Published online Oct 28, 2010. doi: 10.3748/wjg.v16.i40.5077
Revised: June 25, 2010
Accepted: July 2, 2010
Published online: October 28, 2010
AIM: To prospectively compare partially covered vs uncovered sphincterotome use on post-endoscopic biliary sphincterotomy (ES) hemorrhage and other complications.
METHODS: All patients referred for therapeutic endoscopic retrograde cholangiopancreatography (ERCP) were randomly assigned to undergo ES either with a partially covered or an uncovered sphincterotome. Both patient and technical risk factors contributing to the development of post-ES bleeding were recorded and analyzed. The characteristics of bleeding was recorded during and after ES. Other complications were also compared.
RESULTS: Three-hundred and eighty-seven patients were recruited in this study; 194 patients underwent ES with a partially covered sphincterotome and 193 with conventional uncovered sphincterotome. No statistical difference was noted in the baseline characteristics and risk factors for post-ES induced hemorrhage between the 2 groups. No significant difference in the incidence and pattern of visible bleeding rates was found between the 2 groups (immediate bleeding in 24 patients with the partially covered sphincterotome vs 19 patients with the uncovered sphincterotome, P = 0.418). Delayed bleeding was observed in 2 patients with a partially covered sphincterotome and in 1 patient with an uncovered sphincterotome (P = 0.62). No statistical difference was noted in the rate of other complications.
CONCLUSION: The partially covered sphincterotome was not associated with a lower frequency of bleeding. Also, there was no difference in the incidence of other significant complications between the 2 types of sphincterotome.
-
Citation: Katsinelos P, Paroutoglou G, Kountouras J, Chatzimavroudis G, Zavos C, Terzoudis S, Katsinelos T, Fasoulas K, Gelas G, Tzovaras G, Pilpilidis I. Partially covered
vs uncovered sphincterotome and post-endoscopic sphincterotomy bleeding. World J Gastroenterol 2010; 16(40): 5077-5083 - URL: https://www.wjgnet.com/1007-9327/full/v16/i40/5077.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i40.5077
Endoscopic biliary sphincterotomy (ES) is a high-risk procedure with considerable possibility of complications[1]. Endoscopists with experience in ES are well aware that major problems can occur during the procedure, causing morbidity and occasionally death[2]. It is likely that the incidence of ES complications i.e. immediate (intraprocedural) and delayed bleeding (1%-10%)[2-6], pancreatitis (1%-3%)[4,7,8] or perforation (1%-2%)[4,7,8] can be reduced if cutting is more controlled. Apart from the skill and training of the endoscopist, definite risk factors that increase the risk of post-ES bleeding include coagulopathy, anticoagulation < 3 d after ES, cholangitis prior to ES and low endoscopic retrograde cholangiopancreatography (ERCP) case volume[9-11]. In a recent study[12] to investigate the efficacy of double injection of 50% dextrose plus epinephrine solution (1:10 000) in endoscopic hemostasis of post-ES bleeding, we found the “zipper-cut” phenomenon to be the sole significant risk factor for bleeding. In an effort to achieve a more controlled cutting and to avoid the “zipper-cut” phenomenon, a special sphincterotome has been manufactured having as its main characteristic a half-length of cutting wire insulated with polymeric plastic.
To date, there have been no studies comparing the 2 different types of sphincterotomes with regard to reduction in the incidence of post-ES bleeding or other complications. We hypothesized that, contrary to a conventional uncovered sphincterotome, the use of a partially covered sphincterotome, with the theoretical advantages of more controlled cutting and avoidance of the “zipper-cut” phenomenon, might reduce post-ES bleeding, one of the most frequent post-ES complications associated with morbidity[13]. Therefore, this prospective study was undertaken to compare partially covered vs uncovered sphincterotome use on post-ES hemorrhage and other complications.
The study was carried out between September 2007 and September 2008. The patients enrolled came from our institutions or were referred to us from other hospitals of Northern and Central Greece for therapeutic ERCP. The study was approved by our institutions’ Ethics Committees and each patient or his/her relatives gave written informed consent prior to ERCP. The following inclusion criteria were confirmed by the study coordinators: age > 18 years; no pregnancy; no evidence of significant cardiorespiratory or other medical conditions precluding participation (i.e. deemed able to tolerate routine sedation); and no known documented allergy to lidocaine anesthetic spray, meperidine, midazolam, propofol, or contrast agent. Patients were excluded using the following criteria: refusal to participate, failure to cannulate the common bile duct (CBD), and known gastroduodenal anatomic abnormalities (Billroth II or Roux-en-Y operation and bariatric surgery). Patients undergoing needle-knife papillotomy were also excluded from the study. In contrast, patients undergoing needle-knife fistulotomy with extension of sphincterotomy with a sphincterotome were included in the study. All patients received a pre-procedural assessment which included a detailed medical and drug history, physical examination, complete blood count, serum liver biochemistry, coagulation studies, abdominal ultrasound or computed tomography and magnetic resonance imaging cholangiography. Specifically, demographic data recorded before ERCP included indications for ES and risk factors for post-ES bleeding as demonstrated in Table 1. In cases of prolonged prothrombin time (> 3 s from upper limit of normal) or platelet count < 70 000/mm3 the patients received plasma or platelet transfusions, respectively, until normal prothrombin time and platelet count > 70 000/mm3 were achieved. During ERCP, endoscopic evidence of periampullary diverticulum, length of ES, extension of previous ES and use of precut papillotomy (transpancreatic, needle-knife) or suprapapillary fistulotomy with extension of ES were also recorded.
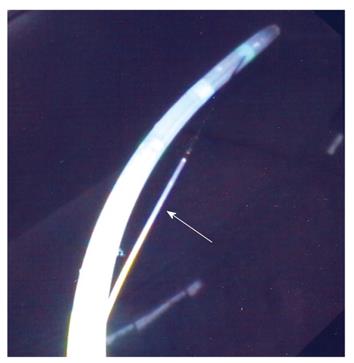
Patients were randomized to undergo ES with either a partially covered sphincterotome (Clever-cut, Olympus, Tokyo, Japan; cost 220 euros) or an uncovered sphincterotome (Autotome, Microvasive, Boston Scientific, USA; cost 160 euros). The proximal half-length of the cutting wire of the partially covered sphincterotome is insulated (Figure 1). The insulation is bonded to the cutting wire and is made of polymeric plastic, which has good electrical insulating properties, is heat resistant and can be autoclaved. The insulated section is colored to minimize iris closure when using a videoscope and contrasts with the silver color of the cutting wire for easy visibility. The device is designed to take a 0.035 inch guidewire, but any wire of lesser diameter can be used. Contrast medium can be injected through the guidewire channel using a side arm valve with the guidewire in place. The proximal part of the wire is not used for cutting. Insulation is provided to avoid contact with the endoscope, but more importantly, to avoid contact with the medial wall of the duodenum anterior to the papilla, and to avoid inadvertent cutting of a low-lying duodenal fold or of the roof of a periampullary diverticulum. Proximal insulation also encourages the endoscopist to use only the distal portion of the wire for cutting, thereby maximizing current density, and minimizing the injury of the surrounding tissues even in exceptional difficult cases as reported by the manufacturer.
Assignments were prepared in a 1:1 proportion by an independent biostatistician using a computer-generated random numbers program. Allocation was concealed using an opaque envelope system. A trainee who was not participating in the assessment of the study outcomes carried out the randomization based on the assigned study number, which allowed allocation to remain concealed from the investigators, patients and research coordinators, who completed the assessment of the clinical outcomes. It was not possible for the endoscopists to be blinded to the study group allocation following randomization, as the 2 sphincterotomes can be readily distinguished based on their appearance.
All procedures were performed by 2 experienced endoscopists (PK, GP), without the participation of trainees. Procedures were performed with the patients in the oblique left lateral position using different types of Olympus duodenoscopy. The patients were given a topical throat spray with 10% lidocaine and sedated with intravenous midazolam and meperidine or propofol, all titrated according to the age and tolerance of each patient. Bowel relaxation was achieved with intravenous administration of hyoscine butylbromide or glucagon. The patients were given continuous nasal oxygen and their hemoglobin saturation and pulse rate were monitored with pulse oximetry. Cannulation of CBD was attempted initially with the sphincterotome, then with the assistance of a hydrophilic guidewire (Jagwire, Microvasive, Boston Scientific, USA) if cannulation by sphincterotome was unsuccessful. When the cannulation with both methods was unsuccessful, precut techniques (suprapapillary fistulotomy, transpancreatic and needle-knife sphincterotomy) were used. ES was performed by a controlled cut in a stepwise fashion using short pulses of current. The length of the ES was dependent upon the indication: small for stent placement or the maximum possible for choledocholithiasis. It is a policy in our units to perform ES by completely dividing the sphincter and by extending the incision to the maximum safe length in patients with choledocholithiasis. We believe this minimizes the risk of subsequent ampullary stenosis. All ESs were performed using an Olympus electrosurgical unit (PSD 30) and a blended current. The power output was set at 45-30 W. The device was periodically assessed for proper functioning, according to the protocol of our hospitals’ biomedical services.
The primary endpoint was the assessment of post-ES bleeding, according to the risk factors for hemorrhage after ES published by Freeman (Table 1)[11], with the addition of the “zipper-cut” phenomenon in the definite risk factors for post-ES bleeding on the basis of the findings of our previous study[12].
Postsphincterotomy bleeding was classified as immediate (intraprocedural) or delayed bleeding. Immediate bleeding was defined as: “none” if no blood was seen; “trickle” if blood was evident; “oozing” if a perceptible blood stream was present; and “pulsatile” if arterial bleeding was evident. Delayed bleeding was defined as hemorrhage not evident at the time of sphincterotomy but which presented subsequently as melena or hematemesis associated with a reduction in hemoglobin. The severity of the bleeding was graded as mild: clinical or endoscopic evidence of bleeding only without blood transfusion; moderate: endoscopic evidence of bleeding requiring endoscopic therapy and blood transfusion of 4 units or less; and severe: significant bleeding requiring surgery or radiographic embolization for control of bleeding or a total blood transfusion of 5 units or more.
The secondary outcome measures included any complication occurring secondary to the ERCP procedure. Post-ERCP pancreatitis was defined as new or exacerbated abdominal pain attributable to pancreatitis, together with a need for an unplanned hospitalization or an extension of a planned hospitalization and a serum amylase at least 3 times above the upper limit of normal at 24 h after the procedure. Perforation was defined as the presence of free air on any radiographic test. Cholangitis was defined as a fever of > 38°C for more than 24 h that was thought to be due to biliary causes.
After the discharge from our endoscopic units, each patient’s clinical status was monitored by a phone call; if the patient showed clinical symptoms and signs of bleeding, including hematemesis, melena, hematochezia or hypertension, an emergency endoscopic evaluation was carried out for further management.
Clinical factors assessed to ensure equal distribution between study groups included demographic characteristics, indication for procedure, risk factors for post-ES bleeding and malfunction of any endoscopic equipment or sphincterotome.
The data were analyzed using the Statistical Package for Social Sciences (SPSS, version 13.0, Chicago, IL, USA). The continuous variable of age was examined with the Student t-test, whereas all categorical variables were analyzed with the χ2 and Fisher’s exact tests, as appropriate. Statistical significance was set at P < 0.05.
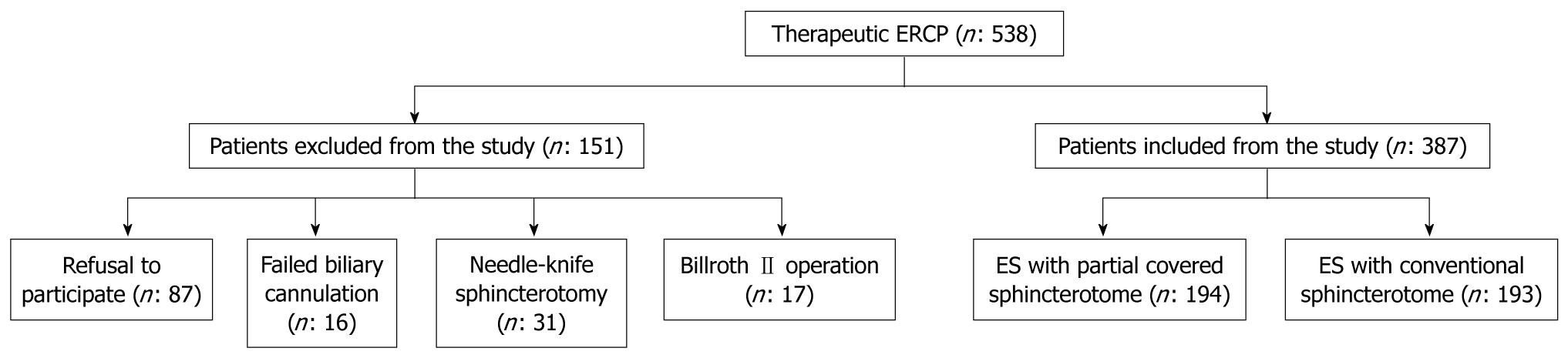
During the study period 538 patients underwent therapeutic ERCP. Of these, 151 were excluded due to refusal to participate (87), previously performed needle-knife sphincterotomy (31), previous Billroth II operation (17) and failed biliary biliary cannulation (16) (Figure 2).
A total of 387 patients were included in the study. Of these, 193 underwent ES using the uncovered sphincterotome and 194 patients underwent ES using the partially covered sphincterotome. Baseline characteristics of the patients and risk factors of post-ES hemorrhage were similar in both groups (Tables 2 and 3).
| Partially covered sphincterotome (n = 194) | Uncovered sphincterotome (n = 193) | P | |
| Sex (M/F) | 85/109 | 89/104 | 0.640 |
| Age (mean ± SD) (yr) | 69.93 ± 13.85 | 70.71 ± 13.75 | 0.590 |
| Indications for ES | 0.304 | ||
| Choledocholithiasis | 143 | 136 | 0.382 |
| Pancreatic malignancy | 20 | 26 | 0.356 |
| Biliary malignancy | 11 | 8 | 0.473 |
| Papillary cancer | 7 | 6 | 0.771 |
| SOD | 8 | 5 | 0.392 |
| Bile leak | 2 | 3 | 0.657 |
| Metastatic lymphadenopathy | 1 | 4 | 0.187 |
| Others | 2 | 5 | 0.255 |
| Associated comorbid disease | 0.561 | ||
| Coronary artery disease | 11 | 7 | 0.329 |
| Hypertension | 31 | 42 | 0.160 |
| Diabetes | 22 | 24 | 0.768 |
| Heart failure | 21 | 24 | 0.647 |
| COPD | 9 | 10 | 0.823 |
| Renal failure | 4 | 6 | 0.527 |
| Renal failure in hemodialysis | 2 | - | 0.155 |
| Others | 53 | 61 | 0.390 |
| Partially covered sphincterotome (n = 194) | Uncovered sphincterotome (n = 193) | P | |
| Definite | |||
| Anticoagulation < 3 d after ES | 3 | 5 | 0.365 |
| Bleeding during ES | 24 | 19 | 0.408 |
| Cholangitis prior to ERCP | 26 | 21 | 0.425 |
| "Zipper cut" phenomenon | 4 | 3 | 0.497 |
| Maybe | |||
| Papillary cancer | 7 | 6 | 0.771 |
| Cirrhosis | 5 | 3 | 0.359 |
| Dilated CBD | 72 | 74 | 0.865 |
| CBD stone | 143 | 136 | 0.382 |
| Periampullary diverticulum | 38 | 44 | 0.472 |
| Precut (suprapapillary) sphincterotomy with extension | 10 | 6 | 0.302 |
ES-visible hemorrhage was seen in 24 patients (12.37%) of the partially covered and in 19 patients (9.84%) of the uncovered group (P = 0.418). The endoscopic-visible bleeding patterns were: uncovered sphincterotome group [12 trickle (6.22%) and 7 oozing (3.63%)]; partially covered sphincterotome group [14 trickle (7.22%), 9 oozing (4.64%) and 1 pulsatile (0.52%)] (Table 4). Risk factors for post-ES hemorrhage in bleeders between the 2 groups were similar (Table 5). Three patients (2 in the partially covered with renal failure undergoing hemodialysis and 1 in the uncovered group) who underwent ES for choledocholithiasis and had no evidence of bleeding at the time of ES, presented with melena after 6, 7 and 9 d respectively. Bleeding from the ES site was confirmed at repeat endoscopy with raw red surfaces at the edges of the ES in 2 patients and trickling in one. The patient with trickling responded to injection treatment with 2 mL D50+E solution. Hemostasis was successfully treated by spray irrigation of D50+E solution in all patients (26/26) with trickle and in 9 of 16 patients (56.25%) with oozing. Injection of D50+E solution was required in 7 patients (43.75%) with oozing who did not respond to solution irrigation. The spurting bleeding in 1 patient was treated with injection of 3 mL D50+E solution and electrocoagulation with the tip of a polypectomy of the bleeding vessel. The volume of D50+E solution injected ranged from 2 to 5 mL (median volume 3.1 mL). Overall successful hemostasis was achieved in all patients. None of the ES-induced bleeding episodes required transfusions or repeat endoscopy for hemostasis and therefore none was classified as a significant complication. None of the patients with ES-bleeding had a platelet count less than 100 × 109/L and an international normalized ratio greater than 1.5. No patient treated with D50+E injection developed pancreatitis or cardiac complications. In addition, the incidence of pancreatitis, perforation or other complications did not differ between the 2 groups (Table 6).
| Partially covered sphincterotome (n = 26) | Uncovered sphincterotome (n = 20) | P | |
| Immediate | 24 | 19 | 0.418 |
| Trickle | 14 | 12 | 0.748 |
| Ooze | 9 | 7 | 0.965 |
| Pulsatile | 1 | - | 0.558 |
| Delayed | 2 | 1 | 0.620 |
| Partially covered sphincterotome (n = 24) | Uncovered sphincterotome (n = 19) | P | |
| Definite | |||
| Anticoagulation < 3 d after ES | 2 | 3 | 0.373 |
| Cholangitis prior to ERCP | 7 | 5 | 0.883 |
| "Zipper cut" phenomenon | 1 | 1 | 1.000 |
| Maybe | |||
| Cirrhosis | 1 | - | 0.565 |
| Dilated CBD | 12 | 14 | 0.106 |
| CBD stone | 21 | 16 | 0.590 |
| Periampullary diverticulum | 6 | 7 | 0.373 |
| Precut (suprapapillary) sphincterotomy with extension | - | 1 | 0.435 |
| Partially covered sphincterotome(n = 194) | Uncoveredsphincterotome(n = 193) | P | |
| Pancreatitis | 13 | 10 | 0.23 |
| Mild | 10 | 8 | |
| Moderate | 2 | 1 | |
| Severe | 1 | 1 | |
| Perforation | 1 | - | 0.50 |
| Cholangitis | 1 | 3 | 0.31 |
| Bleeding | 26 | 20 | 0.36 |
| Mild | 26 | 20 | |
| Moderate | - | - | |
| Severe | - | - | |
| Basket impaction | 1 | - | 0.50 |
Short-term complications of ES, including bleeding, pancreatitis, and perforation can vary widely in different circumstances, and appear to be related principally to patient-related factors and the technical skill of the endoscopists[13,14]; both technical risk factors and patient risk factors contribute to the development of post-ES bleeding. The endoscopic technique is a major factor in complications, and this is in turn related to the case volume, and presumably the skill and training of the endoscopist. Endoscopists with adequate experience by performing large volumes of ERCP, learn to initiate cutting by using a short length of wire-tissue contact and reduce inefficient expenditure of energy by identifying optimal generator settings. After the first few millimeters of cutting, if a large length of cutting wire is in contact with papillary tissue, speed can be counterproductive, leading to rapid uncontrolled acceleration of cutting and increased risk of hemorrhage because the vessels are larger in the upper half of papilla[15,16]. Also, rapid cutting is related to less hemostasis because the wire is not in contact with tissue long enough to produce an effective zone of coagulative dissipation[17]. Another factor that increases the cutting rate may be the replacement of the liquid phase contact of the wire inside the bile duct with air as cutting progresses[18]. It is our belief, as well as that of others, that sphincterotomy is most safely and precisely achieved when only a short segment of cutting wire is in contact with the papilla. High current density is maintained over a very short segment of tissue allowing sphincterotomy to be performed under precise visual control and thereby minimizing total electrical energy requirement for sphincterotomy. To avoid an uncontrolled rapid cutting (the zipper effect) a sphincterotome has been introduced in the market with its main characteristic that half the length of the cutting wire is covered with polymeric plastic.
To our knowledge, the present study is the first that compares a partially covered vs an uncovered sphincterotome on the incidence of post-ES-induced hemorrhage and other complications. With the exception of the significantly cheaper cost of the uncovered sphincterotome (160 euros vs 220 euros for the partially covered sphincterotome), we found no difference in the incidence, pattern (Table 4) and severity of post-ES bleeding between the 2 groups (Table 6). The risk factors for post-ES-induced hemorrhage of the bleeders of the 2 groups were comparable, presenting no statistical difference (Table 5). This can be explained by: performance of all procedures by 2 experienced endoscopists resulting in a controlled and safe cut; type of cutting current (blended 40-30 W); and study design (prospective). It must be emphasized that, of all the risk factors for post-ES complications, the skill of the endoscopist who carries out the procedure is the most important one in determining the outcome of the procedure. Rabenstein et al[19] demonstrated that the case volume per year, rather than the total number or ERCPs performed, is one of the most important factors influencing the complication rate. A similar observation was made in the study of Freeman et al[4] in which endoscopists from 17 institutions who performed ES at least once per week had significantly better results compared with those at 12 centers who performed ES less than once per week. Salminen et al[10] also demonstrated that the rate of severe complications of ERCP is low in experienced hands at a high-volume center, comparing favorably to corresponding complication rates of multicenter series, which further supports the worth of centralizing ERCP procedures in high-volume advanced centers.
Two patients of the partially covered sphincterotome group with renal failure undergoing hemodialysis presented delayed post-ES hemorrhage (on the 7th and 11th day, respectively). Hemodialysis has not been previously recognized as a risk factor, possibly owing to the low proportion of hemodialysis patients referred for therapeutic ERCP. However, Nelson and Freeman, in an analysis of risk factors for major post-ES bleeding in 189 patients undergoing 191 ESs, found chronic hemodialysis to be the strongest predictor of major hemorrhage following ES (4/10)[20]. Renal failure and hemodialysis are associated with a number of hemostatic defects. Platelet function is altered in uremia and is only partially corrected by hemodialysis[21,22]. The routine use of anticoagulants during hemodialysis may further increase the risk of bleeding. Both patients in our study who presented mild delayed post-ES bleeding received heparin during the hemodialysis session prior to ES and low-dose heparin during dialysis after ES. We cannot determine whether bleeding resulted from underlying uremia or the systemic anticoagulation accompanying hemodialysis. In addition, the 3 patients with delayed post-ES bleeding did not present visible hemorrhage during ES, supporting the previous observation that the presence of oozing or trickling at the time of incision does not appear to be predictive of clinically significant delayed hemorrhage[12,17].
An interesting finding of our study was that immediate (trickling or oozing) or delayed post-ES hemorrhage did nor relate to the use of non-steroidal antiinflammatory drugs (NSAIDs) and clopidogrel. Equally, a case-control study provided controlled data suggesting that antiplatelet agents did not significantly increase the risk of clinically-important bleeding related to ES[23]. However, the low prevalence of these drugs in our study limits a definite conclusion on their elective use before ES. Guidelines from the American Society for Gastrointestinal Endoscopy published in 2002 concluded that aspirin and NSAIDs given in standard doses do not appear to increase the risk of significant post-ES bleeding[24]. Also, there is no consensus on whether it is necessary to discontinue NSAIDs and other antiplatelet agents, particularly in patients with other risk factors for post-ES bleeding.
Another interesting finding of our study was that immediate (oozing) post-ES hemorrhage responded to injection treatment with D50+E solution, and overall successful hemostasis was achieved in all patients. The mechanism of action of E injection in post-ES bleeding is by exerting a local tamponade effect as well as localizing the E to cause constriction of the bleeding vessels and platelet aggregation to promote hemostasis. The addition of D50, which has high viscosity, prolongs swelling and therefore both the tamponade and E effect.
In our study we observed only 1 case of perforation in the partially covered sphincterotomy group, reflecting the experience of the endoscopists. The patient was treated conservatively with a good outcome. Duodenal perforation during ES often occurs during a rapid, poorly controlled cut of the sphincter beyond the boundaries of the intramural common bile duct. Clinically significant perforation occurs in less than 1% of ES although the risk for significant perforation may be as high as 8% in patients with small papilla or papillary stenosis[25,26]. Early diagnosis of duodenal perforation is essential for an optimum outcome, and subcutaneous emphysema may be a sensitive sign[27]. As in our case, others also reported that most duodenal perforations secondary to periampullary endoscopic interventions can be managed nonoperatively[28]. In addition, we found that endoclipping of duodenal perforation induced by ES is a safe, effective alternative to surgery treatment[29]. No statistical difference was noted in the rate of pancreatitis and cholangitis or other complications between the 2 groups (Table 6).
In conclusion, our study suggests no significant difference in post-ES hemorrhage and other complications between the partially covered and uncovered sphincterotome groups when ES is performed by experienced endoscopists. Because uncovered sphincterotome use appears to be cost-saving, further studies, which will include trainees or endoscopists with less experience, are needed to investigate the advertized theoretical beneficial role of the partially covered sphincterotome in post-ES complications.
Post-endoscopic biliary sphincterotomy (ES) bleeding is one of the most frequent post-ES complications associated with morbidity and rarely, with death. Uncontrolled cutting, known as the “zipper cut” phenomenon is the technical factor which contributes to the development of post-ES hemorrhage. In an effort to achieve a more controlled cutting and avoid the “zipper cut” phenomenon, a special sphincterotome has been manufactured having as its main characteristic half the length of the cutting wire insulated with a polymeric plastic.
There are no studies comparing a partially covered vs uncovered sphincterotome with regard to reduction in the incidence of post-ES bleeding and other complications.
The present prospective randomized study investigated whether the use of a partially covered sphincterotome with the theoretical advantage of avoidance of the “zipper cut” phenomenon might reduce the incidence of post-ES hemorrhage. No significant differences in the incidence of intraprocedural and delayed post-ES bleeding was found between the partially covered and uncovered sphincterotome group. In addition, no difference was noted in the rate of other complications.
When ES is performed by experienced endoscopists, using a partially covered or uncovered sphincterotome, no significant difference in post-ES bleeding and other complications are observed. However, further studies which will include trainees or endoscopists with less experience are needed to investigate the advertized theoretical beneficial role of the partially covered sphincterotome on post-ES complications.
Zipper cut phenomenon is a rapidly poorly controlled cutting leading to post-ES hemorrhage; a partially covered sphincterotome, with half the length of the cutting wire insulated with a polymeric plastic, has an advertized advantage of avoidance of uncontrolled cutting during ES.
The authors compared partially covered vs uncovered sphincterotome use on post-ES hemorrhage and other complications. They found that there was no significant difference between the 2 groups. The study is well designed and the data collected properly. Companies may claim that the partially covered sphincterotome has lower complications rate and they also encourage endoscopists to use it.
| 1. | Leung J, Foster E. How do we ensure that trainees learn to perform biliary sphincterotomy safely, appropriately, and effectively? Curr Gastroenterol Rep. 2008;10:163-168. |
| 2. | Freeman ML. Understanding risk factors and avoiding complications with endoscopic retrograde cholangiopancreatography. Curr Gastroenterol Rep. 2003;5:145-153. |
| 3. | Leung JW, Chan FK, Sung JJ, Chung S. Endoscopic sphincterotomy-induced hemorrhage: a study of risk factors and the role of epinephrine injection. Gastrointest Endosc. 1995;42:550-554. |
| 4. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. |
| 5. | Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417-423. |
| 6. | Barthet M, Lesavre N, Desjeux A, Gasmi M, Berthezene P, Berdah S, Viviand X, Grimaud JC. Complications of endoscopic sphincterotomy: results from a single tertiary referral center. Endoscopy. 2002;34:991-997. |
| 7. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. |
| 8. | Siegel JH, Veerappan A. Complications of endoscopic sphincterotomy. Gastrointest Endosc Clin N Am. 1991;1:93-104. |
| 9. | Ferreira LE, Fatima J, Baron TH. Clinically significant delayed postsphincterotomy bleeding: a twelve year single center experience. Minerva Gastroenterol Dietol. 2007;53:215-223. |
| 10. | Salminen P, Laine S, Gullichsen R. Severe and fatal complications after ERCP: analysis of 2555 procedures in a single experienced center. Surg Endosc. 2008;22:1965-1970. |
| 11. | Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002;56:S273-S282. |
| 12. | Katsinelos P, Kountouras J, Chatzimavroudis G, Zavos C, Paroutoglou G, Pilpilidis I, Papaziogas B. A novel technique of injection treatment for endoscopic sphincterotomy-induced hemorrhage. Endoscopy. 2007;39:631-636. |
| 13. | Ferreira LE, Baron TH. Post-sphincterotomy bleeding: who, what, when, and how. Am J Gastroenterol. 2007;102:2850-2858. |
| 14. | Freeman ML. Adverse outcomes of endoscopic retrograde cholangiopancreatography. Rev Gastroenterol Disord. 2002;2:147-168. |
| 15. | Elta GH, Barnett JL, Wille RT, Brown KA, Chey WD, Scheiman JM. Pure cut electrocautery current for sphincterotomy causes less post-procedure pancreatitis than blended current. Gastrointest Endosc. 1998;47:149-153. |
| 16. | Norton ID, Petersen BT, Bosco J, Nelson DB, Meier PB, Baron TH, Lange SM, Gostout CJ, Loeb DS, Levy MJ. A randomized trial of endoscopic biliary sphincterotomy using pure-cut versus combined cut and coagulation waveforms. Clin Gastroenterol Hepatol. 2005;3:1029-1033. |
| 17. | Wilcox CM, Canakis J, Mönkemüller KE, Bondora AW, Geels W. Patterns of bleeding after endoscopic sphincterotomy, the subsequent risk of bleeding, and the role of epinephrine injection. Am J Gastroenterol. 2004;99:244-248. |
| 18. | Ratani RS, Mills TN, Ainley CC, Swain CP. Electrophysical factors influencing endoscopic sphincterotomy. Gastrointest Endosc. 1999;49:43-52. |
| 19. | Rabenstein T, Schneider HT, Nicklas M, Ruppert T, Katalinic A, Hahn EG, Ell C. Impact of skill and experience of the endoscopist on the outcome of endoscopic sphincterotomy techniques. Gastrointest Endosc. 1999;50:628-636. |
| 20. | Nelson DB, Freeman ML. Major hemorrhage from endoscopic sphincterotomy: risk factor analysis. J Clin Gastroenterol. 1994;19:283-287. |
| 21. | Remuzzi G. Bleeding disorders in uremia: pathophysiology and treatment. Adv Nephrol Necker Hosp. 1989;18:171-186. |
| 22. | Eberst ME, Berkowitz LR. Hemostasis in renal disease: pathophysiology and management. Am J Med. 1994;96:168-179. |
| 23. | Hussain N, Alsulaiman R, Burtin P, Toubouti Y, Rahme E, Boivin JF, Barkun AN. The safety of endoscopic sphincterotomy in patients receiving antiplatelet agents: a case-control study. Aliment Pharmacol Ther. 2007;25:579-584. |
| 24. | Eisen GM, Baron TH, Dominitz JA, Faigel DO, Goldstein JL, Johanson JF, Mallery JS, Raddawi HM, Vargo JJ 2nd, Waring JP. Guideline on the management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastrointest Endosc. 2002;55:775-779. |
| 25. | Foutch PG. A prospective assessment of results for needle-knife papillotomy and standard endoscopic sphincterotomy. Gastrointest Endosc. 1995;41:25-32. |
| 26. | Leese T, Neoptolemos JP, Carr-Locke DL. Successes, failures, early complications and their management following endoscopic sphincterotomy: results in 394 consecutive patients from a single centre. Br J Surg. 1985;72:215-219. |
| 27. | Mao Z, Zhu Q, Wu W, Wang M, Li J, Lu A, Sun Y, Zheng M. Duodenal perforations after endoscopic retrograde cholangiopancreatography: experience and management. J Laparoendosc Adv Surg Tech A. 2008;18:691-695. |
| 28. | Fatima J, Baron TH, Topazian MD, Houghton SG, Iqbal CW, Ott BJ, Farley DR, Farnell MB, Sarr MG. Pancreaticobiliary and duodenal perforations after periampullary endoscopic procedures: diagnosis and management. Arch Surg. 2007;142:448-454; discussion 454-455. |
| 29. | Katsinelos P, Paroutoglou G, Papaziogas B, Beltsis A, Dimiropoulos S, Atmatzidis K. Treatment of a duodenal perforation secondary to an endoscopic sphincterotomy with clips. World J Gastroenterol. 2005;11:6232-6234. |
Peer reviewers: Tarkan Karakan, Associate Professor, Department of Gastroenterology, Gazi university, Ankara, 06500, Turkey; Dr. Ahmet Tekin, MD, Department of General Surgery, IMC Hospital, Istiklal Cad no 198, Mersin, 33100, Turkey
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH