INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has become the most common liver disease in the United States and other developed countries[1,2]. Approximately 30% of adults in the USA have NAFLD[3], and the incidence of NAFLD in Shanghai, Guangzhou and Hong Kong of China is roughly 15%[4]. With the rise in the incidence of metabolic syndrome in recent years, the incidence of NAFLD is expected to increase in many countries[5]. In the past, NAFLD was considered to be a benign liver disease. However, recent data have shown that some patients with NAFLD can progress to nonalcoholic steatohepatitis (NASH) and then to cirrhosis and even hepatocellular carcinoma (HCC). One study showed that 20% of patients with NASH eventually progressed to cirrhosis, and among them, 8% would go on to develop a potentially fatal liver disease, such as liver cancer[2]. In the United States, about 2%-3% of adults have NASH, and approximately 3% of patients diagnosed with NAFLD develop cirrhosis or a liver-related complication[6,7]. The World Health Organization estimates that at least two million patients will develop cirrhosis following hepatic steatosis in the years to come[8]. Additionally, NAFLD can promote the development and progression of diseases in other organs. Recent studies show that NAFLD is associated with a higher prevalence of cardiovascular disease and that this association is independent of classical risk factors, such as the presence of metabolic syndrome[9,10]. NAFLD also significantly increases the risk of diabetes[11] and the development of chronic kidney disease in individuals with type 2 diabetes[12]. Thus, understanding the pathogenesis of NAFLD is of great clinical importance and is critical for the prevention and treatment of the disease.
The pathogenesis of NAFLD is described by the “two-hit” hypothesis. The “first hit” (i.e. fat accumulation) sensitizes the liver to the injurious effects of one or more additional factors, while the “second hit” leads to the development of steatohepatitis and fibrosis[13]. A variety of factors that may be involved in the development of inflammation and fibrosis may comprise the “second hit”. These factors include cytokine overproduction, hepatocyte organelle (particularly mitochondria) malfunction, lipid peroxidation, reactive oxygen species (ROS) and peroxisome proliferator-activated receptor (PPAR) dysfunction in the cell nucleus[14,15]. The exact pathogenesis of NAFLD is, to date, not well understood. The innate immune system is responsible for the rapid, initial response of the organism to potentially dangerous stressors, including pathogens, tissue injury and malignancy[16]. As pathophysiological research on NAFLD continues, a considerable amount of the current data shows that innate immune processes both within and outside the liver are involved with NAFLD[17]. In this review, we will summarize the information concerning the contributions of liver innate immune cells, such as Kupffer cells (KCs), natural killer T (NKT) cells and natural killer (NK) cells to the development of NAFLD and will discuss the possible role of their involvement in the disease. This is the first paper to comprehensively review the role of various liver innate immune cells in NAFLD.
LIVER IS AN IMPORTANT INNATE IMMUNE ORGAN
The innate immune system responds to potential attacks by pathogens against the organism and is the first line of defense against infection. Innate immunity consists of lymphocytic cells, phagocytic cells, physical barriers, chemical barrier and humoral factors. Recent evidence suggests that the liver is a major immune organ and functions predominantly in innate immunity. The liver is an important part of the body’s immune response[18,19]. From the perspective of its anatomical location, the liver is exposed to a large variety of antigens from the gastrointestinal tract, including dietary antigens, pathogens and toxins. The liver rapidly removes these harmful particles from the intestinal tract. Studies have shown that liver lymphocytes are primarily located around the portal tracts. This distribution of lymphocytes in the liver aids in the rapid removal of gastrointestinal antigens from the circulation. The liver is responsible for the biosynthesis of 80%-90% of innate proteins, including acute-phase proteins (APPs), complement factors and secreted pattern recognition receptors. APPs are the key to the innate defenses against infection and reduce tissue damage through inactivation of proteinases, which are released by pathogens and dead or dying cells. Over 35 proteins and protein fragments make up the complement system, including serum proteins, serosal proteins and cell membrane receptors. These proteins are synthesized primarily in the liver and account for about 5% of the globulin fraction of blood serum. These proteins interact with each other to protect against infection. In addition, the complement system contributes to the pathogenesis of many liver disorders, including liver fibrosis and alcoholic liver disease. The liver contains large numbers of innate immune cells, including phagocytic cells (e.g. KCs) and lymphocytic cells. Liver KCs account for about 80%-90% of the total fixed tissue macrophages in the body. In an average human liver of 1.5 kg, there are about 1 × 1010 lymphocytes[20]. The liver lymphocyte population is enriched with NK cells and NKT cells[21]. For example, in the mouse liver, NK and NKT cells account for approximately 10% and 30% of all lymphocytes; however, in the rat and human liver, NK cells account for 30%-50% of all lymphocytes[22]. In comparison with other organs, such as the spleen, the proportion of innate immune cells is much higher in the liver. The large quantities of these cells comprise the cellular basis of the liver’s innate immune response. Several studies in both experimental animal models and human clinical studies have shown that nearly all innate immune cells in the liver are involved in liver injury[23,24]. It has been proposed that an imbalance of liver cytokines (e.g. Th1 cytokine excess) produced by liver innate immune cells could be the common pathogenic mechanisms for hepatic insulin resistance and NASH[21].
Th1-PREDOMINATED CYTOKINE RESPONSE IN NAFLD
T helper cells (Th cells) are a sub-group of lymphocytes that play an important role in maximizing the capability of the immune system. Th cells can be categorized into two groups: (1) T helper 1 (Th1), which produces proinflammatory cytokines including tumor necrosis factor-β (TNF-β) and induces cellular immunity; and (2) T helper 2 (Th2), which produces anti-inflammatory cytokines and induces humoral immunity primarily due to antibody production[25]. The balance between Th1 and Th2 is believed to play an important role in the immune response against invading microbes[26]. Recently, more studies have addressed the role of proinflammatory cytokines in fatty livers. Although NASH is not classically considered to be a Th1-polarized disease, several recent studies show that an imbalance between a relative excess in proinflammatory Th1 cytokines and a relative deficiency of anti-inflammatory cytokines can affect fatty liver disease[17]. Hepatic innate immune cell activation due to Th1-predominated cytokine responses, therefore, could be one mechanism by which NAFLD occurs. Food-derived fatty acids, intestinal bacteria-derived fatty acids and adipose tissue-derived fatty acids contribute to activation of innate immune cells in the liver[27]. Fatty acids bind to toll-like receptors (TLRs) expressed on immune cells, resulting in activation of the immune system. Adipose tissue-derived cytokines may also promote activation of innate immune cells in the liver. For example, TNF-α activates the KCs by interacting with its specific receptors on these cells. In addition, the effects of different types of liver innate cells on each other also play an important role in the activation of hepatic innate cells. KCs are able to activate liver NK cells directly by interaction between retinoic acid early inducible-1 (Rae1) on KCs and natural killer group 2, member D (NKG2D) on NK cells; they also indirectly interact with liver NK cells via interleukin (IL)-12, IL-18 and TNF-α[28].
KCs
KCs reside in liver sinusoids and are derived from circulating monocytes that probably originate from bone marrow progenitors. The liver contains a large number of KCs, which constitute approximately 20% of hepatic nonparenchymal cells (hepatic nonparenchymal cells include endothelial cells, KCs, lymphocytes, hepatic stellate cells and biliary ductal cells)[19]. KCs possess scavenger receptors which are responsible for eliminating blood-borne pathogens[29] and are essential in the clearance of bacteria from the blood-stream. KCs also generate various mediators, including proinflammatory cytokines and ROS. These mediators can act either locally or systemically to mediate immune responses[16]. These immune responses directly leads to hepatocyte injury.
KCs are closely involved in the liver’s response to infection, toxins, transient ischemia and a variety of other stressors[30]. Recent studies have revealed that KCs also participate in the pathogenesis of NAFLD. For example, in a rat model of NASH induced by a high fat diet KCs are largely recruited and activated[31]. Indeed, the number of KCs seen in the liver of rats with NAFLD has been shown to be high[32]. Adachi et al[33] reported that KCs were inactivated by gadolinium chloride, and the inactivation of KCs could prevent the development of fatty liver and inflammation in rats chronically exposed to ethanol via intragastric feeding. In experimental liver transplantation, Frankenberg et al[34] observed that depletion of Kupffer cells in donor animals prevents primary nonfunction of fatty livers after transplantation and diminishes amino acid release at harvest. Meanwhile, the increased expression of the adhesive molecule Intercellular Adhesion Molecule-1 was inhibited only after transplantation, indicating that the increased proteolysis in marginal donor livers is not induced by cytokines, but is Kupffer cell-dependent. In experimental models of NASH in mice, Rivera et al[35] found that destruction of Kupffer cells can attenuate the histological appearance of hepatic steatosis, inflammation and necrosis. These results suggest that KCs contribute to the pathogenesis of NAFLD.
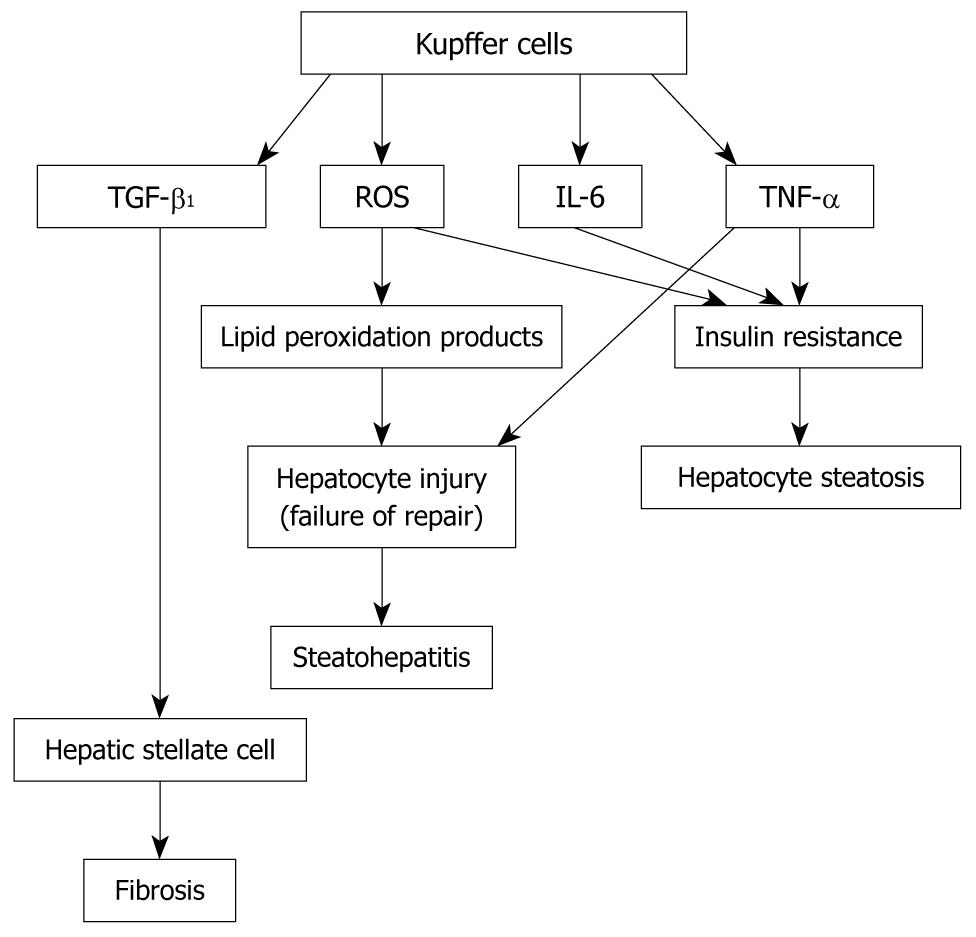
The characteristics of macrophages include plasticity and functional polarization. The macrophage phenotype has been defined at two separate polarization states, i.e. M1 and M2. M1 (i.e. classically activated macrophages) is induced by proinflammatory mediators, such as interferon-γ (IFN-γ). M1 macrophages have a high capacity to present antigen, to induce the release of large amounts of some cytokines (IL-12, IL-6, TNF-α, and IL-23) and to activate polarized Th1 responses; M1 macrophages also produce ROS. M2 (i.e. alternatively activated) macrophages respond to IL-4 and IL-13, thus promoting a Th2 response. M2 cells express high levels of the anti-inflammatory cytokines IL-10 and IL-1 decoy receptor. Recent studies show that adipose tissue macrophages from lean mice have the characteristics of the M2 phenotype, while macrophages from obese mice present the characteristics of the M1 phenotype. KCs also display great plasticity in their activation programs, ranging from the proinflammatory classical state to the anti-inflammatory alternative state[36]. It is possible that M1 or “classically activated” KCs play an important role in the development of NAFLD by producing TNF-α, IL-12, IL-6, and ROS. TNF-α is critical to the pathogenesis of NASH. Crespo et al[37] demonstrated that NASH patients with significant fibrosis exhibited increased expression of TNF-α mRNA when compared with those with minimal or non-existent fibrosis. Li et al[38] reported that treatment with anti-TNF-α antibodies can improve NAFLD induced by a high-fat diet in ob/ob mice. The mechanism of TNF-α’s effect on NAFLD may include the following: (1) TNF-α induces hepatocyte cell death; (2) TNF-α causes insulin resistance, which results in hepatocyte steatosis; and (3) TNF-α regulates KCs’s activation through an autocrine mechanism[39]. While KCs are the primary source of hepatic TNF-α, hepatic TNF-α also comes from visceral adipose tissue, especially in obese human subjects. TNF-α interacts with two specific receptors, TNF receptor 1 (p55) and TNF receptor 2 (p75). KC depletion reduces liver IL-12 expression in choline-deficient diet-induced fatty liver, suggesting that KCs are important cellular sources of liver IL-12 in NAFLD. The fact that hepatic IL-12 mRNA levels significantly increase in choline-deficient diet (CDD)-induced mice suggests that IL-12 participates in the development of NAFLD. IL-12 promotes the production of hepatic Th1-associated cytokines and is involved in hepatic NKT cell depletion. Using IL-12-deficient mice fed with CCD, however, Kremer et al[40] reported that IL-12 does not influence the progression of hepatosteatosis, suggesting an indirect role of KC-derived IL-12 in NAFLD. NAFLD patients with increased systemic IL-6 usually have a higher prevalence of inflammation and fibrosis. IL-6 causes insulin resistance locally. Local insulin resistance may be linked to systemic insulin resistance. The results from considerable investigations suggest that IL-6 is a potential mediator of insulin resistance. In contrast to its role in the liver, IL-6 is believed to be beneficial for insulin-regulated glucose metabolism in muscle. A few studies have shown that IL-6 administration alleviates fatty livers in mice, protects hepatocytes from cellular necrosis and apoptosis, ameliorates hepatic microcirculation and inhibits hepatocyte death, suggesting a beneficial effect of IL-6 on NAFLD[41,42]. The effects of IL-6 are seemingly influenced by whether it is present acutely or chronically; the latter is the setting associated with insulin resistance. KCs also generate hepatic ROS which is involved in the development of NAFLD, as demonstrated by Wei et al[43]. ROS has a causal role in multiple forms of insulin resistance, which can further promote exacerbation of oxidative stress[44]. Oxidative stress increases the release of lipid peroxidation products and cytokines, which together can trigger the liver lesions of NASH[45]. NAFLD is usually caused by two “hits”: the “first hit” is peripheral insulin resistance, which causes steatosis, while the “second hit” is caused by ROS. which induces vicious cycles that lead to inflammation[46]. Therefore, ROS plays an important role in the conversion of simple hepatic steatosis to NASH. However, the molecular mechanism of ROS in NASH formation is still unclear. ROS can directly activate inhibitor of nuclear factor-κB (NF-κB) kinase (IKK) and Jun N-terminal kinase (JNK). JNK can stimulate the transcription of inflammatory target genes through the activation of protein-1, while IKK can stimulate the transcription of inflammatory target genes through the activation of NF-κB[17]. The activated KCs secrete transforming growth factor (TGF)-β1[47], which is one of the key fibrogenic factors of NASH. Other nonparenchymal liver cells, such as hepatic stellate cells and sinusoidal endothelial cells, also produce TGF-β1[48]. All these data suggest that KCs may be implicated in the development of NAFLD via multiple pathways (Figure 1).
Figure 1 Mechanisms for the role of Kupffer cells in the development of nonalcoholic fatty liver disease.
(1) Activated Kupffer cells (KCs) produce reactive oxygen species (ROS), interleukin-6 and tumor necrosis factor-α (TNF-α), which induce insulin resistance, leading to hepatocyte steatosis; (2) Activated KC-derived ROS causes lipid peroxidation production. Lipid peroxidation products and KC-derived TNF-α result in hepatocyte injury. Failure to repair hepatocytes after injury promotes the development of steatohepatitis; and (3) Activated KCs produce transforming growth factor-β1, which activates hepatic stellate cells. Activated hepatic stellate cells produce large quantities of extracellular matrix, leading to fibrosis.
The mechanism of KC activation in the development of NAFLD remains unclear. A number of studies have suggested that a gut-derived endotoxin could play a role in the pathogenesis of insulin resistance and NAFLD. In patients with NAFLD, the increase of gut permeability may induce bacterial overgrowth in the small intestine and lead to endotoxin production[49]. Gut-derived endotoxin can bind to TLR4 to induce KC activation[50]. Endotoxin can also activate the complement system. The activated complement system releases the anaphylatoxins C3a and C5a, which activate KCs through their receptors, C3aR and C5aR[51]. Visceral adiposity tissue in patients with NAFLD produces numerous proinflammatory cytokines, including TNF-α and IL-6, and these cytokines are involved in the recruitment and activation of liver KCs[52]. Therefore, we speculate that gut-derived endotoxin and visceral adiposity tissue-derived proinflammatory cytokines may contribute to M1 phenotype activation of KCs. While PPARδ plays a critical role in alternative phenotype activation of KCs, the KCs prepared from PPARδ-deficient mice (PPARδ-/-) are unable to maintain the alternative phenotype[36]. NAFLD results in a high level of serum fatty acids. Fatty acids and their metabolites are ligands for the PPARs[53]. It is possible that binding of fatty acid to PPARδ on KCs can result in the alternative phenotype activation of KCs. Effective mechanism of PPARs agonists on NAFLD may be involved in the alternative phenotype activation of KCs.
NKT CELL
NKT cells are a unique subset of lymphocytes that express NK cell markers, such as CD161 and CD94, as well as a T-cell receptor (TCR) α/β[54]. NKT cells differentiate from NK cells and are different from NK cells in that NKT cells have a restricted repertoire and have TCR. NKT cells can be categorized into three subclasses: class I (classical, Vα14-Jα18+TCR/CD1-dependent); class II (non-classical, all other CD1d-dependent T cells); and class III, i.e. NKT-like cells (CD1d-independent NK1.1+ T cells)[51]. Evidence suggests that NKT cells develop in the thymus and migrate to peripheral organs, including the spleen and the liver. Reconstitution of adult thymectomized irradiated mice with syngeneic bone marrow cells gives rise to NKT cells in the recipient organs, including the liver[55]. Hence, liver NKT cells may originate from both the thymus and bone marrow. Because NKT cells have numerous functions related to innate and adaptive immunity, NKT cells are critical in the immune response against viral infections and malaria, as well as in tumor immunity and autoimmune diseases[54,56]. The current studies show that NKT cells modulate inflammatory and fibrogenic responses in various liver diseases, such as viral hepatitis, various autoimmune liver disease, metabolic liver disease and hepatic malignant tumor[57].
In recent years, there has been increasing evidence of NKT cell population or function abnormalities in NAFLD patients. Observation suggests an inverse correlation between hepatic NKT cell populations and the accumulation of hepatic lipid. Guebre-Xabier et al[58] showed that NKT cells were selectively reduced in the fatty livers of obese, leptin-deficient ob/ob mice. This reduction was also confirmed in different models of diet-induced hepatic steatosis[59]. Utilizing biopsies from patients with mild to severe hepatic steatosis, Kremer et al[40] found that the NKT cell population in the human liver decreased when hepatosteatosis was moderate to severe. Xu and colleagues[57] also demonstrated a reduction in the numbers of peripheral NKT cells in patients with NAFLD. Adoptive inoculation of a relatively small aliquot (1 × 106 cells) of NKT cells into ob/ob mice leads to a significant reduction in hepatic fat content. Within 12 d of transplantation, an estimated 12% of hepatic fat content in NKT cell-transplanted mice was decreased, as compared with the control-treated
ob/ob mice, and resulted in a shift from a mixed microvesicular-macrovesicular steatosis pattern to a microvesicular steatosis pattern[60]. These results further suggest that there is a negative correlation between hepatic NKT cell populations and the severity of NAFLD and that enhancing the activity of NKT cells may become a therapeutic tool for the treatment of NASH.
It remains unclear how hepatic steatosis reduces hepatic and peripheral NKT cells. The fact that apoptosis of hepatic NKT cells is significantly increased in ob/ob mice suggests that apoptosis of this cell line may be critical in NAFLD. Deng found that a high-fat diet triggers an accumulation of immature myeloid cells in B6 mice livers, e.g. CD11b+Ly6ChiLy6G- cells, and that these cells can induce NKT cell apoptosis[61]. In addition, IL-12 can promote NKT cell death. A study of CDD-induced mice fatty liver shows that up to 98% of the hepatic NKT cell population in wild-type mice was depleted after 20 wk. However, hepatic NKT cells in IL-12-deficient (IL-12-/-) mice was preserved. Further observations show that KC depletion blunts hepatic IL-12 and leads to a complete repopulation of hepatic NKT cells in CDD-induced mice fatty liver. Accordingly, KC-derived IL-12 may be involved in the loss of NKT cells in fatty livers[40]. Additionally, hepatic lipid accumulation might be directly responsible for the reduced NKT cell population in NAFLD.
Activated NKT cells express FAS ligand on their cell surface. FAS ligand can interact with FAS on hepatocytes, resulting in hepatocyte apoptosis. NKT cells can also release perforin and granzyme from cytoplasmic granules which can destroy hepatocyte cells[62]. Based on this finding, reductions in NKT cell accumulation in the liver could potentially become a therapeutic strategy to minimize liver damage in patients with NASH.
Hepatic NKT cells modulate liver injury primarily by balancing local production of Th1 and Th2 cytokines. NKT cells can generate a lot of Th1 cytokines, such as IFN-γ, TNF-α and Th2 cytokines, such as IL-4, IL-10 and IL-13[63]. Hepatic NKT cell-derived Th2 cytokine quantity may be greater than NKT cell-derived Th1 cytokine quantity. Therefore, hepatic NKT cell depletion or reduction may lead to Th1 polarization of hepatic cytokine production, increasing TNF-α, IL-12 and IFN-γ[64]. The Th1 cytokine polarization caused by hepatic NKT cell reductions appears to play an important role in the pathogenesis of NASH. Therefore, the net effect of hepatic NKT cell reductions may be harmful for NASH patients.
However, in contrast to the past studies, recent studies support the concept that NKT cells accumulate in progressive fatty liver disease. Tajiri et al[65] noted that hepatic CD3+CD56+ NKT cells increased as NAFLD progressed. Most recently, Syn et al[66] reported that NKT cells expressing CD56/CD3 or CD57/CD3 were largely accumulated in the livers of patients with progressive and chronic diseases, suggesting that NKT cells contribute to the progression of NASH into cirrhosis. Different study results in the NKT cell population of livers with NAFLD may be due to multiple factors, including different fatty liver models used and the different NKT cell subtypes checked. The different study results suggest a complex role of NKT cells in the development of NAFLD.
NK CELL
NK cells are an important component of the innate immune response against many viruses. NK cells have the ability to lyse virus-infected cells and to secrete cytokines. Cytokines can inhibit viral replication and activate and recruit cells of the adaptive immune response[67]. NK cells play a critical role in bridging the innate and adaptive arms of the immune response[68]. NK cells originate from the bone marrow; undergo a complex maturation process, which leads to the acquisition of their effector functions; and then redistribute from the bone marrow and lymph nodes to blood, spleen, liver and lung[69]. NK cells are abundant in the liver and are relatively rare in peripheral lymphoid organs. Hepatic NK cells are large granular cells in the liver sinusoids and were originally termed Pit cells. Many studies have shown that liver NK cells may be involved in the pathogenesis of liver injury, fibrosis and regeneration[70-72]. Recent findings revealed that NK cells may also participate in the development of NAFLD. Obesity is the most common cause of NAFLD. By determining the cytotoxic activity of peripheral blood NK cells, Lamas et al[73] found that rats with diet-induced overweight had significantly lower NK cytotoxic activity compared with control rats. O’Shea et al[74] also demonstrated that obese human subjects had significantly lower circulating NK (CD56+CD3-) cells (7.6% of all lymphocytes) when compared with lean healthy controls (16.6% of all lymphocytes). In addition, the cytotoxic function of NK cells was significantly lower in obese human subjects compared with lean healthy controls (30% vs 42% tumor cells lysed). However, Kahraman and colleagues[75] recently reported that hepatic NK cells were increased in patients with NASH, while only a few of these cells were found in patients with NAFLD, and almost no NK cells were found in healthy controls. It is possible that there is a difference in the NK cell population found in peripheral blood and that found in the liver of patients with NAFLD.
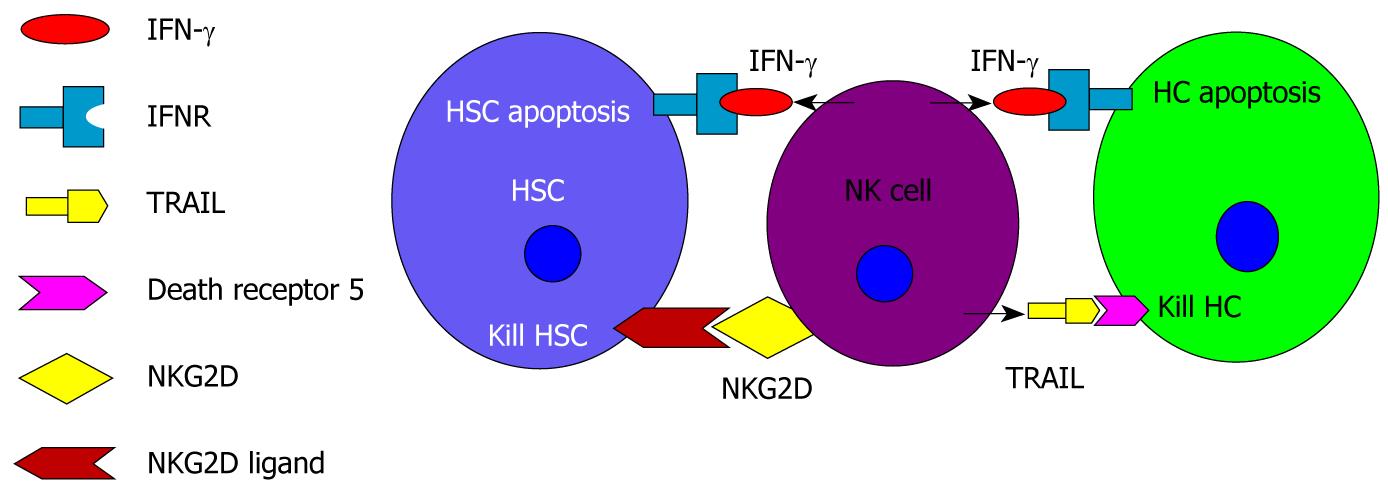
Hepatic NK cells may have two different roles in the pathogenesis of NAFLD (Figure 2). First, a recent study on liver fibrosis in mice suggests that NK cells have an anti-fibrotic effect[76]. The mechanisms of NK cells on anti-fibrosis may be two-fold. NK cells can directly kill early activated hepatic stellate cells (HSCs), which are the principal fibrogenic cell type in the liver. Activation of the HSC is the central event in hepatic fibrosis[77-79]. Activated HSCs can produce a great deal of extracellular matrix, which leads to hepatic fibrosis. Second, hepatic NK cells release IFN-γ, inducing HSC cell cycle arrest and apoptosis[80]. Of immune cells in the liver, NK cells are the primary producers of IFN-γ[81]. A recent study showed that IFN-γ is effective in inhibiting hepatic fibrosis induced by intraperitoneal injections of dimethylnitrosamine in nonobese diabetic mice[82]. In addition to their anti-fibrotic effect, NK cells may also protect hepatocytes from NASH injury. Kahraman et al[75] reported that mRNAs expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was significantly enhanced in NASH livers. Activated NK cells are able to kill hepatocytes and cholangiocytes via TRAIL. Because hepatic apoptosis is one of the prominent features of liver injury during the pathogenesis of NASH[83], IFN-γ-induced hepatocyte apoptosis in response to activation of NK cells seems to be a primary mechanism explaining liver injury in this disease. IL-12 and IL-18 can induce IFN-γ production and the secretion of NK cells[84]. Many recent studies have identified the critical role of IL-18 as a potent IFN-γ inducer in NK cells[85].
Figure 2 Potential roles of hepatic natural killer cells in the development of nonalcoholic fatty liver disease.
Hepatic natural killer (NK) cells may have two different roles in the pathogenesis of nonalcoholic fatty liver disease. (1) NK cells have anti-fibrotic effects. NK cells release interferon-γ (IFN-γ), which combines with its receptor to induce hepatic stellate cell (HSC) apoptosis. Early activated HSCs express increased levels of natural killer group 2, member D (NKG2D). NK cells can kill early activated HSCs by binding NKG2D on NK cells with NKG2D ligand on HSCs; and (2) NK cells induce hepatocyte injury. NK cell derived IFN-γ results in hepatocyte apoptosis. NK cell derived-tumor necrosis factor-related apoptosis-inducing ligand combines with its death receptor 5 to kill hepatocytes. HC: Hepatocyte; IFNR: Interferon-γ receptor; TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand.
Cytokines are involved in NASH via modulation of the activation of NK cells. IL-18 is the most important KC-derived cytokine, which in turn promotes NK cell activation. IL-12 is another cytokine that activates NK cells. Accumulation of IL-12 or IL-18 in the liver can stimulate the local expansion of cytotoxic NK cell subpopulations, which produce large amounts of IFN-γ within the hepatic microenvironment[30]. IL-15 is an important cytokine that maintains NK cell activation. Numerous in vitro and in vivo studies have shown that IL-15 plays a critical role in the regulation of development, survival and function of the NK cell lineage[86]. IL-15 prevents NK cells from undergoing apoptosis[87]. In mice lacking IL-15, the numbers of NK cells are found to be severely decreased[88]. However, IL-10 can suppress the activation of the NK cells. NK cells can also be activated through expression of the NKG2D ligand by macrophages or tumor cells and its interaction with NKG2D[89]. The increased level of NKG2D ligands in KCs activates NK cells and induces hepatocyte damage. Likewise, because NKG2D ligands are expressed in HCC and cholangiocarcinoma, the tumor cells could be eradicated by this specific anti-tumor immune response[90].
DYSLIPIDAEMIA, IMMUNE AND INSULIN RESISTANCE
The incidence of dyslipidemia is high in NAFLD patients. Dyslipidemia includes hypertriglyceridemia, hypercholesterolemia and abnormal increases in low-density lipoprotein or decreases in high-density lipoprotein cholesterol. Hypertriglyceridemia is the most common dyslipidemia in NAFLD patients. Triglycerides hydrolyze to form fatty acids, and many studies have found that fatty acids have immunologic effects[91]. Fatty acids can drive macrophages to reside in adipose tissue, where they can recruit more macrophages from the circulation. Macrophages that “reside” in tissue in this manner can produce high levels of TNF-α, which may worsen the insulin resistance. In addition, excessive fat or fatty acids may also affect insulin resistance through the following mechanisms: (1) Stimulation of the IKK and JNK signaling pathway[17]. IKK and JNK can cause insulin resistance by promoting aberrant serine phosphorylation of insulin receptor substrates 1 (IRS-1) and IRS-2, which in turn inhibit insulin receptor signaling. Fatty acids combined with TLR activate IKK and JNK downstream, leading to insulin resistance; (2) Activation of protein kinase C (PKC). Obese diabetic rats have insulin resistance and likely also have elevated free fatty acids (FFAs). Data shows that hepatic PKC activity is greater in obese diabetic rats than in lean rats[92], suggesting that FFAs induce hepatic insulin resistance through the activation of PKC. PKC causes insulin resistance by increasing aberrant serine phosphorylation of IRS-1 and IRS-2 and by activation of the IKK and JNK signaling pathways[17]; (3) Formation of ROS. Oxidation of fatty acids generates ROS, which can activate IKK and JNK[93] to induce insulin resistance; and (4) Endoplasmic reticulum (ER) stress. Excessive fat can promote ER stress. which can increase the JNK-dependent serine phosphorylation of IRS-1 to inhibit insulin receptor signaling[94].
CONCLUSION
NAFLD has become a very common liver disease world-wide. However, the pathogenesis of NAFLD remains unclear. In recent years, hepatologists have studied the roles of liver innate immune cells in the development of NAFLD. However, to our best knowledge, no paper has provided a comprehensive review regarding the roles of liver innate immune cells in the development of NAFLD. Therefore, we reviewed prior studies and provide a holistic framework concerning the relationship between liver innate immune cells and NAFLD. The liver contains a large number of innate immune cells, which are associated with the pathogenesis of NAFLD. Serum fatty acids, adipose tissue-derived cytokines and gut-derived endotoxin could affect liver innate immune cells, and different types of liver innate immune cells affect each other, together leading to the functional abnormalities seen in fatty liver diseases. The Th1 cytokine excessive production in NAFLD results in hepatic insulin resistance and NASH. Activated KC-derived ROS also plays an important role in the development of NAFLD. Additionally, activated NKT cells can directly induce hepatocyte injury. Further studies on the effects and mechanisms of liver innate immune cells in NAFLD will help us better understand the pathogenesis of NAFLD and identify novel targets for the prevention and treatment of NAFLD.
Peer reviewers: Satoru Kakizaki, MD, PhD, Assistant Professor, Department of Medicine and Molecular Science, Gunma University, Graduate School of Medicine, 3-39-15 Showa-machi, Maebashi, Gunma 371-8511, Japan; Dr. Richard A Rippe, Department of Medicine, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7038, United States; MH Ahmed, MD, PhD, Chemical Pathology Department, Southampton University Hospital NHS trust, Mail point 6, Level D, South Academic Block, Southampton SO16 6YD, United Kingdom
S- Editor Wang JL L- Editor Ma JY E- Editor Lin YP