Published online Oct 7, 2010. doi: 10.3748/wjg.v16.i37.4704
Revised: June 13, 2010
Accepted: June 20, 2010
Published online: October 7, 2010
AIM: To conduct a single-stage, combined computed tomography (CT) arterial portography (CTAP) and CT arteriography (CTA) imaging operation, we used Y-shaped sheaths with 2 valves, which allowed the insertion of 2 catheters simultaneously.
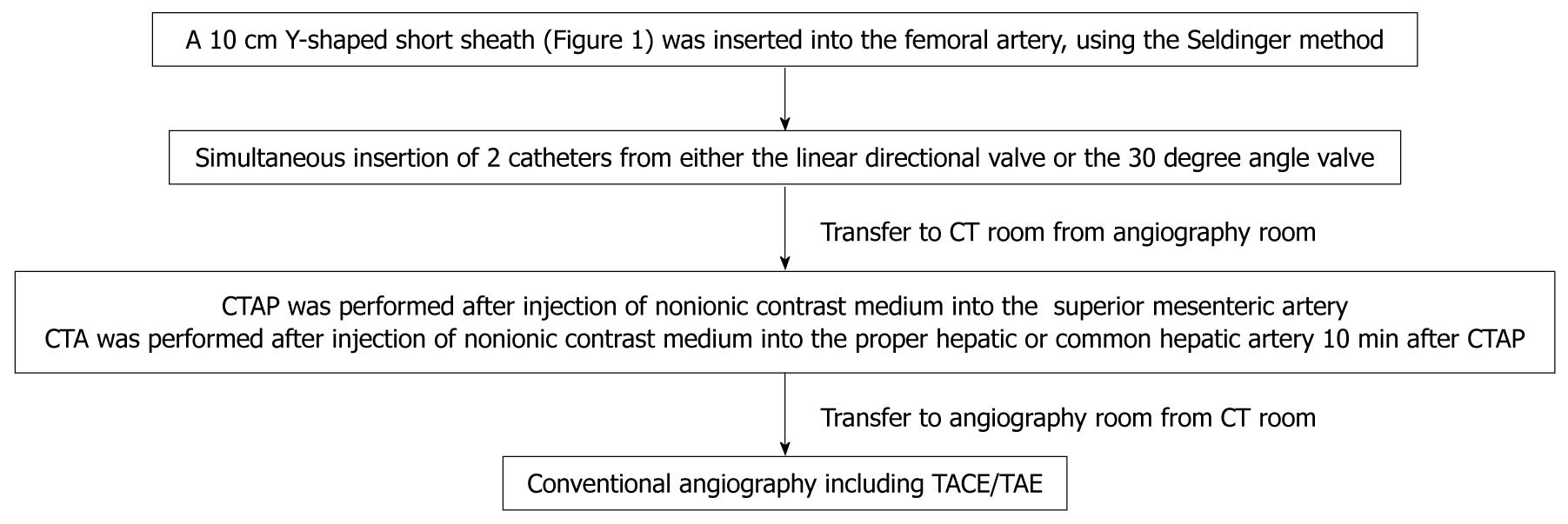
METHODS: Of 1254 patients who underwent abdominal angiography for transarterial embolization and/or intraarterial chemotherapy in our department from May 2002 to November 2009, 664 patients in whom Y-shaped sheaths with 2 valves were used underwent CT angiography using a combination of CTA and CTAP. The Seldinger method was used to insert a 10 cm Y-shaped short sheath with 2 valves into the femoral artery. Under radiographic guidance, a 3.2 French (Fr) catheter was placed in the celiac artery or proper hepatic artery, and a second 3.2 Fr catheter was then placed distal to the inferior pancreaticoduodenal artery of the superior mesenteric artery. CTAP was then performed followed by CTA 10 min later. Photographs were taken during the early and late phases of the procedure.
RESULTS: Insertion of 3.2 Fr catheters was not possible in 6 of 664 (0.9%) patients with strong curvature of the femoral artery and 4 of 664 (0.6%) patients with strong curvature of the abdominal aorta. In addition, performing CTAP and CTA as a single-stage combined intervention was not possible in 14 of 664 (2.1%) patients whose right hepatic artery originated from the superior mesenteric artery and in 8 of 664 (1.2%) patients whose left hepatic artery branched from the left gastric artery. There were no sheath-related complications such as those related to arterial dissection or hemostasis.
CONCLUSION: Although transfers to and from the CT room were necessary for anatomically variant patients, CT angiography using the Y-shaped sheath for combined CTAP and CTA was considered useful.
- Citation: Ishikawa T, Higuchi K, Kubota T, Seki KI, Honma T, Yoshida T, Nemoto T, Takeda K, Kamimura T. Usefulness of Y-shaped sheaths in CT angiography for examination of liver tumors. World J Gastroenterol 2010; 16(37): 4704-4708
- URL: https://www.wjgnet.com/1007-9327/full/v16/i37/4704.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i37.4704
Computed tomography (CT) arterial portography (CTAP) has an extremely high detectability of liver tumors such as hepatocellular carcinoma (HCC) and metastatic liver carcinoma[1-3]. However, the disadvantage of using CTAP alone is that it does not allow for evaluation of arterial blood flow or the existence of non-neoplastic low-concentration regions[3]. Therefore, the combination of CTAP with CT arteriography (CTA) would make it possible to evaluate arterial blood flow, improve the detectability of liver tumors, and reduce the occurrence of false positives[4,5]. Murakami et al[3] revealed that there was no significant difference between the sensitivity of CTAP (85%) and CTA (87%) for HCC nodules. However, they concluded that the combination of CTAP and CTA showed significantly higher sensitivity than either CTAP or CTA alone.
In facilities where interventional radiology CT systems are not available, performing the 2 procedures can be complicated and may include having to transfer patients between the angiography and CT rooms. To conduct a single-stage, combined CTAP and CTA imaging operation, we used Y-shaped sheaths with 2 valves, which allowed the insertion of 2 catheters simultaneously. In the current paper, we report on the usefulness of this approach and the problems associated with it.
CT angiography has been used in our department since May 2002. Of the 1254 patients who underwent abdominal angiography in our department from May 2002 to November 2009, 664 patients (421 men and 243 women) were selected to undergo CT angiography with a combination of CTA and CTAP using Y-shaped sheaths with 2 valves. Exclusion criteria included patients older than 80 years and patients with advanced liver disease (Child-Pugh class C), hepatic encephalopathy, refractory ascites, coagulation abnormality, and portal branch occlusion. Excluded patients in this study underwent abdominal angiography with/without CTAP only through a 5 French (Fr) single sheath. In addition, these patients were excluded from the study because the tumor lesion showed no hypervascularity and CTA was not needed.
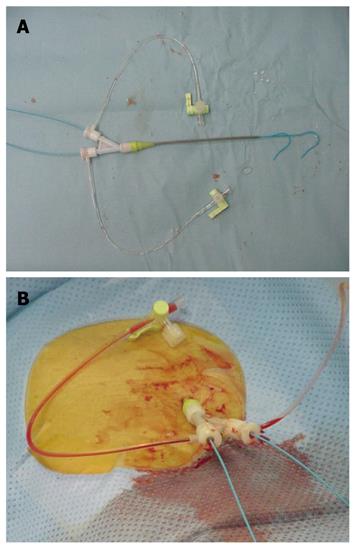
A 10 cm Y-shaped short sheath (6 Fr) with 2 valves (S1 Sheath, Terumo Clinical Supply Co., Ltd, Japan) was inserted into the right or left femoral artery, using the Seldinger method (Figure 1). Angiography then was performed. The Y-shaped sheaths with 2 valves allowed for simultaneous insertion of 2 catheters (3.2 Fr; Selecon PA catheters, Terumo Clinical Supply Co., Ltd, Japan) from either the linear directional valve or the 30 degree angle valve (Figure 1).
Shepherd hook or cobra-type 3.2 Fr catheters (Terumo Clinical Supply Co., Ltd., Japan) were used (Figure 1). The first was placed under radiographic guidance into the proper hepatic artery, and the second was placed distal to the inferior pancreaticoduodenal artery of the superior mesenteric artery. Patients then were moved to the CT room, where CTAP was performed followed by CTA 10 min later. For CTAP, 40 mL of a nonionic contrast medium 140 mg I/mL (Omnipaque 140; Daiichi Sankyo, Tokyo, Japan) was injected into the superior mesenteric artery at a speed of 2.0 mL/s. CT imaging of the entire liver was performed 30 s after the beginning of the injection of the contrast medium. For CTA, 40 mL of a 140 mg I/mL nonionic contrast medium was injected at a speed of 2.0 mL/s after insertion of a catheter into the proper hepatic or common hepatic artery. CT imaging of the entire liver was performed 10 s after the beginning of the injection of the contrast medium for phase I (early arterial phase) and 40 s later for phase II (delayed phase). CT imaging was performed at a voltage of 130 kV, a tube current of 200 mA, an X-ray beam width of 7 mm, and a table speed of 7 mm/s (Figure 2).
A total of 552 patients had HCC and 112 patients had metastatic liver cancer. The primary tumor was colon cancer in 61 cases, gastric cancer in 31 cases, and pancreatic cancer in 20 cases. Average age (± SD) was 69 ± 6 years.
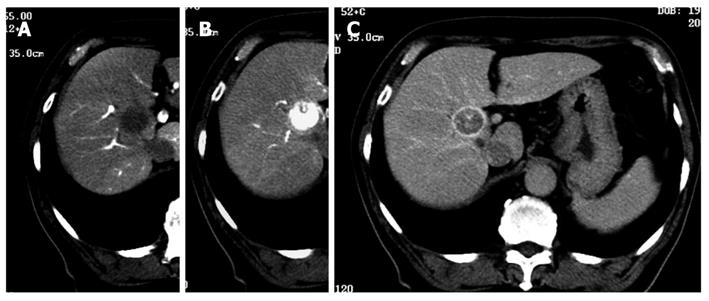
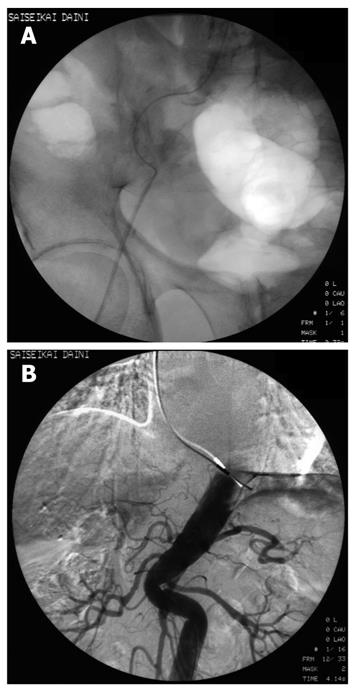
Although 3.2 Fr catheters lack torque ability, we encountered no resistance to their insertion into the 2 Y-shaped valves when a 0.025 inch guidewire was inserted into the catheters. We were able to select the superior mesenteric artery and the common hepatic artery easily. A representative case revealed that classical HCC was diagnosed simultaneously by CTAP and CTA using 3.2 Fr catheters in the Y-shaped sheath (Figure 3). Insertion presented difficulties in only 26 (4.0%) of the 664 patients; in these cases, CTAP and CTA had to be performed separately. Of the 26 patients, 21 (3.8% of 552 cases) had HCC, and 5 (4.46% of 112 cases) had metastatic liver cancer (Table 1). There was one patient in whom the presence of the first catheter impeded smooth sliding of the second catheter, rendering insertion impossible. In this case, manipulation of the second catheter caused the first catheter to move. As a result, it was impossible to perform CTAP and CTA as a single-stage combined operation. In 6 patients with strong curvature of the femoral artery and 3 patients with strong curvature of the abdominal aorta, sheath insertion was difficult and insertion of the 3.2 Fr catheter impossible (Figure 4).
| n (%) | |
| Overall | 26/664 (3.91) |
| Hepatocellular carcinoma | 21/552 (3.80) |
| Metastatic liver tumor | 5/112 (4.46) |
| Reason for insertion difficulty | |
| Sliding by friction of 2 catheters: 1 case | |
| Strong curvature of femoral arteries: 6 cases | |
| Strong curvature of abdominal aorta: 3 cases | |
| Anatomical anatomy | |
| Replaced right hepatic artery: 12 cases | |
| Left hepatic artery from the left gastric artery: 4 cases |
In addition, it was impossible to perform CTAP and CTA in 2 separate rounds in 12 of 18 patients with an anatomical anomaly (replaced right hepatic artery), in which the right hepatic artery branched from the superior mesenteric artery. In addition, CTAP and CTA as a single-stage combined operation was not possible in 6 (0.9%) of 664 patients whose left hepatic artery branched from the left gastric artery.
No arterial spasm or intimal damage was found in any of the patients, and catheter placement was feasible. Time to hemostasis was 15 min or less in all patients and was not different from time to hemostasis using 5 Fr sheaths. However, a marked subcutaneous hematoma was found in one patient with advanced hepatic cirrhosis and a marked decrease of coagulation factors.
CTA and CTAP are useful for understanding the dynamics of blood flow in the diagnosis of liver tumors[1-3]. They are particularly useful as diagnostic imaging methods for evaluating the degree of progression of HCCs. These liver tumor imaging techniques provide transcatheter angiography technology, allowing separate evaluation of hepatic arterial and portal blood flow, in combination with CT technology, resulting in an exhaustive volume of data[6].
In Japan, CT angiography has become the gold standard for the diagnosis of liver tumors since its development by Matsui et al[7,8]. CTA/CTAP have made early diagnosis of early HCC possible. In addition, these techniques have made it possible to detect microscopic HCC that are difficult to detect with conventional CT. Results provide information that is important in determining treatment selection for patients with HCC.
CTA/CTAP selectively enhances the contrast of the portal vein and the hepatic artery through the superior mesenteric artery, and the resulting CT liver images are used for evaluation. During angiography, it is necessary to insert a catheter into the superior mesenteric and hepatic arteries, or, in certain circumstances, into other blood vessels in order to perform a contrast-enhanced CT. For this reason, it is necessary to move between the angiography device and the CT device. When there is a need to perform CTA/CTAP, a catheter is placed into the superior mesenteric artery under radiographic guidance, with the patient positioned on the angiography equipment; the patient then is transferred to a stretcher for transfer via a long corridor to the CT room, where he or she is moved again, this time to the CT machine. During this laborious process, patients sometimes experience dislodgement of the catheter, which may occur after CT images are taken. Therefore, in many facilities without interventional radiology systems, the transfer from the angiography room to the CT room can pose problems when CTAP and CTA are performed together. Consequently, revising the process to include only one transfer is desirable to minimize examination time, the burden on patients, and any associated complications. Thus, various innovations, such as puncture in both inguinal regions[9], and the use of balloon catheters and coaxial systems, have been devised.
Irie et al[10] devised a process for combining CTAP and CTA by performing a single puncture of the femoral artery using balloon catheters. However, the procedure was complicated because of the possibility of inflow of contrast medium into the hepatic artery during CTAP. Therefore, expectations shifted to sheaths allowing insertion of 2 catheters, ideally through a puncture at a single location. In our hospital, the use of Y-shaped sheaths with 2 valves is used in combination with CT angiography, thereby reducing the coming and going from the CT room to the angiography room to a minimum.
To our knowledge, there have no reports on the efficacy and safety of these procedures. CTAP images are not obtained by this method because of the possibility of inflow of the contrast medium to the hepatic artery through the inferior pancreaticoduodenal artery due to the introduction of the contrast medium at the superior mesenteric artery. In addition, collateral circulation, such as a splenorenal shunt accompanying portal hypertension, can reduce the hepatopetal flow from the portal vein and attenuate contrast images of the liver. However, pure CTAP images have been obtained by placing a catheter in the superior mesenteric artery distal to the inferior pancreaticoduodenal artery, thereby avoiding inflow to the hepatic artery. Additionally, because 3.2 Fr catheters have no torque ability, it is necessary to replace the catheters in patients with strong curvature or arteriosclerosis of the abdominal aorta. Murakami et al[11] reported that the triple-lumen balloon catheter technique is useful and convenient in the serial performance of CTAP and CTA.
Although movement to and from the CT room is necessary for some patients with anatomical anomalies, CT angiography using the Y-shaped sheath with 2 valves is still considered useful. These sheaths allow for the simultaneous insertion of 2 catheters (3.2 Fr) from either the linear directional valve or the 30 degree angle valve. In addition, even though the 6 Fr catheter has a bigger diameter than the 5 Fr catheter, no sheath-related complications relating to arterial dissection or hemostasis have been observed. Because 3.2 Fr catheters have low visibility under radioscopy, we selected the celiac artery and the superior mesenteric artery by inserting a 0.025 inch guidewire into the catheter. However, because 3.2 Fr catheters also have no torque ability, some patients with strong curvature or arteriosclerosis of the abdominal aorta also need catheter replacement. In some of these patients, the issue has been resolved by the use of 25 cm long sheaths. There were no sheath-related complications such as those related to arterial dissection or hemostasis. No arterial spasm or intimal damage was found in any of the patients, and catheter placement was feasible.
There have been reports that treatment outcomes have been improved by the use of CTAP and CTA in transcatheter arterial chemoembolization[12]. Determining how to deal with anatomical anomalies of the hepatic artery during CTA remains an ongoing challenge. Of the various modalities for the diagnostic imaging of liver tumors, CTA is a complex and invasive procedure; however, it is a unique and also the most sensitive method for the assessment of blood flow in hepatocellular nodules, and it allows excellent, separate analysis of the blood supply in the hepatic artery and portal vein.
In conclusion, we report that the complicated process involved in the transfer of patients between angiography and CT rooms could be improved by the use of the Y-shaped sheath with 2 valves.
Computed tomography (CT) arterial portography (CTAP) and CT arteriography (CTA) has been widely used for the high detectability of liver tumors. However, in facilities where interventional radiology CT systems are not available, it is necessary to move twice between the angiography room and the CT room to perform CTAP and CTA. Y-shaped sheaths with 2 valves are useful to conduct a single-stage, combined CTAP and CTA imaging operation.
In this study, the usefulness of CTAP and CTA by Y-shaped sheath and the problems associated with the procedure are reported.
This study has confirmed that a Y-shaped sheath with 2 valves could avoid the complicated process involved in the transfer of patients between angiography and CT rooms.
Y-shaped sheaths in CT angiography for examination of liver tumors is useful to conduct a single-stage, combined CTAP and CTA imaging operation.
This is an interesting study. The authors present a series of 664 patients with hepatic tumors who underwent CT arteriography and arterial portography, applying a new approach using Y-shaped sheaths. The authors concluded that using this new device, the complicated process involving the transfer from the angiography CT suite could be improved.
| 1. | Soyer P, Levesque M, Elias D, Zeitoun G, Roche A. Preoperative assessment of resectability of hepatic metastases from colonic carcinoma: CT portography vs sonography and dynamic CT. AJR Am J Roentgenol. 1992;159:741-744. |
| 2. | Small WC, Mehard WB, Langmo LS, Dagher AP, Fishman EK, Heiken JP, Bernardino ME. Preoperative determination of the resectability of hepatic tumors: efficacy of CT during arterial portography. AJR Am J Roentgenol. 1993;161:319-322. |
| 3. | Murakami T, Oi H, Hori M, Kim T, Takahashi S, Tomoda K, Narumi Y, Nakamura H. Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 1997;169:131-135. |
| 4. | Chezmar JL, Bernardino ME, Kaufman SH, Nelson RC. Combined CT arterial portography and CT hepatic angiography for evaluation of the hepatic resection candidate. Work in progress. Radiology. 1993;189:407-410. |
| 5. | Irie T, Takeshita K, Wada Y, Kusano S, Terahata S, Tamai S, Hatsuse K, Aoki H, Sugiura Y. CT evaluation of hepatic tumors: comparison of CT with arterial portography, CT with infusion hepatic arteriography, and simultaneous use of both techniques. AJR Am J Roentgenol. 1995;164:1407-1412. |
| 6. | Takayasu K, Muramatsu Y, Mizuguchi Y, Ojima H. CT Imaging of early hepatocellular carcinoma and the natural outcome of hypoattenuating nodular lesions in chronic liver disease. Oncology. 2007;72 Suppl 1:83-91. |
| 7. | Matsui O, Kadoya M, Suzuki M, Inoue K, Itoh H, Ida M, Takashima T. Work in progress: dynamic sequential computed tomography during arterial portography in the detection of hepatic neoplasms. Radiology. 1983;146:721-727. |
| 8. | Matsui O, Takashima T, Kadoya M, Ida M, Suzuki M, Kitagawa K, Kamimura R, Inoue K, Konishi H, Itoh H. Dynamic computed tomography during arterial portography: the most sensitive examination for small hepatocellular carcinomas. J Comput Assist Tomogr. 1985;9:19-24. |
| 9. | Chezmar JL, Bernardino ME, Kaufman SH, Nelson RC. Combined CT arterial portography and CT hepatic angiography for evaluation of the hepatic resection candidate. Work in progress. Radiology. 1993;189:407-410. |
| 10. | Irie T, Takeshita K, Makita K, Yamauchi T, Kusano S. A one-stage method for obtaining CT during arterial portography and hepatic arteriography. Acta Radiol. 1994;35:135-137. |
| 11. | Murakami T, Oi H, Hori M, Kim T, Takahashi S, Matsushita M, Narumi Y, Nakamura H. CT arterial portography and CT arteriography with a triple-lumen balloon catheter. Acta Radiol. 1997;38:553-557. |
Peer reviewer: Beat Schnüriger, MD, University of Southern California, Keck School of Medicine, Department of Surgery, Division of Acute Care Surgery, (Trauma, Emergency Surgery and Surgical Critical Care), 1200 North State Street, Inpatient Tower (C), 5th Floor, Room C5L100, Los Angeles, CA 90033-4525, United States
S- Editor Wang YR L- Editor Cant MR E- Editor Ma WH