Published online Sep 21, 2010. doi: 10.3748/wjg.v16.i35.4400
Revised: April 19, 2010
Accepted: April 26, 2010
Published online: September 21, 2010
AIM: To analyze the efficacy and safety of a combination therapy of pegylated interferon (PEG-IFN) α-2b plus ribavirin (RBV) in older Japanese patients (65 years or older) infected with hepatitis C virus (HCV).
METHODS: This multicenter study included 938 patients with HCV genotype 1 who received 1.5 μg/kg per week PEG-IFN α-2b plus RBV 600-1000 mg/d for 48 wk and 313 HCV genotype 2 patients who received this treatment for 24 wk.
RESULTS: At 24 wk after the end of combination therapy, the overall sustained virological response (SVR) for genotypes 1 and 2 were 40.7% and 79.6%, respectively. The SVR rate decreased significantly with age in each genotype, and was markedly reduced in genotype 1 (P < 0.001). Moreover, the SVR was significantly higher in patients with genotype 1 who were less than 65 years (47.3% of 685) than in those 65 years or older (22.9% of 253) (P < 0.001) and was higher in patients with genotype 2 who were less than 65 years (82.9% of 252) than in those 65 years or older (65.6% of 61) (P = 0.004). When patients received a dosage at least 80% or more of the target dosage of PEG-IFN α-2b and 60% or more of the target dosage of RBV, the SVR rate significantly increased to 66.5% in patients less than 65 years and to 45.2% in those 65 years or older (P < 0.001). Adverse effects resulted in treatment discontinuation more often in patients with genotype 1 (14.4%) than in patients with genotype 2 (7.3%), especially by patients 65 years or older (24.1%).
CONCLUSION: PEG-IFN α-2b plus RBV treatment was effective in chronic hepatitis C patients 65 years or older who completed treatment with at least the minimum acceptable treatment dosage.
- Citation: Kainuma M, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Tanabe Y, Satoh T, Maruyama T, Nakamuta M, Kotoh K, Azuma K, Shimono J, Shimoda S, Hayashi J, Group TKULDS. Pegylated interferon α-2b plus ribavirin for older patients with chronic hepatitis C. World J Gastroenterol 2010; 16(35): 4400-4409
- URL: https://www.wjgnet.com/1007-9327/full/v16/i35/4400.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i35.4400
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease, affecting 170 million individuals worldwide[1]. It is well known that patients with chronic hepatitis C eventually develop hepatocellular carcinoma (HCC)[2]. Previous studies have made clear that interferon (IFN) treatment is effective for eliminating HCV[3,4] and that it significantly reduces the progression of liver fibrosis and the risk of HCC[5,6]. Antiviral treatment for chronic hepatitis C has greatly improved, and the combination treatment of pegylated (PEG)-IFN α-2b plus ribavirin (RBV) has been approved and recommended in Japan since 2004, as the first choice for chronic hepatitis C. This combination treatment attained a sustained virological response (SVR) rate of 50%-60% for genotype 1 in the United States and Europe[7]. However, SVR was relatively low (42.4%) in Japan[8], where chronic hepatitis C patients are older, indicating that older patients did not respond well to IFN treatment[9]. Moreover, the combination treatment was associated with more adverse effects than IFN monotherapy[7,10]. Older patients who have decreased cardiovascular, pulmonary and renal function have a higher incidence of adverse effects than younger patients. The rate of discontinuation due to adverse effects was reported to be significantly higher in patients aged 65 years or more than in those less than 65 years[11]. Older patients with HCV infection are at risk for progressive liver disease. It was reported that clearance of HCV after IFN therapy significantly reduces the incidence of HCC and death in older chronic hepatitis C patients[6,12]. Ikeda et al[13] demonstrated that IFN treatment is needed for 65-70-year-old patients with chronic hepatitis C to prevent the occurrence of HCC. We also consider older patients to be acceptable candidates for antiviral treatment to prevent the development of HCC, and previously reported that monotherapy with natural IFN α was not effective in older patients[9]. Therefore, in an attempt to ameliorate these problems, we decided to treat older patients with a combination of PEG-IFN plus RBV therapy.
Little data concerning the response and safety of this combination treatment in a large number of older patients with chronic HCV infection has been published. A multicenter study of the efficacy and safety of antiviral treatments for Japanese patients with chronic liver disease, the Kyushu University Liver Disease Study (KULDS), was launched in 2003[8,14]. The present prospective study was carried out to analyze the efficacy and safety of the combination treatment of PEG-IFN α-2b plus RBV in older patients.
Treatment of chronic hepatitis C with a combination of PEG-IFN α-2b plus RBV was accepted by the Japanese Ministry of Health in October, 2004. We used this combination treatment from December 2004 to July 2008, and enrolled chronic hepatitis C patients with exclusion criteria which included: (1) clinical or biochemical evidence of hepatic decompensation, advanced cirrhosis identified by bleeding, high-risk esophageal varices, history of gastrointestinal bleeding, ascites, encephalopathy, or HCC; (2) hemoglobin level < 11.5 g/L, white blood cell count < 3 × 109/L, and platelet count < 50 × 109/L; (3) concomitant liver disease other than hepatitis C (hepatitis B surface antigen positive or HIV positive); (4) excessive active alcohol consumption > 60 g/d or drug abuse; (5) severe psychiatric disease; or (6) antiviral or corticosteroid treatment within 12 mo prior to enrollment. Patients who fulfilled the above criteria were recruited at Kyushu University Hospital and 32 affiliated hospitals in the northern Kyushu area of Japan. We have treated 2270 Japanese patients aged 18 years or older with PEG-IFN α-2b plus RBV. All patients who were positive for both antibody to HCV and HCV RNA for over 6 mo were enrolled in KULDS. Three months before the start of treatment and every 3 mo during the treatment period, each patient was tested for α-fetoprotein (AFP) and had an abdominal ultrasonographic examination. If an abnormal AFP level of 40 ng/mL and/or focal lesions on ultrasonographic examination were found at any testing, further testing for HCC was carried out, which included dynamic computed tomography, and angiography. Patients confirmed to have HCC within 3 mo after starting treatment were excluded from this study (n = 14). Of 2270 patients, 1021 were currently under combination treatment or we were not yet able to judge the effect of the combination treatment. This left the data of 1251 patients (938 with genotype 1 and 313 with genotype 2) available for analysis.
Informed consent was obtained from all patients before enrollment in this study. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and the International Conference on Harmonization of guidelines for good clinical practice.
Table 1 (genotype 1) and Table 2 (genotype 2) show the baseline characteristics of the enrolled patients, who were further classified into four groups according to age and genotype status: group A, genotype 1 aged less than 65 years (n = 685); group B, genotype 1 aged 65 years or older (n = 253); group C, genotype 2 aged less than 65 years (n = 252); and group D, genotype 2 aged 65 or older (n = 61). In group B, body mass index, prior combined IFN plus RBV treatment, alanine aminotransferase, albumin, white blood cell count, hemoglobin, platelet count, and creatinine clearance calculated using the Modification of Diet in Renal Disease equation[15] were significantly lower than in group A (P < 0.010). In group D, albumin, hemoglobin, platelet count, creatinine clearance and serum HCV RNA level were significantly lower than in group C (P < 0.010). The percentage of patients with platelet counts below 10 × 1010/L was significantly higher in group B (36 of 253, 14.2%) than in group A (56 of 685, 8.2%) (P = 0.006), however, there was no significant difference between group C (16 of 252, 6.3%) and group D (7 of 61, 11.5%).
| Group A (age < 65 yr) (n = 685) | Group B (age≥65 yr) (n = 253) | P-value | |
| Age (yr) | 53.1 ± 8.9 | 68.6 ± 3.1 | < 0.001 |
| Male/female | 374/311 | 122/131 | 0.090 |
| Body mass index (kg/m2) | 23.7 ± 3.3 | 22.8 ± 2.7 | < 0.001 |
| Prior IFN monotherapy, n (%) | 163 (23.8) | 76 (30.0) | 0.052 |
| Prior combined IFN plus RBV treatment, n (%) | 51 (7.4) | 20 (7.9) | < 0.001 |
| Alanine aminotransferase (IU/L) | 80.2 ± 62.0 | 67.9 ± 46.6 | 0.004 |
| γ-glutamyltranspeptidase (IU/L) | 60.2 ± 56.6 | 57.1 ± 49.2 | 0.708 |
| Albumin (g/dL) | 4.1 ± 0.4 | 4.0 ± 0.4 | < 0.001 |
| White blood cell count (/mm3) | 5200.0 ± 1476.7 | 4756.3 ± 1458.9 | < 0.001 |
| Hemoglobin (g/dL) | 14.1 ± 1.4 | 13. 5 ± 1.4 | < 0.001 |
| Platelet count (109/L) | 16.6 ± 5.3 | 15.0 ± 5.2 | < 0.001 |
| Creatinine (mg/dL) | 0.7 ± 0.6 | 0.8 ± 1.4 | 0.107 |
| Creatinine clearance (mL/min) | 105.5 ± 28.7 | 75.8 ± 17.5 | < 0.001 |
| Serum HCV-RNA level (kIU/mL) | 1776.1 ± 1500.0 | 1986.9 ± 1604.5 | 0.125 |
| Histological fibrosis | 0.008 | ||
| F0/F1/F2/F3/F4 | 36/155/121/61/30 | 9/46/49/31/17 |
| Group C (age < 65 yr) (n = 252) | Group D (age≥65 yr) (n = 61) | P-value | |
| Age (yr) | 47.7 ± 10.4 | 69.2 ± 3.4 | < 0.001 |
| Male/female | 124/128 | 28/33 | 0.671 |
| Body mass index (kg/m2) | 23.1 ± 3.5 | 22.8 ± 2.9 | 0.577 |
| Prior IFN monotherapy, n (%) | 47 (18.7) | 16 (26.2) | < 0.001 |
| Prior combined IFN plus RBV treatment, n (%) | 5 (2.0) | 4 (6.6) | 0.056 |
| Alanine aminotransferase (IU/L) | 79.9 ± 78.7 | 68.9 ± 52.9 | 0.821 |
| γ-glutamyltranspeptidase (IU/L) | 55.8 ± 64.7 | 44.3 ± 34.7 | 0.937 |
| Albumin (g/dL) | 4.2 ± 0.4 | 3.9 ± 0.5 | < 0.001 |
| White blood cell count (/mm3) | 5276.3 ± 1636.3 | 4958.0 ± 1495.6 | 0.005 |
| Hemoglobin (g/dL) | 14.1 ± 1.4 | 13.4 ± 1.3 | < 0.001 |
| Platelet count (109/L) | 18.9 ± 6.3 | 15.6 ± 4.7 | < 0.001 |
| Creatinine (mg/dL) | 0.8 ± 1.5 | 0.7 ± 0.2 | 0.581 |
| Creatinine clearance (mL/min) | 112.1 ± 31.4 | 74.6 ± 17.2 | < 0.001 |
| Serum HCV-RNA level (kIU/mL) | 1588.3 ± 1628.7 | 1195.4 ± 1645.5 | 0.038 |
| Histological fibrosis | < 0.001 | ||
| F0/F1/F2/F3/F4 | 30/77/39/10/10 | 1/21/9/2/12 |
Liver biopsy was performed in 555 patients (59.2%) with genotype 1 and 209 patients (66.8%) with genotype 2. The other patients refused liver biopsy. Fibrosis was staged on a 0-4 scale as follows: F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = portal fibrosis with few septa, F3 = numerous septa without cirrhosis, F4 = cirrhosis. Liver fibrosis was more advanced in group B than in group A and was more advanced in group D than in group C (P = 0.008, P < 0.001, respectively).
All patients were treated with a weight-based, 1.5 μg/kg weekly dose of subcutaneous PEG-IFN α-2b (PegIntron, Schering-Plough, Osaka, Japan), in combination with RBV (Rebetol, Schering-Plough), which was given orally at a daily dose of 600-1000 mg based on body weight (600 mg for patients weighing less than 60 kg, 800 mg for those weighing 60-80 kg, and 1000 mg for those weighing 80 kg or over). The length of treatment was 48 wk for patients with HCV genotype 1 and 24 wk for patients with genotype 2. The above duration and dosage are those approved by the Japanese Ministry of Health, Labor and Welfare. Patients were considered to have RBV-induced anemia if the hemoglobin level decreased to less than 100 g/L. In such cases, a reduction in the dose of RBV was required. Patients aged 65 years or older had a significantly higher frequency of RBV dose reduction during the treatment period than those aged less than 65 years old (HCV genotype 1: group A vs group B, 41.2% vs 49.0%, P = 0.032, genotype 2: group C vs group D, 28.6% vs 54.1%, P < 0.001). Some patients also had PEG-IFN α-2b-induced psychological adverse effects or a decrease in white blood cell and platelet counts. In such cases, a reduction in the dosage of PEG-IFN α-2b was required. Both PEG-IFN α-2b and RBV were discontinued if the hemoglobin level, white blood cell count, or platelet count fell below 85 g/L, 1 × 109/L, and 25 × 109/L, respectively. The treatment was discontinued if severe general fatigue, hyperthyroidism, interstitial pneumonia, or severe hemolytic disorders developed, continuation of treatment was judged not to be possible by the attending physician, or if the patient desired discontinuation of treatment.
The pretreatment, baseline, serum HCV RNA level was measured by a quantitative HCV RNA polymerase chain reaction (PCR) assay (COBAS Amplicor HCV Monitor Test v 2.0 using the 10-fold dilution method; Roche Diagnostics, Tokyo, Japan), which has a lower limit of quantitation of 5000 IU (13 500 copies)/mL (5 kIU/mL) and an outer limit of quantitation of 5 100 000 IU/mL (5100 kIU/mL). The HCV genotype was determined by type-specific primers of the core region of the HCV genome. The protocol for genotyping was carried out as previously described[3].
End of treatment (EOT) response and SVR were defined as serum HCV RNA undetectable at the end of treatment and at 24-wk follow-up after the end of treatment, respectively. EOT response and SVR were defined as non-detectable HCV-RNA as measured by qualitative COBAS Amplicor HCV Monitor Test v 2.0, with the results labeled as positive or negative. The lower limit of detection was 50 IU/mL (0.5 kIU/mL). The analysis of EOT and SVR was performed on an intention-to-treat basis.
Continuous data are expressed as mean ± SD. The statistics were carried out using a commercially available software package (BMDP Statistical Software Inc., Los Angeles, CA, USA) for the IBM 3090 system computer. The χ2 test, Fisher’s exact test and Kruskal-Wallis test were used to determine the differences in baseline clinical characteristics, safety, efficacy of the combination therapy, adherence to the total dose, and the association between the adherence and SVR. Logistic regression analysis was used to identify the association between age and SVR. A P < 0.05 was considered significant.
Among patients with genotype 1, the EOT response rate was significantly higher in group A (497 of 685, 72.5%) than in group B (129 of 253, 45.0%) (P < 0.001). Among patients with genotype 2, there was no significant difference between groups C (239 of 252, 94.8%) and D (55 of 61, 90.1%).
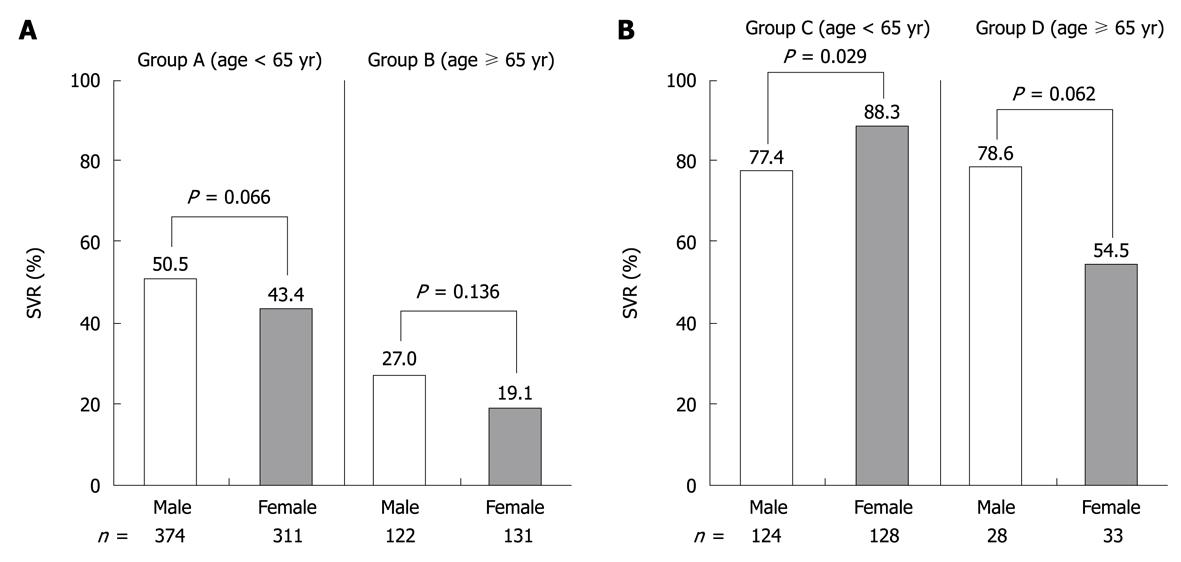
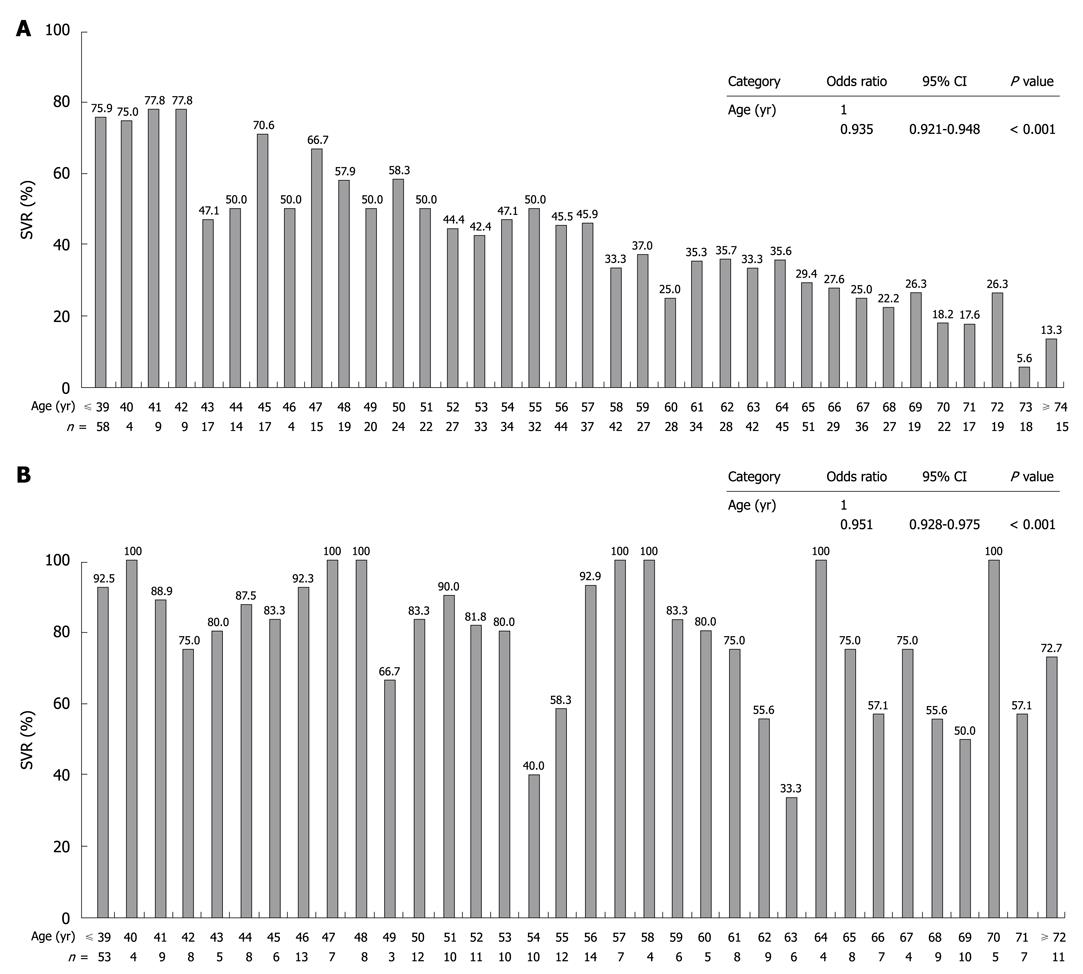
Of 1251 patients, 631 (50.4%) achieved SVR in the intention-to-treat analysis. The SVR rate was significantly higher for genotype 2 (249 of 313, 79.6%) than for genotype 1 patients (382 of 938, 40.7%) (P < 0.001). Among patients with genotype 1, the SVR rate was significantly higher in group A (324 of 685, 47.3%) than in group B (58 of 253, 22.9%) (P < 0.001). Among patients with genotype 2, SVR was also significantly higher in group C (209 of 252, 82.9%) than in group D (40 of 61, 65.6%) (P = 0.004). The rate of SVR was significantly higher for females (113 of 128, 88.3%) than for males (96 of 124, 77.4%) in group C only (Figure 1). Furthermore, we analyzed whether or not the SVR rate differed according to the age at which the combination treatment of PEG-IFN α-2b plus RBV was started. The results showed that the SVR rate decreased significantly with age for both genotype 1 and 2. SVR was achieved by 5.6%-26.3% of genotype 1 patients aged 70 years or older, and by 57.1%-100% of genotype 2 patients aged 70 years or older (Figure 2).
We previously reported a minimum acceptable dose of at least 80% or more of the target dosage of PEG-IFN α-2b and 60% or more of the target dosage of RBV for the successful treatment of Japanese patients with genotype 1[8]. Therefore, we analyzed the SVR rates in patients with genotype 1 by the dosage they actually received during treatment (a total dose of at least 80% or more of PEG-IFN α-2b and 60% or more of RBV) (Table 3). The number who received at least this minimum acceptable dosage during treatment were 278 (40.6%) of 685 patients in group A and 62 (24.5%) of 253 in group B, significantly lower in group B than in group A (P < 0.001). Compared with patients who received less than the minimum acceptable dosage, in patients who received at least this minimum dosage, the SVR rates increased from 34.2% to 66.5% in group A patients and from 15.7% to 45.2% (P < 0.001) in group B patients. No significant difference between groups C and D was observed. On comparing patients whose platelet count was under 10 × 1010/L, the SVR rate for genotype 1 was significantly lower in group B (2 of 36, 5.6%) than in group A (16 of 56, 28.6%) (P < 0.001). Among the patients with genotype 2, SVR was not significantly different between group C (9 of 16, 56.3%) and group D (2 of 7, 28.6%).
| Male | Female | Total | ||||
| n | SVR | n | SVR | n | SVR | |
| Group A | ||||||
| Minimum acceptable | 168 | 116 (69.0) | 110 | 69 (62.7) | 278 | 185 (66.5) |
| Reduced | 206 | 73 (35.4) | 201 | 66 (32.8) | 407 | 139 (34.2) |
| Total | 374 | 189 (50.5) | 311 | 135 (43.4) | 685 | 324 (47.3) |
| Group B | ||||||
| Minimum acceptable | 31 | 15 (48.4) | 31 | 13 (41.9) | 62 | 28 (45.2) |
| Reduced | 91 | 18 (19.8) | 100 | 12 (12.0) | 191 | 30 (15.7) |
| Total | 122 | 33 (27.0) | 131 | 25 (19.1) | 253 | 58 (22.9) |
In a comparison of the SVR rate in patients with or without one or more previous courses of IFN plus RBV, there was no significant difference between the genotypes (genotype 1: 118 of 310, 38.1% vs 264 of 628, 42.0%, genotype 2: 44 of 72, 61.1% vs 141 of 241, 58.5%). Furthermore, we compared the EOT response rate and SVR rate of cirrhosis patients whose liver fibrosis was F4, and found no significant difference between groups A (EOT: 16 of 30, 53.3%, SVR: 7 of 30, 23.3%) and B (EOT: 6 of 17, 35.3%, SVR: 2 of 17, 11.8%). In addition, no significant difference was found between groups C (EOT: 8 of 10, 80.0%, SVR: 6 of 10, 60.0%) and D (EOT: 9 of 12, 75.0%, SVR: 5 of 12, 41.7%).
Of 1251 patients, 314 (25.1%) did not complete PEG-IFN α-2b plus RBV treatment due to adverse effects or other reasons. The discontinuation rate was significantly higher in patients with genotype 1 (273 of 938, 29.1%) than in those with genotype 2 (41 of 313, 13.1%) (P < 0.001) (Tables 4 and 5). Furthermore, the rate of discontinuation due to adverse effects was significantly higher in patients with genotype 1 (135 of 938, 14.4%) than in those with genotype 2 (23 of 313, 7.3%) (P < 0.010). The rates of discontinuation due to lack of treatment efficacy and for economic reasons (loss of job, inability to pay the medical costs) were also significantly higher in patients with genotype 1 (55 of 938, 5.9%, 15 of 938, 1.6%) than in those with genotype 2 (1 of 313, 0.3%, 0 of 938, 0%) (P < 0.001 and P = 0.025, respectively).
| Group A (age < 65 yr) | Group B (age≥65 yr) | Total | |||
| Male (n = 374) | Female (n = 311) | Male (n = 122) | Female (n = 131) | ||
| Discontinued number | 101 | 66 | 52 | 54 | 273 |
| Adverse effects | 43 | 31 | 33 | 28 | 135 |
| General fatigue | 17 | 7 | 12 | 11 | 47 |
| Depression | 3 | 11 | 4 | 5 | 23 |
| Appetite loss | 1 | 0 | 1 | 0 | 2 |
| Rash | 3 | 2 | 3 | 4 | 12 |
| Encephalopathy | 1 | 0 | 0 | 0 | 1 |
| Neutropenia | 2 | 0 | 0 | 0 | 2 |
| Anemia | 3 | 2 | 4 | 1 | 10 |
| Thrombocytopenia | 1 | 0 | 3 | 1 | 5 |
| Elevation of ALT | 1 | 0 | 0 | 0 | 1 |
| Hyperthyroidism | 3 | 2 | 0 | 1 | 6 |
| Hypothyroidism | 0 | 1 | 0 | 0 | 1 |
| Retinopathy | 1 | 0 | 1 | 0 | 2 |
| Interstitial pneumonia | 2 | 0 | 1 | 1 | 4 |
| Pulmonary disease (others)1 | 0 | 1 | 1 | 1 | 3 |
| Psychoneurotic disorder2 | 2 | 0 | 2 | 0 | 4 |
| Nervous disease3 | 1 | 1 | 0 | 1 | 3 |
| Autoimmune disease4 | 0 | 2 | 0 | 1 | 3 |
| Metabolic disease5 | 0 | 2 | 0 | 0 | 2 |
| Digestive disorder6 | 2 | 0 | 1 | 1 | 4 |
| Hepatocellular carcinoma | 2 | 0 | 4 | 1 | 7 |
| Malignancy (extra-liver) | 0 | 1 | 1 | 0 | 2 |
| No effect of treatment | 22 | 18 | 7 | 8 | 55 |
| Economic problem | 9 | 3 | 0 | 3 | 15 |
| Others7 | 25 | 13 | 7 | 14 | 59 |
| Group C (age < 65 yr) | Group D (age≥65 yr) | Total | |||
| Male (n = 124) | Female (n = 128) | Male (n = 28) | Female (n = 33) | ||
| Discontinued number | 18 | 15 | 4 | 4 | 41 |
| Adverse effects | 6 | 11 | 3 | 3 | 23 |
| General fatigue | 1 | 3 | 1 | 0 | 5 |
| Depression | 0 | 2 | 0 | 0 | 2 |
| Appetite loss | 0 | 0 | 0 | 0 | 0 |
| Rash | 2 | 1 | 0 | 2 | 5 |
| Encephalopathy | 0 | 0 | 0 | 1 | 1 |
| Neutropenia | 0 | 2 | 0 | 0 | 2 |
| Anemia | 0 | 0 | 2 | 0 | 2 |
| Thrombocytopenia | 2 | 0 | 0 | 0 | 2 |
| Elevation of ALT | 0 | 0 | 0 | 0 | 0 |
| Hyperthyroidism | 0 | 1 | 0 | 0 | 1 |
| Hypothyroidism | 0 | 1 | 0 | 0 | 1 |
| Retinopathy | 0 | 0 | 0 | 0 | 0 |
| Interstitial pneumonia | 0 | 0 | 0 | 0 | 0 |
| Pulmonary disease(others) | 0 | 0 | 0 | 0 | 0 |
| Psychoneurotic disorder | 0 | 0 | 0 | 0 | 0 |
| Nervous disease1 | 1 | 1 | 0 | 0 | 2 |
| Autoimmune disease | 0 | 0 | 0 | 0 | 0 |
| Metabolic disease | 0 | 0 | 0 | 0 | 0 |
| Digestive disorder | 0 | 0 | 0 | 0 | 0 |
| Hepatocellular carcinoma | 0 | 0 | 0 | 0 | 0 |
| Malignancy (extra-liver) | 1 | 0 | 0 | 0 | 1 |
| No effect of treatment | 1 | 0 | 0 | 0 | 1 |
| Economic problem | 0 | 0 | 0 | 0 | 0 |
| Others2 | 10 | 4 | 1 | 1 | 16 |
For genotype 1 patients, the discontinuation rate was significantly higher in group B (106 of 253, 42.9%) than in group A (167 of 685, 24.4%) (P < 0.001), and the rate of discontinuation due to adverse effects was also significantly higher in group B (61 of 253, 24.1%) than in group A (74 of 685, 10.8%) (P < 0.001). General fatigue was the most frequent adverse effect, and was significantly more frequent in group B than in group A (P < 0.001). However, in these group 1 patients, RBV was reduced due to anemia in 12.5% (3 of 24) of group A and in 30.4% (7 of 23) of group B. Furthermore, rash and thrombocytopenia were significantly more frequent in group B than in group A (P = 0.014 and P = 0.007, respectively). In group A, depression was significantly more frequent in females than in males (P = 0.012). In genotype 2 patients, treatment discontinuation did not differ between group C (33 of 252, 13.1%) and group D (8 of 61, 13.1%), and the rate of discontinuation due to adverse effects did not differ between these groups (17 of 252, 6.7%, 6 of 61, 9.8%, respectively).
The mean time to discontinuation in group A (21.6 ± 11.9 wk) was not significantly different from group B (21.5 ± 12.6 wk), and the mean time in group C (11.0 ± 6.8 wk) was also not significantly different from group D (11.6 ± 6.0 wk). There was no significant difference between male and female patients in each group (male: 21.0 ± 12.4 vs female: 22.1 ± 11.8 in group 1, male: 11.3 ± 7.1 vs female: 10.9 ± 6.1 in group 2).
HCC was not seen in genotype 2 patients; only in patients with genotype 1 (29.5 ± 9.9 wk) and was more frequent in group B (5 of 253, 2.0%) than in group A (2 of 685, 0.3%) (P = 0.008).
In a large, national, multicenter Greek study involving 993 treated and 734 untreated patients with chronic hepatitis C, patients with cirrhosis, showed a protective effect of treatment even among those without SVR. For patients without cirrhosis, the beneficial effect of IFN α treatment was particularly evident in older patients; patients with the worst prognosis if left untreated. Therefore, IFN α-based treatment should be offered to older persons, as these are the patients with the greatest potential benefit and may achieve SVR[16]. In Japan, the prevalence of chronic HCV infection increases with age, however, the optimal management of older patients has not yet been accurately defined. Whether or not to treat patients older than 65 years with antiviral treatment is highly debated, especially in terms of cost/benefit ratio. In addition, the natural history of chronic hepatitis C in elderly patients is not accurately known, as the presence of comorbidity can affect illness progression and life expectancy. HCV became more prevalent in Japan decades before the United States[17]. Japanese patients with chronic hepatitis C treated with IFN are currently 10 to 15 years older than corresponding patients in the United States and European countries, where patients treated with antiviral treatment tend to average 45 years of age[18-20]. Therefore, our results can serve as a world-wide model for the treatment of older chronic hepatitis C patients.
It has been well documented that the combination therapy of PEG-IFN α-2b plus RBV is more effective than previous IFN monotherapy in chronic hepatitis C patients[7,8]. There have been four studies on the efficacy of PEG-IFN plus RBV therapy in patients 65 years or older with genotype 1, which revealed low rates of SVR (31.1%-51.9%)[21-24]. However, these studies were too small (11-93 patients) for conclusive recommendations to be made. Because the present study was a large multicenter design, it is useful for clarifying the efficacy and safety of PEG-IFN plus RBV combination therapy in older patients. The present study confirmed the results of our previous study which showed that the SVR rate was significantly higher for genotype 2 than for genotype 1 patients[8]. Another important result was that the ability to take at least a minimum acceptable dosage during treatment increased the SVR rate by about three times in older patients with genotype 1. This result also confirmed previous studies which indicated the importance of giving at least the minimum acceptable treatment dosage in patients infected with HCV genotype 1, especially older patients[23,24].
Secondly, we compared discontinuation of treatment by genotype and sex. In genotype 1 patients, adverse effects were seen more often in older than in younger patients. This was the most important reason why the rate of treatment discontinuation was higher in older than in younger patients, and affected the outcome of PEG-IFN α-2b plus RBV combination therapy. General fatigue was the most common adverse effect in older patients. Because older patients often have impaired renal function, they have increased blood levels of RBV[25,26]. They are also inclined to be anemic and to have general fatigue. However, only a small number of older patients in the present study had reduced RBV due to anemia. Therefore, general fatigue is probably a direct adverse effect of PEG-IFN α-2b. We previously reported that herbal medicine relieved the adverse effects of IFN, including general fatigue[27]. Herbal medicine may be useful for mitigating general fatigue during PEG-IFN α-2b plus RBV combination treatment, especially in older patients.
The rate of discontinuation was lower in patients with genotype 2 than in patients with genotype 1, and there was no difference between the older and the younger patients with genotype 2. These results are possibly a consequence of the shorter term of treatment in genotype 2 and the many genotype 1 patients who discontinued due to lack of efficacy.
Two of the characteristics of older patients in the present study were that both hemoglobin and platelet count were significantly lower than in younger patients. The SVR rate was significantly lower when the platelet count was less than 10 × 1010/L. Furthermore, the older genotype 1 patients were often forced to discontinue treatment due to thrombocytopenia and the occurrence of HCC. These findings appear to result from advanced liver fibrosis in older chronic hepatitis C patients. Therefore, the possibility of HCC during long-term IFN treatment in older patients must be considered.
We previously reported that older female patients had a low response to IFN-α monotherapy[9], and other investigators have reported that older female patients have a poor response to PEG-IFN α-2b plus RBV[22,28]. Although our data showed that sex was not related to SVR, the reason for this finding was not fully elucidated. In any case, studies have conclusively shown that it is important to begin treatment with PEG-IFN α-2b plus RBV combination therapy as soon as possible. Our data suggest that age may be a more important factor than sex for increasing the rate of SVR. Resistance to treatment in older patients may be due to IFN-immunomodulation, advanced liver fibrosis, or reduced dosage.
To maximize adherence to the optimal treatment regimen, the treatment schedule can be modified or other therapeutic modalities added, such as hematopoietic growth factors[29] or the new thrombopoietin-receptor agonist, eltrombopag, for the antiviral treatment of older patients with chronic hepatitis C[30]. A further individualized treatment protocol based on viral kinetics might be more practical[31].
In conclusion, PEG-IFN α-2b plus RBV treatment was effective in the treatment of older chronic hepatitis C patients when they received at least the minimum acceptable treatment dosage. However, there were frequent adverse effects and treatment discontinuation. It is necessary to control for adverse effects that might interrupt treatment and to begin this combination therapy as soon as possible, especially in older patients.
Whether or not to treat patients older than 65 years with antiviral treatment is highly debated, especially in terms of cost/benefit ratio. However, there is little data concerning the response and safety of combination treatment for a large number of older patients with chronic hepatitis C virus infection. Therefore, in an attempt to ameliorate these problems, the authors decided to treat older patients with pegylated interferon (PEG-IFN) α-2b plus ribavirin (RBV) combination therapy.
The combination treatment of PEG-IFN α-2b plus RBV improved the sustained virological response rate in chronic hepatitis C patients. However, the current issue is whether or not to treat older patients because of low response and high dropout rate.
There have been four studies on the efficacy of PEG-IFN plus RBV therapy in patients 65 years or older with genotype 1. However, these studies were too small (11-93 patients) for conclusive recommendations to be made. This study is very useful for clarifying the efficacy and safety of PEG-IFN plus RBV combination therapy in older patients, because of its large scale, multicenter design.
The study demonstrated that PEG-IFN α-2b plus RBV treatment was effective in chronic hepatitis C patients 65 years or older who completed treatment with at least the minimum required treatment dosage. Furthermore, this study suggested that the combination treatment and beginning this therapy as soon as possible are important, especially in older patients.
The study has been well conducted and includes a large number of patients. Results have been described in a lucid and informative manner and are of clinical relevance.
| 1. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. |
| 2. | Hayashi J, Furusyo N, Ariyama I, Sawayama Y, Etoh Y, Kashiwagi S. A relationship between the evolution of hepatitis C virus variants, liver damage, and hepatocellular carcinoma in patients with hepatitis C viremia. J Infect Dis. 2000;181:1523-1527. |
| 3. | Hayashi J, Ohmiya M, Kishihara Y, Tani Y, Kinukawa N, Ikematsu H, Kashiwagi S. A statistical analysis of predictive factors of response to human lymphoblastoid interferon in patients with chronic hepatitis C. Am J Gastroenterol. 1994;89:2151-2156. |
| 4. | Furusyo N, Hayashi J, Ohmiya M, Sawayama Y, Kawakami Y, Ariyama I, Kinukawa N, Kashiwagi S. Differences between interferon-alpha and -beta treatment for patients with chronic hepatitis C virus infection. Dig Dis Sci. 1999;44:608-617. |
| 5. | Furusyo N, Hayashi J, Ueno K, Sawayama Y, Kawakami Y, Kishihara Y, Kashiwagi S. Human lymphoblastoid interferon treatment for patients with hepatitis C virus-related cirrhosis. Clin Ther. 1997;19:1352-1367. |
| 6. | Kashiwagi K, Furusyo N, Kubo N, Nakashima H, Nomura H, Kashiwagi S, Hayashi J. A prospective comparison of the effect of interferon-alpha and interferon-beta treatment in patients with chronic hepatitis C on the incidence of hepatocellular carcinoma development. J Infect Chemother. 2003;9:333-340. |
| 7. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. |
| 8. | Furusyo N, Kajiwara E, Takahashi K, Nomura H, Tanabe Y, Masumoto A, Maruyama T, Nakamuta M, Enjoji M, Azuma K. Association between the treatment length and cumulative dose of pegylated interferon alpha-2b plus ribavirin and their effectiveness as a combination treatment for Japanese chronic hepatitis C patients: project of the Kyushu University Liver Disease Study Group. J Gastroenterol Hepatol. 2008;23:1094-1104. |
| 9. | Hayashi J, Kishihara Y, Ueno K, Yamaji K, Kawakami Y, Furusyo N, Sawayama Y, Kashiwagi S. Age-related response to interferon alfa treatment in women vs men with chronic hepatitis C virus infection. Arch Intern Med. 1998;158:177-181. |
| 10. | Iwasaki Y, Ikeda H, Araki Y, Osawa T, Kita K, Ando M, Shimoe T, Takaguchi K, Hashimoto N, Kobatake T. Limitation of combination therapy of interferon and ribavirin for older patients with chronic hepatitis C. Hepatology. 2006;43:54-63. |
| 11. | Arase Y, Suzuki F, Suzuki Y, Akuta N, Kawamura Y, Kobayashi M, Hosaka T, Sezaki H, Yatsuji H, Kobayashi M. Side effects of combination therapy of peginterferon and ribavirin for chronic hepatitis-C. Intern Med. 2007;46:1827-1832. |
| 12. | Arase Y, Ikeda K, Suzuki F, Suzuki Y, Saitoh S, Kobayashi M, Akuta N, Someya T, Koyama R, Hosaka T. Long-term outcome after interferon therapy in elderly patients with chronic hepatitis C. Intervirology. 2007;50:16-23. |
| 13. | Ikeda K, Arase Y, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Saitoh S, Suzuki F. Necessities of interferon therapy in elderly patients with chronic hepatitis C. Am J Med. 2009;122:479-486. |
| 14. | Furusyo N, Katoh M, Tanabe Y, Kajiwara E, Maruyama T, Shimono J, Sakai H, Nakamuta M, Nomura H, Masumoto A. Interferon alpha plus ribavirin combination treatment of Japanese chronic hepatitis C patients with HCV genotype 2: a project of the Kyushu University Liver Disease Study Group. World J Gastroenterol. 2006;12:784-790. |
| 15. | Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982-992. |
| 16. | Manesis EK, Papatheodoridis GV, Touloumi G, Karafoulidou A, Ketikoglou J, Kitis GE, Antoniou A, Kanatakis S, Koutsounas SJ, Vafiadis I. Natural course of treated and untreated chronic HCV infection: results of the nationwide Hepnet.Greece cohort study. Aliment Pharmacol Ther. 2009;29:1121-1130. |
| 17. | Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, Alter HJ. Inaugural Article: A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA. 2002;99:15584-15589. |
| 18. | Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493-1499. |
| 19. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. |
| 20. | Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432. |
| 21. | Antonucci G, Longo MA, Angeletti C, Vairo F, Oliva A, Comandini UV, Tocci G, Boumis E, Noto P, Solmone MC. The effect of age on response to therapy with peginterferon alpha plus ribavirin in a cohort of patients with chronic HCV hepatitis including subjects older than 65 yr. Am J Gastroenterol. 2007;102:1383-1391. |
| 22. | Thabut D, Le Calvez S, Thibault V, Massard J, Munteanu M, Di Martino V, Ratziu V, Poynard T. Hepatitis C in 6,865 patients 65 yr or older: a severe and neglected curable disease? Am J Gastroenterol. 2006;101:1260-1267. |
| 23. | Honda T, Katano Y, Shimizu J, Ishizu Y, Doizaki M, Hayashi K, Ishigami M, Itoh A, Hirooka Y, Nakano I. Efficacy of peginterferon-alpha-2b plus ribavirin in patients aged 65 years and older with chronic hepatitis C. Liver Int. 2010;30:527-537. |
| 24. | Huang CF, Yang JF, Dai CY, Huang JF, Hou NJ, Hsieh MY, Lin ZY, Chen SC, Hsieh MY, Wang LY. Efficacy and safety of pegylated interferon combined with ribavirin for the treatment of older patients with chronic hepatitis C. J Infect Dis. 2010;201:751-759. |
| 25. | Paroni R, Del Puppo M, Borghi C, Sirtori CR, Galli Kienle M. Pharmacokinetics of ribavirin and urinary excretion of the major metabolite 1,2,4-triazole-3-carboxamide in normal volunteers. Int J Clin Pharmacol Ther Toxicol. 1989;27:302-307. |
| 26. | Bruchfeld A, Lindahl K, Schvarcz R, Ståhle L. Dosage of ribavirin in patients with hepatitis C should be based on renal function: a population pharmacokinetic analysis. Ther Drug Monit. 2002;24:701-708. |
| 27. | Kainuma M, Hayashi J, Sakai S, Imai K, Mantani N, Kohta K, Mitsuma T, Shimada Y, Kashiwagi S, Terasawa K. The efficacy of herbal medicine (kampo) in reducing the adverse effects of IFN-beta in chronic hepatitis C. Am J Chin Med. 2002;30:355-367. |
| 28. | Sezaki H, Suzuki F, Kawamura Y, Yatsuji H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitoh S, Arase Y. Poor response to pegylated interferon and ribavirin in older women infected with hepatitis C virus of genotype 1b in high viral loads. Dig Dis Sci. 2009;54:1317-1324. |
| 29. | Afdhal NH, Dieterich DT, Pockros PJ, Schiff ER, Shiffman ML, Sulkowski MS, Wright T, Younossi Z, Goon BL, Tang KL. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302-1311. |
| 30. | McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, Berg T, Gordon SC, Campbell FM, Theodore D. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227-2236. |
| 31. | Berg T, Weich V, Teuber G, Klinker H, Möller B, Rasenack J, Hinrichsen H, Gerlach T, Spengler U, Buggisch P. Individualized treatment strategy according to early viral kinetics in hepatitis C virus type 1-infected patients. Hepatology. 2009;50:369-377. |
Peer reviewer: Emanuel K Manesis, MD, Professor of Medicine, Athens University School of Medicine, Liver Unit, Euroclinic, 19 Mavromateon Street, Athens 10 34, Greece
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP