia reduces hepatic ischemia/reperfusion injury in rats. J Toxicol Sci. 2005;30:103-110. |
| 9. | Demling R, LaLonde C, Knox J, Youn YK, Zhu D, Daryani R. Fluid resuscitation with deferoxamine prevents systemic burn-induced oxidant injury. J Trauma. 1991;31:538-543; discussion 543-544. |
| 10. | Clarke CN, Kuboki S, Tevar A, Lentsch AB, Edwards M. CXC chemokines play a critical role in liver injury, recovery, and regeneration. Am J Surg. 2009;198:415-419. |
| 11. | Liapakis IE, Anagnostoulis S, Karayiannakis AJ, Korkolis DP, Lambropoulou M, Anastakis D, Simopoulos C. Exogenously-administered leptin increases early incisional wound angiogenesis in an experimental animal model. In Vivo. 2007;21:797-801. |
| 12. | Lin J, Yan GT, Xue H, Hao XH, Zhang K, Wang LH. Leptin protects vital organ functions after sepsis through recovering tissue myeloperoxidase activity: an anti-inflammatory role resonating with indomethacin. Peptides. 2007;28:1553-1560. |
| 13. | Massip-Salcedo M, Roselló-Catafau J, Prieto J, Avíla MA, Peralta C. The response of the hepatocyte to ischemia. Liver Int. 2007;27:6-16. |
| 14. | Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156:1781-1787. |
| 15. | Yamada F, Inoue S, Saitoh T, Tanaka K, Satoh S, Takamura Y. Glucoregulatory hormones in the immobilization stress-induced increase of plasma glucose in fasted and fed rats. Endocrinology. 1993;132:2199-2205. |
| 16. | Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169-173. |
| 17. | Pruim J, van Woerden WF, Knol E, Klompmaker IJ, de Bruijn KM, Persijn GG, Slooff MJ. Donor data in liver grafts with primary non-function--a preliminary analysis by the European Liver Registry. Transplant Proc. 1989;21:2383-2384. |
| 18. | Stadler M, Nuyens V, Seidel L, Albert A, Boogaerts JG. Effect of nutritional status on oxidative stress in an ex vivo perfused rat liver. Anesthesiology. 2005;103:978-986. |
| 19. | Muñoz-Calvo MT, Barrios V, García de Alvaro MT, Lefort M, Méndez-Dávila C, Argente J, de la Piedra C. Maintained malnutrition produces a progressive decrease in (OPG)/RANKL ratio and leptin levels in patients with anorexia nervosa. Scand J Clin Lab Invest. 2007;67:387-393. |
| 20. | Arnhold J. Properties, functions, and secretion of human myeloperoxidase. Biochemistry (Mosc). 2004;69:4-9. |
| 21. | Kagan A, Haran N, Leschinsky L, Sarafian R, Aravot D, Dolberg J, Ben-Ary Z, Rapoport J. Serum concentrations of leptin in heart, liver and kidney transplant recipients. Isr Med Assoc J. 2002;4:213-217. |
| 22. | Barazzone-Argiroffo C, Muzzin P, Donati YR, Kan CD, Aubert ML, Piguet PF. Hyperoxia increases leptin production: a mechanism mediated through endogenous elevation of corticosterone. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1150-L1156. |
| 23. | Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2007;175:587-594. |
| 24. | Cakir B, Cevik H, Contuk G, Ercan F, Ekşioğlu-Demiralp E, Yeğen BC. Leptin ameliorates burn-induced multiple organ damage and modulates postburn immune response in rats. Regul Pept. 2005;125:135-144. |
| 25. | Gultekin FA, Kerem M, Tatlicioglu E, Aricioglu A, Unsal C, Bukan N. Leptin treatment ameliorates acute lung injury in rats with cerulein-induced acute pancreatitis. World J Gastroenterol. 2007;13:2932-2938. |
| 26. | Yan GT, Lin J, Hao XH, Xue H, Zhang K, Wang LH. Heart-type fatty acid-binding protein is a useful marker for organ dysfunction and leptin alleviates sepsis-induced organ injuries by restraining its tissue levels. Eur J Pharmacol. 2009;616:244-250. |
| 27. | Caldwell CC, Tschoep J, Lentsch AB. Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol. 2007;82:457-464. |
| 28. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. |
| 29. | Sankary HN, Chong A, Foster P, Brown E, Shen J, Kimura R, Rayudu G, Williams J. Inactivation of Kupffer cells after prolonged donor fasting improves viability of transplanted hepatic allografts. Hepatology. 1995;22:1236-1242. |
| 30. | Jaworek J, Bonior J, Pierzchalski P, Tomaszewska R, Stachura J, Sendur R, Leja A, Jachimczak B, Konturek PC, Bielański W. Leptin protects the pancreas from damage induced by caerulein overstimulation by modulating cytokine production. Pancreatology. 2002;2:89-99. |
| 31. | Moshyedi AK, Josephs MD, Abdalla EK, Mackay SL, Edwards CK 3rd, Copeland EM 3rd, Moldawer LL. Increased leptin expression in mice with bacterial peritonitis is partially regulated by tumor necrosis factor alpha. Infect Immun. 1998;66:1800-1802. |
Peer reviewer: Ren-Xiang Tan, Vice-President, Director, Chair Professor, Institute of Functional Biomolecules, State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, Jiangsu Province, China
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH