INTRODUCTION
Inflammation is a complicated progressive process which is initiated by the body in response to tissue injury or infection. Inflammation proceeds via the sequential release of mediators that leads to vasodilatation and increased blood flow, increased vascular permeability, causing the accumulation of a fluid exudates, and the activation of neurosensory pain fibers giving rise to the classical signs of acute inflammation i.e. heat, redness, swelling, and pain[1]. Acute inflammation is associated with high levels of polymorphonuclear cells, particularly neutrophils, whereas chronic or adaptive immune inflammation has higher levels of mononuclear cells, macrophages, T- and B-lymphocytes.
Regulated release of chemokines and expression and activation of cellular adhesion molecules recruits leukocytes at the site of inflammation. Leukocyte recruitment is a critical step in the inflammatory process[2]. Membrane bound vascular cell adhesion molecule-1 (VCAM-1), intracellular cellular adhesion molecule-1 (ICAM-1), endothelial leukocyte adhesion molecule-1 and E-selectin are expressed on endothelial cells, smooth muscle cells, and tissue macrophages. These adhesion molecules in coordination with others, for example, selectins, allow binding of ligands on leukocytes to mediate rolling, firm attachment and transendothelial migration. Endothelial cells being the interface between the tissue and circulation play an important role in inflammatory and immune-relevant cells[3,4].
Acute pancreatitis (AP) is an inflammatory condition of the pancreas that involves peripancreatic tissue and remote organs. Excessive systemic inflammatory response syndrome (SIRS) in AP leads to distant organ damage and multiple organ dysfunction syndrome (MODS), which is the primary cause of morbidity and mortality in this condition. Mild AP is self limiting but up to 25% of the patients suffer a severe attack and around 30% of these will die. Approximately half of the deaths in AP occur within the first 2 wk of illness and are generally attributed to organ failure. The rest of the deaths occur weeks to months later, characterized by extensive retroperitoneal pancreatic necrosis and septicemia[5]. AP involves a complex cascade of events initializing in pancreatic acinar cells. An unknown trigger within the pancreas leads to conversion of digestive proenzymes into their active form, initiating auto digestion of the gland causing hemorrhage, necrosis, edema and complete destruction of pancreatic parenchyma. Intrapancreatic activation of trypsinogen by lysosomal hydrolases is an early triggering event in AP[6]. Interestingly both pharmacological and genetic deletion of lysosomal hydrolases like cathepsin B can reduce the severity of pancreatitis[7]. Other pharmacological agents which block trypsinogen activation can also modulate the outcome of AP[8,9].
Immune cells involved in elaborating the inflammatory mediators in AP are the pancreatic acinar cells, endothelial cells, neutrophils, lymphocytes, monocytes and macrophages. Inflammatory mediators believed to participate in the pathophysiology of this condition include: tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1β), interleukin-6 (IL-6), platelet activating factor (PAF), ICAM-1, IL-8, growth related oncogene-a/cytokine-induced neutrophils chemo attractant (GRO-α/CINC), monocyte chemotactic protein-1 (MCP-1), IL-10, complement component C5a, substance P (SP), hydrogen sulfide (H2S), and neutral endopeptidase (NEP)[10]. In recent years, it has become clear that the signaling molecule nuclear factor κB (NF-κB) plays a central role in the initiation and progression of AP[11]. The emerging body of evidence suggest that blocking NF-κB activation can markedly reduce the severity of AP[12,13]. These findings have opened a window of opportunity for the use of selective NF-κB inhibitors in regulating the inflammatory process in AP. The expression levels of various proinflammatory mediators like TNF-α and IL-1β in AP are positively regulated by NF-κB[14,15]. Systemic amplification of AP is associated with excessive release of these inflammatory mediators from local tissue and systemically. This systemic amplification is responsible for most of the mortality associated with AP[16]. Studies indicate that both pancreatic and extra pancreatic (lung, liver, monocytes, macrophages and endothelial cells) activation of NF-κB is associated with development of MODS in AP[17,18].
In this review we discuss the recent advances, till date, pointing towards the fundamental role of different monocyte/macrophage populations in the progression of AP.
MONOCYTE/MACROPHAGE SYSTEM
Macrophages are among the most versatile cells of the body. Compelling evidence has emerged in recent years with respect to their roles in innate immunity, inflammation, and tumor progression. Macrophages are released from bone marrow into the bloodstream as promonocytes which develop into monocytes and migrate to tissue and undergo final differentiation into specific types of tissue resident macrophages[19]. The phenotypes of these tissue resident macrophages vary markedly within tissues, including Kupffer cells in liver, alveolar macrophages in lungs, osteoclasts in bone, microglial cells in brain, Langerhans cells in skin.
Monocyte and macrophage in AP
Since macrophages orchestrate both the initiation and the resolution of inflammation, they are an interesting target for designing a therapeutic strategy focused on the control of systemic effects of AP. Macrophage activation constitutes a key component of the immune response and several proinflammatory cytokines and bacterial products participate actively in the triggering of this process.
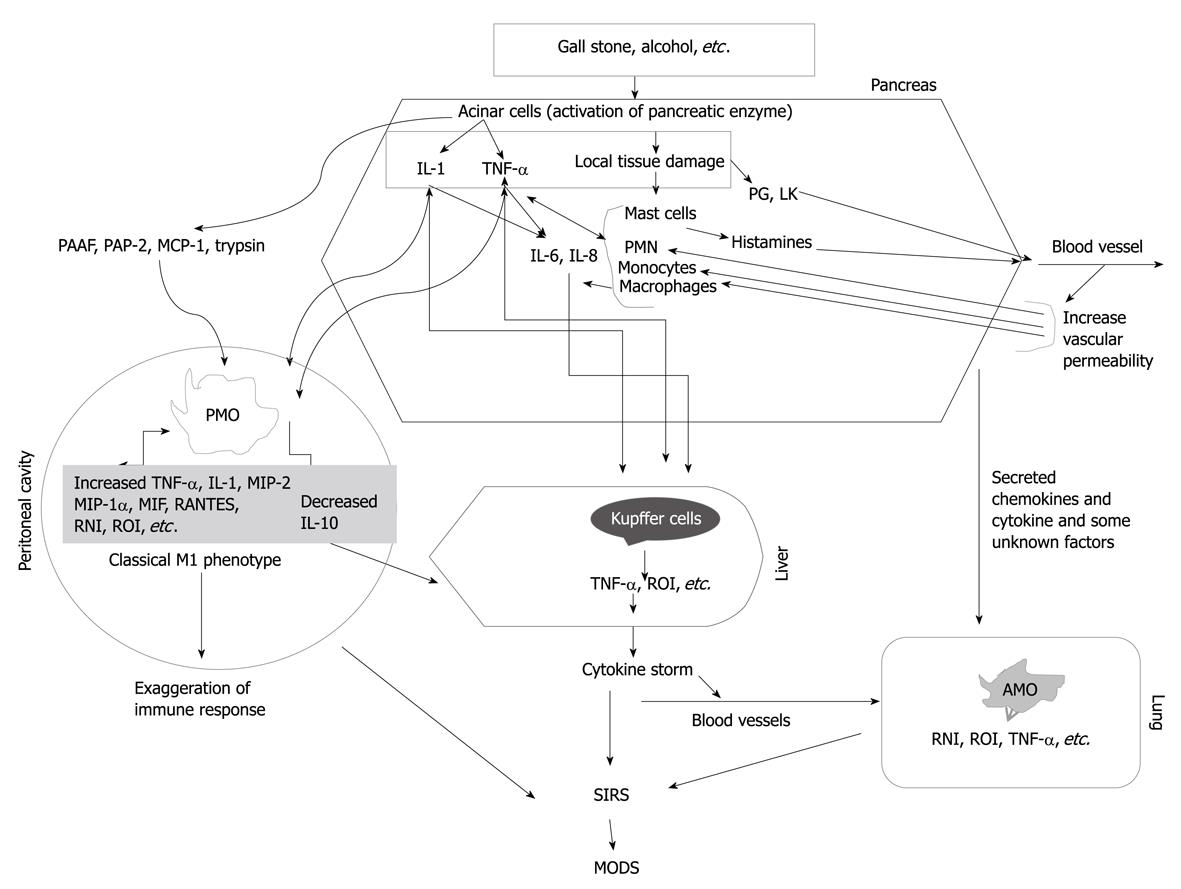
The degree of macrophage activation might be one of the important factors that determine the severity of AP[40]. Besides acinar cells, monocytes/macrophages are the main inflammatory cell population involved in the pathogenesis of AP (Figure 1). In AP, the inflammatory process starts with the migration of monocytes and neutrophils in the circulation into the pancreatic interstitial space, mediated by adhesion molecules on leukocytes. These infiltrating cells assist the production of different cytokines and various inflammatory mediators. As a result, amplification of non-infectious inflammation initiated in the pancreas to specific distant organs such as the lung, liver and spleen occurs[20]. The CC chemokines such as MCP-1, macrophage inflammatory protein (MIP)-1α and RANTES (CCL5) are believed to activate primarily monocytes, whereas the CXC chemokines, such as IL-8, tend to preferentially activate neutrophils. Bhatia et al[21] have shown that administration of bindarit, a blocker of MCP-1 synthesis (prophylactically or therapeutically), significantly reduces the severity of AP suggesting that MCP-1 may be an early inflammatory mediator in AP.
Figure 1 Our current understanding of the role of monocytes and macrophages in the pathogenesis of acute pancreatitis.
Local inflammation in the pancreas leads to secretion of pro-inflammatory cytokines and some unknown factors. These inflammatory mediators activate peritoneal macrophages (PMO), Kupffer cells, and alveolar macrophages (AMO), which if uncontrolled can cause multiple organ dysfunction syndrome (MODS). PMO display a classical M1 type activation in acute pancreatitis thus supporting the inflammatory process. TNF: Tumor necrosis factor; RNI: Reactive nitrogen intermediates; ROI: Reactive oxygen intermediates; PAAF: Pancreatitis associated ascitic fluid; PAP: Pancreatitis-associated protein; MCP: Monocyte chemotactic protein-1; PG: Prostaglandins; LK: Leukotrienes; SIRS: Systemic inflammatory response syndrome; PMN: Polymorphonuclear leukocytes; MIP: Macrophage inflammatory protein.
CD14, is a glycosyl-phosphatidylinositol anchored cell surface molecule expressed on the cell surface of immune effector cells of myeloid and monocytes hematopoietic lineage, which acts in concert with mammalian Toll-like receptors (TLR). It exists in 2 forms - soluble and membrane bound - and the soluble form has both immune stimulatory and inhibiting activities. Increased soluble CD14 expression and expansion of the proinflammatory CD14/CD16 monocytes subset has been reported in AP, suggesting that soluble CD14 receptor may serve as a key mediator of the systemic endothelial response, and its targeted disruption with anti-CD14 monoclonal antibodies may afford some therapeutic benefit in preventing the development of MODS and septic complications associated with AP[22]. Li et al[23] demonstrated that during early stages of AP, expression of TLR4 is upregulated in peripheral blood mononuclear cells (PBMC) with a simultaneous increase in TNF-α expression suggesting an important role of TLR-4 in pathogenesis of AP. TNF-α and IL-1β are regarded as most prominent “first-line” cytokines. Studies have indicated that during the course of pancreatitis, initially IL-1β and TNF-α are produced in the pancreas by acinar cells, in a process mediated by leukocytes, and organs such as liver, lungs and spleen which have large populations of macrophages, serve as subsequent sources of production of proinflammatory cytokines[24]. The pancreas itself contains an unknown proportion of resident macrophages. The liver and lung are susceptible to injury and organ failure during AP. Peritoneal macrophages, Kupffer cells and alveolar macrophages play a pivotal role in controlling the progression of AP, due to their ability to generate pro- and anti-inflammatory mediators.
Peritoneal macrophages
Peritoneal inflammatory cells play an important role in the production of chemical mediators and in the defense against infection of the abdominal cavity. Macrophages are the major resident immune cells within the pancreas and in the peritoneal cavity. Severe AP (SAP) and early multi system organ failure are associated with the sustained release of both pro- and anti-inflammatory cytokines in the ascitic fluid, the thoracic lymph and the systemic circulation. The peritoneal compartment which is in the close vicinity to the site of pancreatic inflammation and necrosis is the site for net proinflammatory reaction. Release of IL-1β and TNF-α by peritoneal macrophages in early stages of AP induces a cascade of other inflammatory cytokines, activation of neutrophils, and induction of the pro-inflammatory response whereas the anti-inflammatory response is mainly systemic in nature. Preventing the activity of both cytokines concurrently has no additional effect on the degree of pancreatitis but does attenuate the systemic stress response and is associated with an additional but modest decrease in mortality[10]. Thus, in AP, peritoneal macrophages act as a principal contributor to the acute systemic inflammatory response that in turn determines the severity of disease. Peritoneal macrophages isolated from rats with SAP showed over production of nitric oxide. Increased nitric oxide secretion is implicated in progression of AP[25]. Abnormal trypsin activation in the pancreas contributes to the pathogenesis of AP. Lundberg et al[26] showed that trypsin stimulates the production of cytokines in peritoneal macrophages and that injection of trypsin into the peritoneal cavity induces lung injury. Hori et al[27] demonstrated that TGF-β produced by peritoneal macrophages induces hepatocellular injury via apoptosis in the rat SAP model.
Studies demonstrate that pancreatitis associated ascitic fluid (PAAF) of SAP affects peritoneal macrophage functions thereby contributing to the pathologic course of disease. They showed that incubation of peritoneal macrophages with PAAF leads to rapid and prolonged activation of NF-κB and TNF-α production[28]. The major site of TNF-α gene transcription in AP is the pancreatic activated macrophages[29]. Their deactivation in the early course of AP increases survival and decreases the severity of disease. Liu et al[30] suggested that activation of NF-κB and p38 MAPK in monocytes/macrophages from animals with AP might play a role in transcription and biosynthesis of TNF-α and IL-6. Animal experiments indicated that sterile ascites without cytokines from AP can stimulate production of cytokines from macrophages derived from spleen and lung in vitro and can induce cytokine production systemically in vivo[31].
Mikami et al[32,33], by depleting peritoneal macrophages using liposome encapsulated dichloromethylene biphosphonate, suggested that peritoneal macrophages extend inflammation from the pancreas to the peritoneal cavity and subsequently induce lung injury leading to SIRS and multiple organ failure opening the possibility that therapeutic modification of peritoneal macrophages may be a new therapeutic approach in patients with AP. The immune response in AP depends to a larger extent on macrophages as they represent about a third of infiltrating mononuclear cells. Macrophages are the main source for the production of inflammatory mediators; their presence in pancreatitis might contribute to the amplification of the immune response during disease progression.
With the progression of AP, there is a change in number and ratio of CD4+ and CD8+ lymphocytes indicating the possible involvement of T-lymphocytes. Observation by Demlos et al[34] demonstrated that CD4+ T cell depletion reduces the severity of pancreatitis. CD4+ T cells act as a costimulator for macrophage activation via antigen presentation and proinflammatory cytokine release[35].
Activation and trafficking of inflammatory cells involves chemokines and their receptors. Interestingly deletion of the receptor for MIP-1α and RANTES, the CCR1 receptor, was associated with protection from pulmonary inflammation but did not reduce the severity of cerulein-induced pancreatitis. This protection from lung injury is associated with decreased levels of TNF-α in a temporal sequence indicating the critical role of CCR1 receptor in the extension of pancreatic injury to the systemic response. The authors underline these findings by suggesting that CCR1 may be activated on either peritoneal or lung macrophages, leading to an autocrine process whereby increased levels of TNF-α drive further induction of both α and β chemokines, resulting in recruitment of inflammatory cells to the pancreas and lung[36]. However, in the same model, inhibition of CXCL2 (MIP-2) protected partially against both pancreatic and lung injury[37]. Attenuation of pancreatic injury in this study may be due to the fact that CXCL2 (MIP-2), in contrast to CCL5 (RANTES) and CCL3 (MIP-α), also attracts neutrophils in addition to monocytes. Macrophage migration inhibitory factor (MIF), a proinflammatory cytokine released by macrophages and lymphocytes, is emerging as an important player in the pathogenesis of AP. Pre-treatment with anti-MIF antibodies improved the survival in rats with AP[38]. Recently, work by Sorli et al[39] suggested that peritoneal macrophages showed a classical M1 phenotype in AP, characterized by high expression of TNF-α and lack of changes in the mannose receptor. TNF-α can in turn activate macrophages for release of other macrophage derived inflammatory cytokines thus exaggerating the inflammatory response. Their study also indicates that peritoneal macrophages could be reprogrammed in vitro to reparative alternatively activated (M2) macrophages by IL-4 and IL-13 thus opening the possibility that therapeutic modulation of macrophage activation can help in the treatment of AP[39].
In any immune response dying cells are normally phagocytosed by macrophages; this elimination of dying cells is associated with anti-inflammatory cytokine switching. Recent observations by Liang et al[40] have suggested that there is a close relation between modes of pancreatic acinar cell death, the release of cell contents and the inflammatory reaction of peritoneal inflammatory cells.
In recent years, neuropeptide SP has gained considerable attention as a mediator of neurogenic regulation of inflammation. Earlier work by Sun et al[41] has suggested that neuropeptide SP is a pro-inflammatory mediator involved in pancreatitis and during acute inflammation, SP induces chemokine release from macrophages infiltrating into local and distant damaged tissues. Mice lacking NK-1Rs which bind to SP are protected against cerulein induced pancreatitis and associated lung injury. Macrophages have receptors for SP. Recently we have also shown the effect of SP on the murine macrophage cell line RAW264.7, as well as isolated primary macrophages, indicating that SP, at nanomolar concentrations, elicited selective chemokine production from murine macrophages[42]. Indeed, unpublished observations from our lab indicate that macrophages isolated from mice lacking NK-1R receptors show M2 phenotype displaying an anti-inflammatory cytokine switch. These observations led us to conclude that SP, acting via NK-1R on macrophages, plays an important role in regulating the severity of pancreatitis and associated lung injury.
Pancreatitis-associated proteins (PAP) are members of the Reg gene family; these 14-17 kDa proteins have been shown to be strongly induced during AP[43]. Although originally identified during AP, they have been reported in other inflamed pathologic organ systems including Crohn’s disease, inflammatory bowel disease, liver injury, neuronal, ovarian, and cardiac tissue damage[44,45]. Recent investigation suggests that PAP2 is a potential mediator of early inflammation in AP, its act specifically by orchestrating the macrophage inflammatory response, and may do so by working in concert with other PAP isoforms[46]. They also demonstrated that macrophages may express a potential PAP2 receptor[46].
Kupffer cells
Liver macrophages or Kupffer cells are the most abundant mononuclear phagocytes in the body. They are a predominant source of inflammatory cytokine released in the systemic circulation. Cytokine release from the liver represents about 50% of total cytokine release from the body[47]. Recent studies have shown the involvement of the liver in complex networks of events triggering the multiorgan dysfunction associated with AP. During AP the inflamed pancreas generates soluble inflammatory mediators. Once pancreatic mediators reach the liver, they strongly activate Kupffer cells (resident macrophages), they then greatly amplify the release of cytokines into the blood stream and thus contribute to the systemic manifestation of AP. Activated Kupffer cells release different inflammatory mediators, immunoregulatory and inflammatory cytokines, reactive nitrogen intermediates (RNI), reactive oxygen intermediates (ROI), hydrogen peroxide, etc. which play a significant role in progression of pancreatic inflammation into a systemic process[48]. TNF-α released from the pancreas triggers the early events in AP, as pancreatitis progresses TNF-α reaches the liver, further manifesting the disease outcome by causing a cytokine storm. This cytokine is considered as one of the most important mediators of the systemic complications associated with AP[49]. The observation that in native pancreatitis the systemic manifestation of AP is more severe as compared to graft pancreatitis (as the mediators released by pancreas in graft pancreatitis are sent directly through the iliac vein) suggested that the liver plays an active role in the development of lung injury secondary to AP[50]. Gloor et al[51,52] further confirm this observation by preventing the passage of blood coming from pancreas to liver. Interestingly it has been shown that blocking the activity of Kupffer cells by gadolinium chloride in a sodium taurocholate AP model significantly reduces the increased TNF-α in serum during pancreatitis to its control levels, followed by reducing the inflammatory response in the lung, and in turn reducing the systemic complications associated with AP and improving the survival rate in mice. The authors confirm their observation in vitro, by using cultured Kupffer cells[53,54]. Additionally, inhibiting the Kupffer cell activity before induction of AP significantly diminished the associated lung injury with a decrease in NF-κB activity[55]. Altogether, these results show a link between the local inflammatory process in the pancreas and its manifestation by liver cells, especially Kupffer cells, causing the appearance of systemic organ dysfunction secondary to AP.
Alveolar macrophages
Patients with AP may develop acute lung injury, manifest clinically as the adult respiratory distress syndrome (ARDS). Most patients who die during the early stages of SAP die either with or as a result of this lung injury. However, the events that link AP to acute lung injury are not fully understood. It has been postulated that alveolar macrophages (AM) are involved in development of acute local disorders as a consequence of extra pulmonary stimuli like pancreatitis, peritonitis, or trauma. AM have the capacity to secrete a very large number of inflammatory mediators, including lipid mediators, chemokines, cytokines, growth factors and reactive oxygen and nitrogen species. They may therefore play multiple roles in the respiratory tract and may be pro-inflammatory or anti-inflammatory. They may be activated by several stimuli, including cigarette smoke, pro-inflammatory cytokines, endotoxin and immune stimuli. Their capacity to release multiple chemokines leads to the recruitment of several cell types from the circulation, including monocytes, neutrophils and T lymphocytes. Polymorphonuclear neutrophils play an important role in the development of ARDS and activation of the complement system, and generation of AM derived factors promotes neutrophil aggregation into lungs. The capacity of AM to mobilize a large amount of leukocyte and to release secretory products such as RNI and ROI suggests that these cells can be involved in lung damage associated with AP. The activation of AM seems to be regulated by cytokines and inflammatory mediators, which are generated during the course of AP. Sailai et al[56] suggested that the inhibition of NF-κB activation may reverse the lung injury of acute necrotizing pancreatitis (ANP) by inhibiting the release of inflammatory mediators by AM. Nitric oxide (NO) is an important marker of oxidative injury in the lung. High TNF-α and NO released by activated AM in ANP suggest a role for AM in inducing lung injury associated with ANP[57]. Studies have suggested that, during the early phase of AP, AM-derived NO contributes to lung injury. Administration of the NOS inhibitor L-NMMA prevented lung injury in this model. In conclusion, this study shows that lung damage induced by experimental AP develops with AM activation. The liver plays an active role in the activation of AM in this experimental model[58]. In addition, neutrophil recruitment into the lungs during AP seems to be mediated by chemotactic mediators (TNF-α and MIP-2) released by activated AM.
In AP, endothelial cells, polymorphonuclear neutrophils and macrophages release PAF, which has been implicated as a key mediator in the progression of AP, leading to complications and unacceptably high mortality rates. PAF participates in the occurrence and development of AP and administration of platelet-activating factor receptor antagonists (PAF-RAs) could significantly reduce local and systemic events after AP[59]. BN52021 (PAF-RA) prevented the labilization of the lysosomal membrane of AM thus suggesting that complications of AP could be dependent on the stabilization of AM lysosomes[60]. Another PAF antagonist (TCV-309) prevented hyperactivity of AM by reducing cytokine induced neutrophil chemoattractant expression by AM[61]. These studies further point to the use of PAF antagonist in reducing the secondary complications associated with AP.
Hsp72 induction is associated with the early stages of lung neutrophil infiltration. In a study using a 5% sodium taurocholate model of AP it was found that Hsp72-was over expressed in bronchial epithelium, alveolar macrophages, infiltrating neutrophils, blood vessels and blocking of P-selectin activity diminished the expression of Hsp72 in lungs thus suggesting that Hsp72 induction is mediated by neutrophil infiltration into the lungs[62].
Macrophages generate ROI and therefore contribute to the increased oxidative stress. Increased levels of pancreatic phospholipase A2 (PLA2) are detected in the systemic circulation and bronchoalveolar lavage fluid from patients with lung injury in AP. Reports indicate that PLA2 regulates the cytokine production of monocyte/macrophages and the phagocytosis and superoxide (O2-) generation of neutrophils[63]. AMs are activated by PLA2 and produce a large amount of NO that contributes to lung injury in AP. Blocking of PLA2 activity prevented the lung injury associated with pancreatitis[64].
The secretion of multiple inflammatory proteins by AM is largely a result of increased expression of inflammatory genes orchestrated by proinflammatory transcription factors, such as NF-κB and AP-1. Sphingosine-1-phosphate (S1P), a biological active lipid generated by numerous cell types, significantly reduced the NF-κB activation of the AM, ameliorating pulmonary injury[65], thus suggesting that AM could be a therapeutic target of S1P for combating pulmonary injury associated with pancreatitis.