INTRODUCTION
Hepatocellular carcinoma (HCC) is an important global health issue. Owing to the dissemination of hepatitis B and C virus infection, the overall incidence of HCC remains alarmingly high in developing countries and increases steadily in most developed countries[1,2]. HCC is the sixth most common cancer and the third leading cause of cancer-related death worldwide[3]. Its incidence rate is different in various countries, accounting for 55% of all cases (and deaths) in China[3]. Its age-adjusted annual incidence is 5.5-14.9 people per 100 000 population in the world[4,5]. The prognosis of HCC patients is generally very poor and their 5-year relative survival rate is 3%-5% in most countries[3]. Current major treatment modalities for HCC include surgical resection, liver transplantation and local ablation therapies[6]. Many patients who are diagnosed with HCC at its advanced stage, are only candidates for palliative care[2,7]. Furthermore, since no effective palliative chemotherapy is available, the prognosis of advanced HCC patients is dismal. Therefore, it is necessary to find new effective medications for HCC. Development of pharmacologically effective agents with little toxicity or few side effects from natural products has become a new trend.
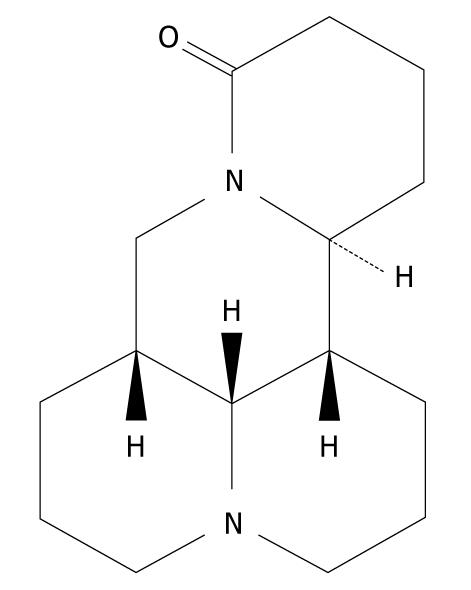
Matrine is one of the main alkaloid components extracted from the Sophora root, with a molecular formula of C15H24N2O (Figure 1)[8], which was first isolated and identified in 1958 from Sophora flavescens Ait (also known as kushen), subprostrata (shandougen) and alopecuroides (kudouzi)[9-12]. Matrine has been widely used in treatment of viral hepatitis, hepatic fibrosis, cardiac arrhythmia and skin diseases, such as atopic dermatitis and eczema in China, because it has a wide range of pharmacological effects, such as anti-inflammatory[13,14], antiviral[15,16], immunoinhibitory[17], antifibrotic[18,19], analgesic[20], antiarrhythmic[21-23] and anti-diarrhea effects[24]. Recently, interest has been generated in its antitumor activity. It has been reported that matrine exerts its antitumor effects by inhibiting proliferation and inducing apoptosis of gastric and cervical cancer cells, leukemia and glioma cells[10,12,25-29]. Matrine can also inhibit invasiveness and metastasis of human malignant melanoma cell line A375 and cervical cancer HeLa cells, and induce differentiation of leukemia K-562 cells[12,30]. In addition, matrine-induced autophagy in rat C6 glioma cells has been observed by electronic microscopy[29]. However, the precise mechanism underlying the anticancer activity of matrine remains unclear. Therefore, we designed this study to investigate the antitumor effect of matrine in human hepatoma G2 cells, and further elucidate its molecular mechanism involved in antineoplastic activities.
Figure 1 Chemical structure of matrine with a molecular formula of C15H24N2O and a molecular weight of 248.
37.
MATERIALS AND METHODS
Reagents
Fetal bovine serum was purchased from Sijiqing Biological Engineering Company Limited (Hangzhou, China). RPMI medium 1640 was bought from Gibco (USA). Sodium dodecyl sulfate (SDS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), L-glutamine, Annexin V-FITC/PI (propidium iodide) apoptosis detection kit, and monodansylcadaverine (MDC) were purchased from Sigma (USA). TRIzol reagent was bought from Invitrogen (USA). Primescript™ reverse transcription (RT) reagent kit and SYBR® Premix Ex Taq™ II were purchased from TaKaRa (Dalian, China).
Matrine was purchased from Xi’an Tianyuan Biologics Plant (China), with a purity of over 99% as proved by high-performance liquid chromatography. Matrine was dissolved in sterile double distilled water at a stock concentration of 40 mg/mL, stored at -20°C in the dark, and then diluted in RPMI-1640 medium to obtain the desired concentration. 3-methyladenine (3-MA) (Sigma, USA) was dissolved in heated sterile double distilled water to make a 100 mmol/L stock solution and then added to the medium after heated for a final concentration of 2 mmol/L. Three hours later, matrine was added for treatment.
Cell line and cell culture
Human hepatoma G2 (HepG2) cell line was purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). The cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL of penicillin and 100 μg/mL of streptomycin at 37°C in a 5% CO2 incubator. The cells in mid-log phase were used in experiments.
Cell viability assay
Viability of HepG2 cells was assessed by MTT assay as previously described[31]. The cells were seeded in 96-well flat bottom microtiter plates (Costar 3599, Corning Inc., Corning, NY) at a density of 5 × 103 cells per well, allowed to adhere overnight, and then treated with matrine at the concentration of 0.25, 0.5, 1.0, 2.0 mg/mL for 24, 48 and 72 h, respectively. Control group and zero adjustment well were set as well. A MTT solution (5 mg/mL) was added 4 h before the end of incubation and the reaction was terminated by adding 10% acidified SDS. The absorbance value per well at 570 nm was read using an automatic multiwell spectrophotometer (PowerWave x, Bio-Tek Instruments Inc, USA). All MTT assays were performed in triplicate. The inhibitory rate for the proliferation of HepG2 cells was calculated according to the formula: (1-experimental absorbance value/control absorbance value) × 100%.
Cell cycle analysis
To determine cell cycle distribution, HepG2 cells were treated with matrine at the concentration of 0, 0.5 and 1.0 mg/mL, respectively. After 24 h of treatment, both floating and attached cells were collected and centrifuged before washed with cold phosphate-buffered saline (PBS), and then fixed in 70% cold ethanol overnight at 4°C. A fluorochrome solution containing 50 μg/mL PI, 3.4 mmol/L sodium citration, 20 μg/mL RNase A and 1% Triton X-100 was added and then incubated in the dark at room temperature for 30 min. Cell cycle analysis was performed using an EPICS XL flow cytometer (Beckman Coulter, California, USA). All experiments were performed in triplicate.
Detection of apoptosis
Annexin-V-FITC/PI double staining assay was performed to detect apoptosis of HepG2 cells. The cells were exposed to matrine at the concentration of 0, 0.5, 1.0 and 2.0 mg/mL, respectively, for 24 h, then harvested and resuspended in Annexin-V binding buffer. The suspension was incubated with 5 μL of Annexin V-FITC and 10 μL of PI for 10 min at room temperature in the dark, followed by cytometric analysis (EPICS XL, Beckman Coulter, USA) within 30 min of staining (as soon as possible). All experiments were performed in triplicate.
Morphologic observation under inverted phase contrast microscope
HepG2 cells were equally seeded in 24-well flat bottom microtiter plates (Costar 3524, Corning Inc., Corning, NY), and then treated with matrine at the concentration of 0, 0.25, 0.5, 1.0 and 2.0 mg/mL, respectively. After 24 h of treatment, the morphology of HepG2 cells was observed under an inverted phase contrast microscope (Olympus, Tokyo, Japan).
Observation of cell ultrastructure under transmission electron microscope
HepG2 cells were fixed with 2.5% glutaraldehyde in 0.1 mol/L PBS (pH 7.4) for 90 min at room temperature, and post-fixed in 1% osmium tetraoxide for 30 min. After washed with PBS, the cells were progressively dehydrated in a 10% graded series of 50%-100% ethanol and propylene oxide, and embedded in Epon 812 resin. The blocks were cut into ultrathin sections with a microtome, which were then stained with saturated uranyl acetate and lead citrate. The ultrastructure of the cells was then observed under a transmission electron microscope (JEM-1230, JEOL, Japan).
MDC staining of autophagic vacuoles
MDC staining of autophagic vacuoles was performed for autophagy analysis as previously described[32]. HepG2 cells were divided into control group, 3-MA treatment group, matrine treatment group, and combined 3-MA and matrine treatment group. The cells were incubated for 48 h on coverslips. Autophagic vacuoles were labeled with 0.05 mmol/L MDC in PBS at 37°C for 10 min. After incubation, the cells were washed three times with PBS and immediately analyzed under a fluorescence microscope (IX-81; Olympus, Japan). Fluorescence of MDC was measured at the excitation wavelength 380 nm with an emission filter at 530 nm.
Real-time quantitative RT-polymerase chain reaction
HepG2 cells were cultured in 35 mm dishes and then collected after treatment with matrine for 24 and 72 h, respectively. Total RNA was isolated from cells using Trizol reagent (Invitrogen, USA) according to its manufacturer’s protocol. RNA concentration and purity were measured with a spectrophotometer at A260 and A260/280, respectively. RNA was reverse-transcribed into cDNA using a Primescript™ RT reagent kit (TaKaRa, Dalian, China) according to its manufacturer’s instructions. Real-time quantitative polymerase chain reaction (PCR) was carried out with the SYBR Green I fluorescent dye method (SYBR® Premix Ex TaqTM II, TaKaRa, Dalian, China) and a Rotor Gene 3000 real-time PCR apparatus (Corbett Research Company, Australia). The sequences of primers used are as follows: forward: 5′-TGCTTCAGGGTTTCATCCAG-3′ and reverse: 5′-GGCGGCAAT CATCCTCTG-3′ for Bax; forward: 5′-GAGGGATGGAAGGGTCTAAG-3′ and reverse: 5′-GCCTGGGCTGTGGTAAGT-3′ for Beclin 1; forward: 5′-TGGCACCCAGCACAATGAA-3′ and reverse: 5′-CTAAGTCATAGT CCGCCTAGAAGCA- 3′ for β-actin. β-actin was used as an internal control to evaluate the relative expressions of Bax and Beclin 1. The PCR conditions were as follows: a pre-denaturing at 95°C for 2 min, followed by 45 cycles of denaturation at 95°C for 10 s, annealing/extension at 60°C for 20 s. The amplification specificity was checked by melting curve analysis. The PCR products were visualized by gel electrophoresis to confirm the presence of a single product with a correct size. The 2-∆∆CT method was used to calculate the relative abundance of target gene expression generated by Rotor-Gene Real-Time Analysis Software 6.1.81. For each cDNA, the target gene mRNA level was normalized to β-actin mRNA level. Results were expressed as the ratio of normalized target gene mRNA level in cells treated with matrine to that in cells not treated with matrine. The experiments were performed in triplicate.
Statistical analysis
All data were expressed as mean ± SD. Statistical analysis was performed using the SPSS 16.0 for Window. One-way analysis of variance (ANOVA) was used to analyze statistical differences between groups under different conditions. P < 0.05 was considered statistically significant.
RESULTS
Matrine inhibited proliferation of HepG2 cells in a dose- and time-dependent manner
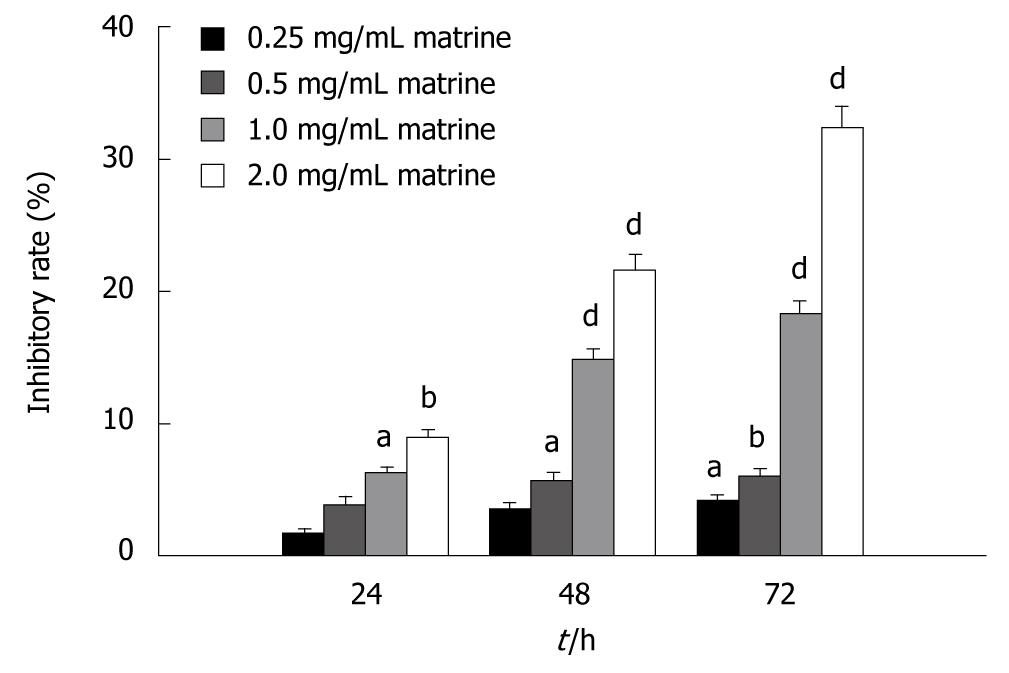
The antiproliferative effect of matrine on HepG2 cells was detected by MTT assay. The results showed that matrine inhibited the proliferation of HepG2 cells in a dose-dependent and time-dependent manner. The inhibitory rate of matrine on growth of HepG2 cells was 6.28% ± 0.42%, 14.81% ± 0.81%, and 18.25% ± 0.99%, respectively, after the cells were treated with matrine at the concentration of 1.0 mg/mL for 24, 48 and 72 h (Figure 2).
Figure 2 MTT assay showing the inhibitory effect of matrine on growth of HepG2 cells.
HepG2 cells were treated with matrine at the concentration of 0.25, 0.5, 1.0 and 2.0 mg/mL, respectively for 24, 48 and 72 h. Matrine inhibited the growth of HepG2 cells in a dose- and time-dependent manner. aP < 0.05, bP < 0.01, dP < 0.001 vs control group.
Matrine induced G1-phase cell cycle arrest in HepG2 cells
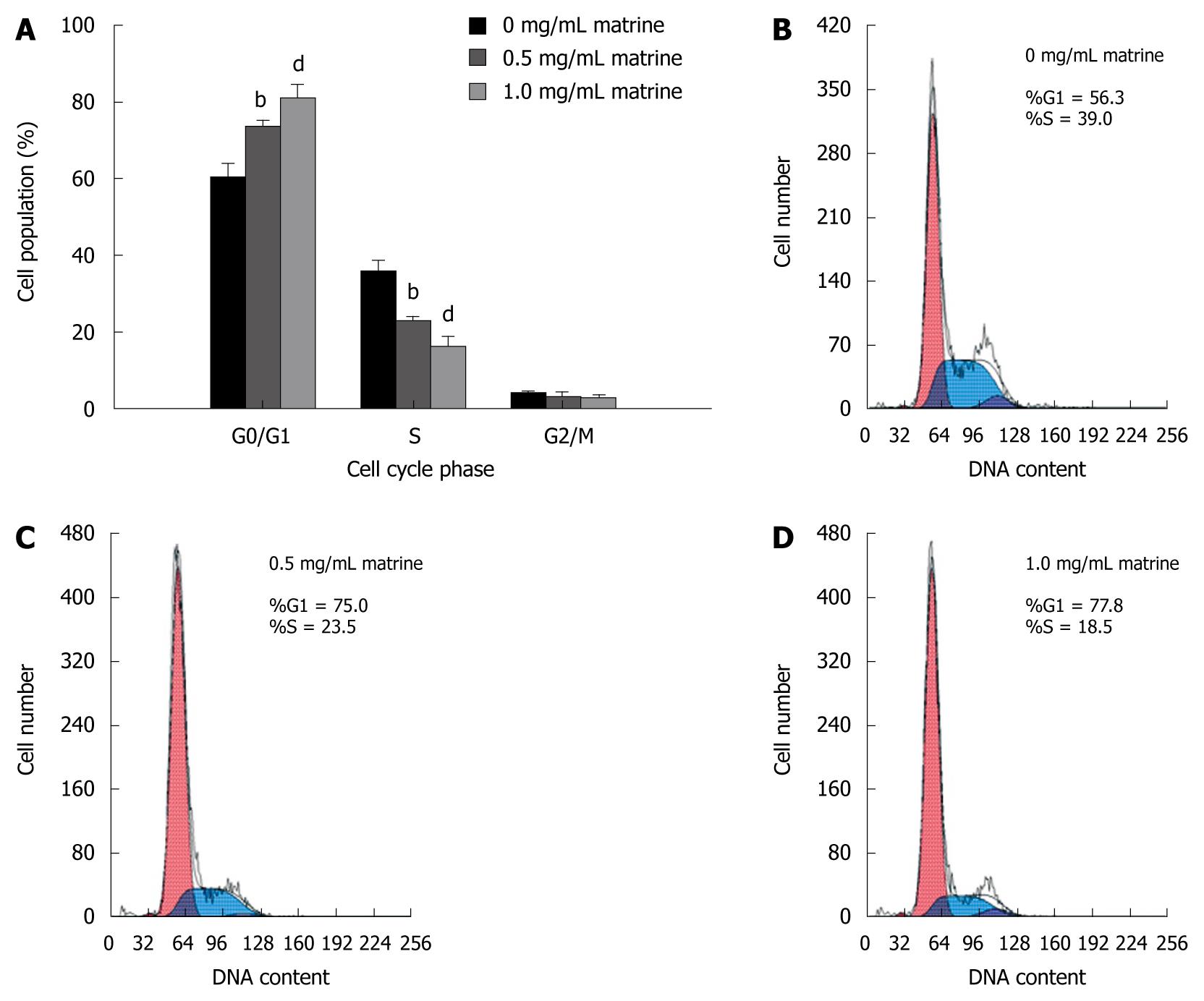
To better understand the inhibitory effect of matrine on growth of HepG2 cells, cell cycle distribution was analyzed by flow cytometry. Matrine significantly increased the number of cells in G0/G1 phase and decreased the number of cells in the S phase in a dose-dependent manner (Figure 3), indicating that matrine can induce the G0/G1 phase cell cycle arrest in HepG2 cells.
Figure 3 Effect of matrine on cell cycle distribution in HepG2 cells.
A: Matrine treatment significantly increased the proportion of HepG2 cells in G0/G1 phase while decreased the number of HepG2 cells in the S phase. Results were expressed as mean ± SD (n = 3); B-D: Histograms of HepG2 cells incubated with matrine at the concentrations of 0, 0.5 and 1.0 mg/mL. bP < 0.01, dP <0.001 vs control group.
Matrine induced apoptosis of HepG2 cells
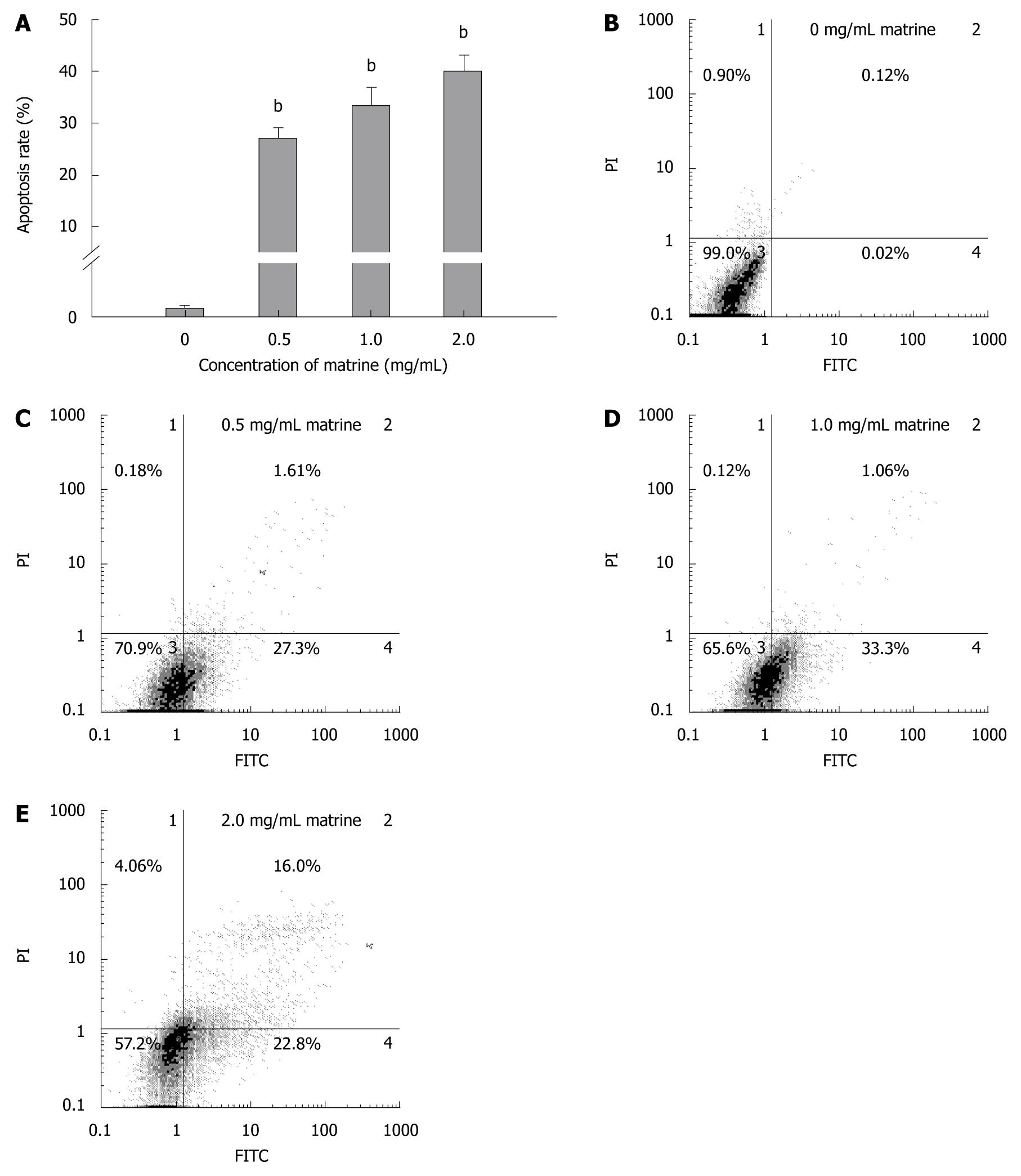
Annexin-V-FITC/PI double staining assay showed that matrine induced apoptosis of HepG2 cells in a dose-dependent manner (Figure 4A). Flow cytometry showed that the total apoptosis rate was 0.14% in HepG2 cells not treated with matrine, and was 28.91%, 34.36%, and 38.80%, respectively, in HepG2 cells treated with matrine at the concentration of 0.5, 1.0 and 2.0 mg/mL (Figure 4B-E). Early apoptosis was observed in HepG2 cells treated with matrine at the concentration of 0.5 and 1.0 mg/mL, while late apoptosis was observed in HepG2 cells treated with matrine at the concentration of 2.0 mg/mL. The late apoptosis rate of HepG2 cells treated with matrine at the concentration of 2.0 mg/mL increased from 1.06% to 16%, which may be due to the direct cytotoxic effect of matrine on HepG2 cells.
Figure 4 Matrine induces apoptosis of HepG2 cells.
A: Apoptosis rate of HepG2 cells treated with matrine at the concentration of 0.5, 1.0 and 2.0 mg/mL, respectively, were significantly different from that in control group. Furthermore, the percentage of apoptotic cells increased when the concentration of matrine was increased. The data are expressed as mean ± SD (n = 3); B-E: Histograms of HepG2 cells incubated with matrine at the concentration of 0, 0.5, 1.0 and 2.0 mg/mL, respectively. Early apoptotic HepG2 cells were observed after incubated with matrine at the concentration of 0.5 and 1.0 mg/mL, the late apoptotic HepG2 cells were observed after incubated with matrine at the concentration of 2.0 mg/mL. bP < 0.001 vs control group.
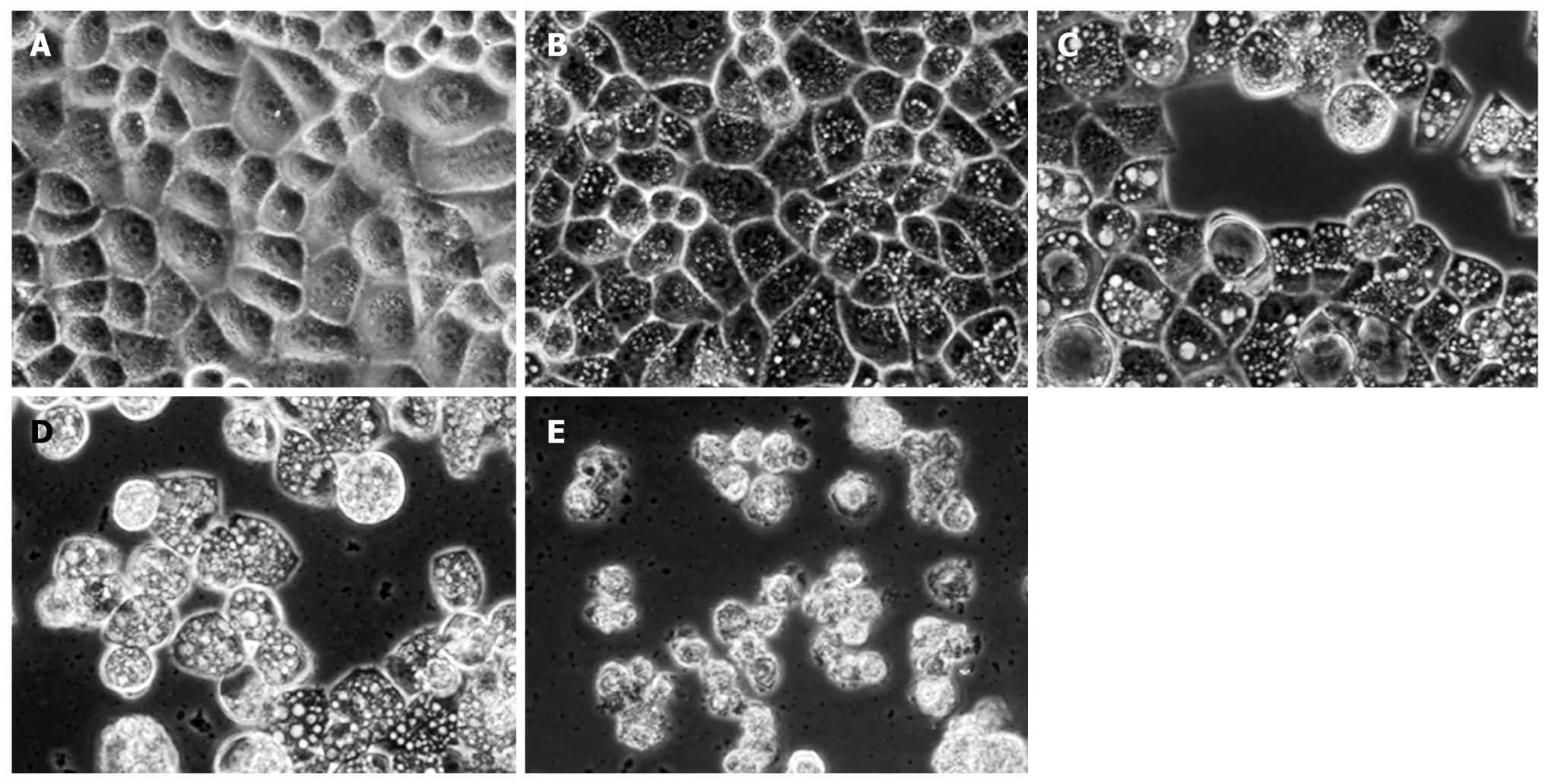
Observation of vacuolization in cytoplasm by inverted phase contrast microscopy
Inverted phase contrast microscopy showed the morphological characteristics of HepG2 cells. Control cells not treated with matrine were well adhered, showing the normal morphology of HepG2 cells, while the tumor cells treated with matrine for 24 h demonstrated remarkable morphological changes (Figure 5). Abundant cytoplasmic vacuoles with varying sizes were observed. Vacuolization in cytoplasm progressively became larger and denser when the concentration of matrine was increased. Moreover, the majority of cells treated with matrine at the concentration of 2.0 mg/mL became round and shrunken, and could not be affixed to the wall and suspended in culture medium.
Figure 5 Inverted phase contrast microscopy showing matrine-induced morphologic changes of HepG2 cells.
The control cells were well adhered, displaying the normal morphology of HepG2 cells. In contrast, abundant cytoplasmic vacuoles were observed in cells treated with matrine. Moreover, vacuolization in cytoplasm progressively became larger and denser when the concentration of matrine was increased. The majority of HepG2 cells treated with matrine at the concentration of 2.0 mg/mL became round and shrunken and could not be affixed to the wall and suspended in culture medium (× 400 magnification). A: 0 mg/mL matrine; B: 0.25 mg/mL matrine; C: 0.5 mg/mL matrine; D: 1.0 mg/mL matrine; E: 2.0 mg/mL matrine.
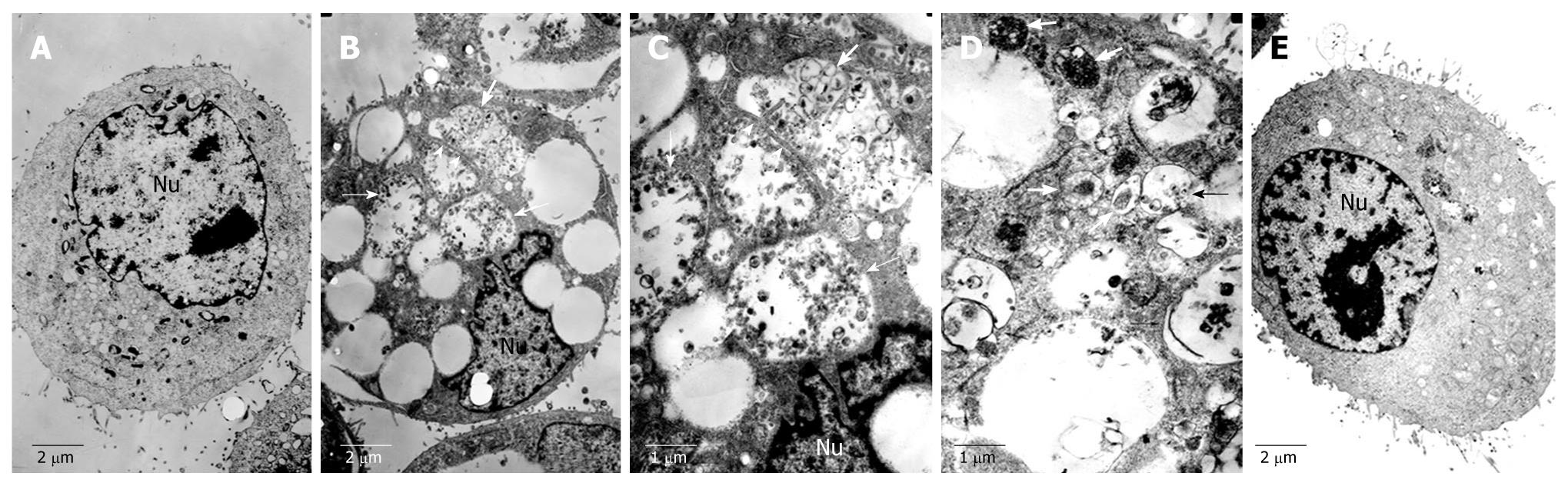
Transmission electron microscopy revealed formation of autophagosomes in matrine-treated HepG2 cells
To further clarify whether the cell vacuolization induced by matrine is involved in autophagy, transmission electron microscopy (TEM) was performed to detect the cells treated with matrine at the concentration of 1.0 mg/mL. The HepG2 cells not treated with matrine exhibited the normal ultrastructural morphology of cytoplasm, organelles and nuclei (Figure 6). The most prominent morphological change in matrine-treated cells was the formation of abundant autophagic vacuoles sequestrating cytoplasm and organelles, such as mitochondria and endoplasmic reticulum. Double-membranes, giant autophagosomes filled with degraded organelles and autolysosomes were frequently observed. TEM, the standard method to detect autophagy[33], was performed to detected the formation of autophagosomes, demonstrating that matrine could induce HepG2 cells to generate autophagy, which was consistent with the vacuolization obtained by inverted phase contrast microscopy. Moreover, 3-MA, a specific inhibitor of autophagy, potently suppressed the matrine-induced autophagy (Figure 6E). The number of autophagic vacuoles was significantly lower while the formed vacuoles were smaller and appeared to be less developed in combined 3-MA and matrine treatment group than in single matrine treatment group.
Figure 6 Transmission electron microscopy showing normal morphology of cytoplasm, cell organelles, and nuclei of HepG2 cells not treated with matrine (A), characteristic ultrastructural morphology of autophagy (B), double-membrane (C) and a large number of autophagic vacuoles (D) of HepG2 cells treated with matrine, and sharply decreased autophagic vacuoles (E) of HepG2 cells treated with combination of 3-MA and matrine (A, B, E, × 4000; C, D, × 8000).
Arrowheads represent double-membrane, thick arrows represent autophagosomes, and thin arrows represent autolysosomes. Nu: Nucleus.
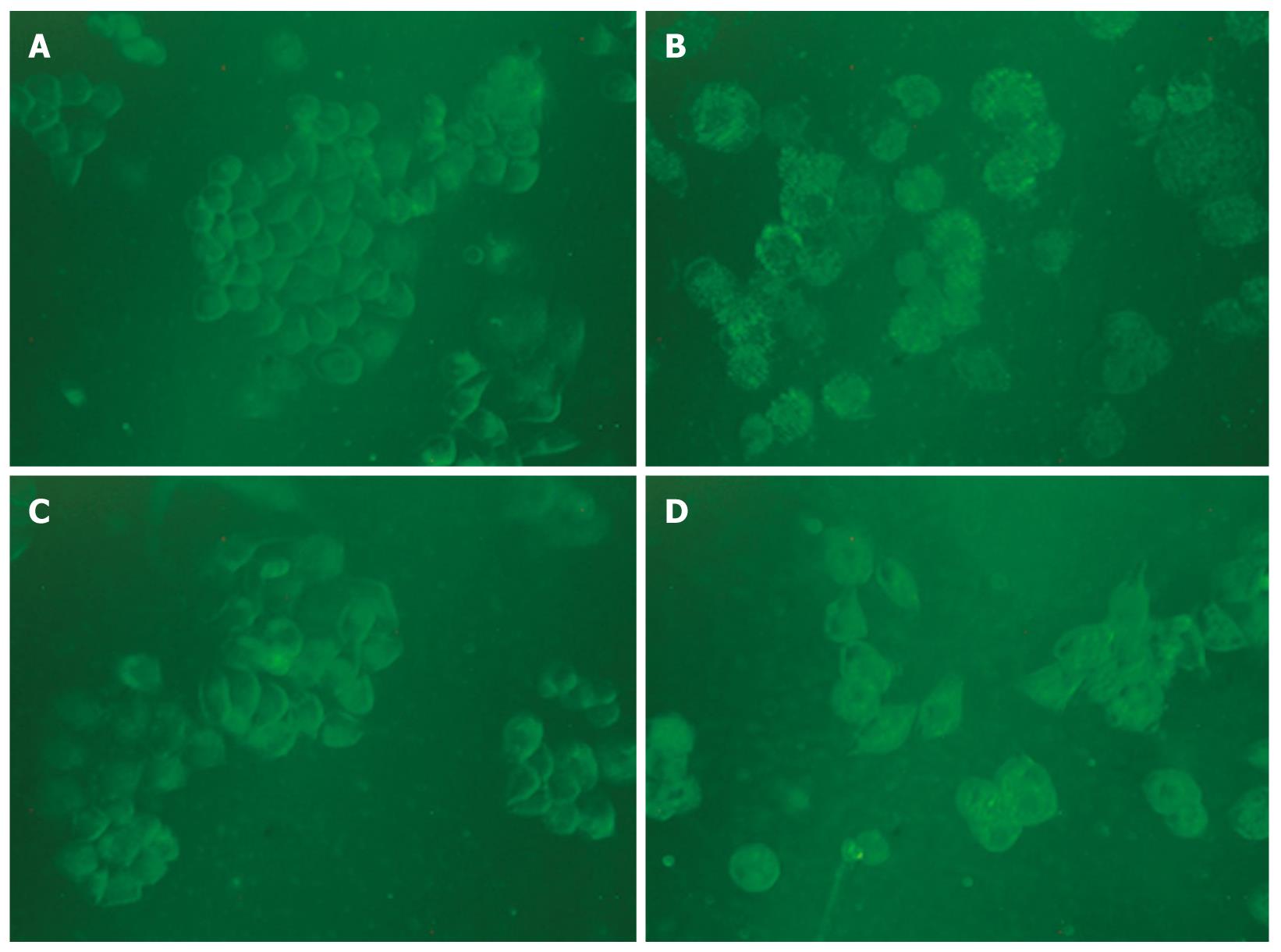
MDC-labeled vacuoles in matrine-treated HepG2 cells
It has been reported that MDC is a specific marker for autophagic vacuoles[34]. When the cells were viewed under a fluorescence microscope, MDC-labeled autophagic vacuoles appeared as distinct dot like structures distributing in cytoplasm or in perinuclear. The fluorescent density and MDC-labeled particles of HepG2 cells were higher in matrine treatment group than in control group (Figure 7), indicating that matrine induces formation of MDC-labeled vacuoles. Fewer autophagic vacuoles were observed in combined 3-MA and matrine treatment group when 3-MA was added before matrine treatment, showing that 3-MA exerts its inhibitory effects on matrine-treated autophagy.
Figure 7 Monodansylcadaverine-labeled vacuoles in HepG2 cells.
Autophagic vacuoles were labeled with 0.05 mmol/L monodansylcadaverine (MDC) in phosphate-buffered saline (PBS) at 37°C for 10 min. The fluorescent density and the MDC-labeled particles in HepG2 cells were higher in matrine treatment group than in control group. The number of MDC-labeled particles in HepG2 cells was significantly lower in combined 3-MA and matrine treatment group than in single matrine treatment group (× 400 magnifications). A: Control; B: Matrine; C: 3-MA; D: 3-MA + matrine.
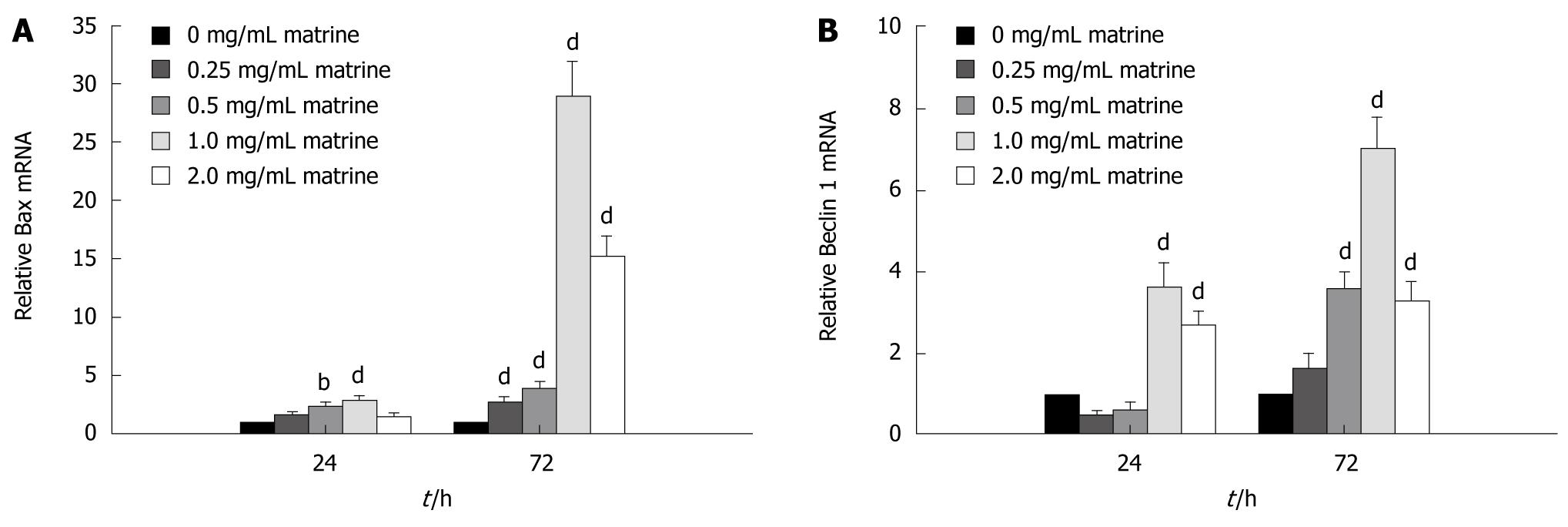
Matrine up-regulated mRNA expression of Bax and Beclin 1 in HepG2 cells
In order to understand the molecular mechanism underlying the apoptosis induced by matrine, the mRNA expression level of Bax gene, an apoptosis-related molecule, in HepG2 cells treated with matrine was measured by real-time quantitative RT-PCR, which showed that matrine up-regulated the Bax mRNA expression in HepG2 cells in a dose- and time-dependent manner (Figure 8A). Following 24 h of treatment, the Bax mRNA expression level increased nearly 3-fold and 30-fold, respectively, when the cells were treated with matrine at the concentration of 1.0 mg/mL for 24 and 72 h. At the same time, matrine gradually increased the Bax mRNA expression level in HepG2 cells when the concentration of matrine was increased.
Figure 8 Matrine up-regulates the mRNA expression level of Bax (A) and Beclin 1 (B) in HepG2 cells.
Data are shown as mean ± SD of three independent experiments. bP < 0.01, dP < 0.001 vs control group.
To elucidate the mechanism underlying the autophagy induced by matrine, real-time quantitative RT-PCR was performed to evaluate the effect of matrine on mRNA expression of Beclin 1, which plays a key role in autophagy[35]. Real-time quantitative RT-PCR showed that matrine activated the Beclin 1 gene expression in a dose- and time-dependent manner (Figure 8B). In other words, the Beclin 1 mRNA expression level steadily increased with the increasing drug concentration and action time. It was interesting that the mRNA expression level of Bax and Beclin 1 was lower in HepG2 cells treated with matrine at the concentration of 2.0 mg/mL than in those treated with matrine at the concentration of 1.0 mg/mL, indicating that matrine at the concentration of 2.0 mg/mL exerts its direct cytotoxic effect on necrosis of HepG2 cells.
DISCUSSION
Both in vivo and in vitro studies showed that matrine inhibits the proliferation of tumor cells[10,27-29]. In this study, matrine obviously inhibited the growth of HepG2 cells in a dose- and time-dependent manner (Figure 2). Flow cytometry showed that matrine markedly arrested HepG2 cells in G0/G1 phase of cell cycle (Figure 3), which is consistent with the reported findings[8], suggesting that retardation of cell cycle progression may be one of the mechanisms underlying the antiproliferative effect of matrine.
It has been reported that matrine induces apoptosis in gastric cancer MKN45 and SGC-7901 cells, leukemia U937 and K562 cells, and C6 glioma cells[25,26,28,29,36]. In this study, matrine induced apoptosis of HepG2 cells in a dose-dependent manner (Figure 4). Consistent with the ability of matrine to kill cells via apoptotic processes, matrine up-regulated the expression of proapoptosis gene, Bax, in a dose- and time-dependent manner (Figure 8A), indicating that the increased Bax expression may trigger matrine-induced apoptosis of HepG2 cells, which is in agreement with the reported findings[28].
In this study, abundant cytoplasmic vacuoles were observed in matrine-treated HepG2 cells under an inverted phase contrast microscope (Figure 5). Electron microscopy showed autophagosomes in HepG2 cells treated with matrine at the concentration of 1.0 mg/mL (Figure 6). When the specific autophagic inhibitor, 3-MA, was applied, the number of autophagic vacuoles greatly decreased. MDC staining demonstrated independent evidence supporting the conclusion that matrine triggers autophagocytosis (Figure 7). The results of our study demonstrated that both autophagy and apoptosis were activated when death of HepG2 cells occurred after matrine treatment, revealing that the mechanism underlying the cytocidal action of matrine may be more complex than it has been reported. Autophagy, an evolutionarily conserved process, regulates cell death in both physiological and pathophysiological conditions[37-39]. In normal cells, autophagy contributes to the turnover of long-lived proteins and elimination of damaged or aged organelles to maintain cell homeostasis[40,41]. While under pathological conditions, autophagy is generally considered to play a prosurvival role. However, extensive autophagy or inappropriate activation of autophagy results in autophagic cell death (type II programmed cell death), which is an important cell death process besides apoptosis. Recently, increasing evidence indicates that autophagy is closely associated with tumors and plays an important role in human tumor suppression[35,41,42]. Autophagy has been observed in response to anticancer agents, such as vitamin D analogues[43], resveratrol[44], arsenic trioxide[45], tamoxifen[46], temozolomide[47] and rapamycin[48], indicating that autophagy can be potentially used in treatment of cancer. Furthermore, it has been reported that agents can directly lead to autophagic cell death. For example, caspase inhibitor induces autophagic cell death of L929 and U937 cells [49], which is consistent with that of human leukemia HL60 cells treated with Eupalitin A[50]. In addition, 5-fluorouracil induces autophagic cell death of Bax or PUMA deficient human colon cancer cells[51], indicating that induction of autophagy may be a promising new approach to treatment of tumor cells. In this study, a novel activity of matrine was identified in HepG2 cells, namely the ability of matrine to induce autophagy. In line with our data, matrine-induced autophagy in rat C6 glioma cells has been reported[29], suggesting that autophagic cell death induced by matrine underlines its potential utility as a new cancer treatment modality.
In this study, the mRNA expression level of Beclin 1 in HepG2 cells was measured. Beclin 1, a mammalian orthologue of the yeast Apg6/Vps30 gene, is the first identified mammalian gene to induce autophagy[35]. The Beclin 1 gene is mapped to the human chromosome 17q21[52], which is monoallelically deleted in 40%-75% of human prostate, breast, and ovarian cancers[53]. Ectopic expression of Beclin 1 restores full autophagy potential in Beclin 1 deficient MCF-7 cells[35]. Moreover, the incidence of spontaneous tumors is high in Beclin 1+/- heterozygous mice and Beclin 1-/- homozygous embryonic stem cells exhibit a decreased number of autophagic vesicles, establishing that Beclin 1 is a critical component of mammalian autophagy and a haploinsufficient tumor suppressor gene[42,54]. Beclin 1 functions in autophagy as part of class III phosphatidylinositol 3-kinase (PI3k) complex, which is necessary for the formation of autophagosome during the autophagic sequestration process[55,56]. In our study, matrine treatment increased the expression of Beclin 1 in HepG2 cells in a dose- and time-dependent manner (Figure 8B), indicating that Beclin 1 is involved in matrine-induced autophagy.
Additionally, although matrine simultaneously induced both apoptosis and autophagy in HepG2 cells in our study, the relation between apoptosis and autophagy remains unknown. Further study is needed to analyze the relation between related molecules at protein level.
In conclusion, matrine is a potent antitumor agent and exerts its antineoplastic action by inhibiting cell proliferation and inducing cell apoptosis and autophagy. Autophagic cell death induced by matrine underlines its potential utility as a new cancer treatment modality. In particular, autophagy may provide leverage to treat HCC that is chemoresistant on the basis of ineffective apoptosis. Beclin 1 is involved in matrine-induced autophagy and the pro-apoptotic mechanism of matrine may be related to its upregulation of Bax expression.
COMMENTS
Background
The incidence of hepatocellular carcinoma (HCC) increases in the world while many patients are diagnosed with HCC at its advanced stage. Since no effective palliative chemotherapy is available for HCC, the prognosis of patients with advanced HCC is dismal. Recently, interest has been generated in the antitumor activity of matrine, which has been widely used in treatment of various diseases.
Research frontiers
Autophagy is closely associated with tumors and plays an important role in human tumor suppression. Furthermore, it has been reported that agents directly cause autophagic cell death. Matrine induces autophagy in human hepatoma G2 (HepG2) cells. This study investigated the effect of matrine on the proliferation, cell cycle, apoptosis and autophagy of HepG2 cells and its molecular mechanism involved in antineoplastic activities.
Innovations and breakthroughs
In this paper, the authors identified a novel activity of matrine in HepG2 cells, namely the ability of matrine to induce autophagy, revealing that the mechanism underlying the cytocidal action of matrine may be more complex than it has been previously reported. The authors further demonstrated that Beclin 1 was involved in matrine-induced autophagy and the pro-apoptotic mechanisms of matrine might be related to its upregulation of Bax expression. Autophagic cell death induced by matrine underlines its potential utility as a new cancer treatment modality.
Applications
Matrine may be used as a potentially promising reagent in treatment of hepatocellular carcinoma. In particular, autophagy may provide leverage to treat HCC that is chemoresistant on the basis of ineffective apoptosis.
Terminology
Autophagy, an evolutionarily conserved process, regulates cell death in both physiological and pathophysiological conditions. Autophagic cell death (type II programmed cell death) is an important cell death process besides apoptosis. Beclin 1, a mammalian orthologue of the yeast Apg6/Vps30 gene, is the first identified mammalian gene to induce autophagy and a haploinsufficient tumor suppressor gene. Bax, a pro-apoptotic molecule, is closely related to apoptosis.
Peer review
This is an interesting study. The study investigated the effect of matrine, one of the main alkaloid components extracted from the Sophora root, on the proliferation, apoptosis and autophagy of HepG2 cells. Matrine has been widely used in treatment of various diseases. The authors showed that matrine inhibited the proliferation and stimulated the apoptosis and autophagy of HepG2 cells, indicating that matrine can be used as a potentially promising agent in treatment of cancer.