Published online Aug 21, 2010. doi: 10.3748/wjg.v16.i31.3928
Revised: April 26, 2010
Accepted: May 3, 2010
Published online: August 21, 2010
AIM: To investigate whether irradiation (IR) and partial hepatectomy (PH) may prepare the host liver for non-parenchymal cell (NPC) transplantation.
METHODS: Livers of dipeptidyl peptidase IV (DPPIV)-deficient rats were pre-conditioned with external beam IR (25 Gy) delivered to two-thirds of the right liver lobules followed by a one-third PH of the untreated lobule. DPPIV-positive liver cells (NPC preparations enriched for liver sinusoidal endothelial cells (LSECs) and hepatocytes) were transplanted via the spleen into the recipient livers. The extent and quality of donor cell engraftment and growth was studied over a long-term interval of 16 wk after transplantation.
RESULTS: Host liver staining demonstrated 3 different repopulation types. Well defined clusters of donor-derived hepatocytes with canalicular expression of DPPIV were detectable either adjacent to or in between large areas of donor cells (covering up to 90% of the section plane) co-expressing the endothelial marker platelet endothelial cell adhesion molecule. The third type consisted of formations of DPPIV-positive duct-like structures which co-localized with biliary epithelial CD49f.
CONCLUSION: Liver IR and PH as a preconditioning stimulus enables multiple cell liver repopulation by donor hepatocytes, LSECs, and bile duct cells.
- Citation: Krause P, Rave-Fränk M, Wolff HA, Becker H, Christiansen H, Koenig S. Liver sinusoidal endothelial and biliary cell repopulation following irradiation and partial hepatectomy. World J Gastroenterol 2010; 16(31): 3928-3935
- URL: https://www.wjgnet.com/1007-9327/full/v16/i31/3928.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i31.3928
Hepatocyte transplantation is considered to be a promising option for the treatment of both acute and chronic liver failure as well as for the correction of end stage metabolic liver disease[1]. However, host liver repopulation by transplanted hepatocytes requires a special preparative regimen combining the induced failure of endogenous cell proliferation with some strong mitogenic stimulus. There are a number of experimental protocols based on the application of DNA-damaging toxins such as the pyrrolizidine alkaloids retrorsine[2] or monocrotaline[3], which efficiently inhibits the proliferative capacity of endogenous hepatocytes; however these systemically harmful and potentially carcinogenic substances are not suitable for preparation of the human liver.
Recently, we developed a preclinical rat model using external beam liver irradiation (IR) with 25 Gy administered to the right liver lobules (2/3 of liver mass) in combination with 1/3 partial hepatectomy (PH) of the untreated left liver lobule. In that study, the aim was to prime the residual host liver mass for selective donor cell growth, leading to significant liver repopulation by donor hepatocytes[4]. In a further study, we investigated the underlying molecular effects, demonstrating that IR suppressed the regeneration of endogenous hepatocytes in response to PH through a persistent block of the cell cycle[5]. Additionally, we were able to reveal considerable damage to the liver sinusoidal endothelial cells (LSECs), contributing to the notion that these cells are particularly vulnerable to IR[6,7]. However, there is a paucity of data regarding the possible IR damage to bile duct cells and its consequences for cell therapy purposes.
Therefore, the aim of this study was to investigate whether liver injury following IR and PH could not only enable donor hepatocyte proliferation, but more importantly the growth of transplanted non-parenchymal cells (NPCs) in the host parenchyma. With this in mind, we hypothesized that host liver IR permits the replacement and reconstitution of endogenous endothelial as well as biliary cells by donor cells.
Medium and buffers were supplied by Gibco Brl, Germany. All further chemicals were reagent grade and, unless specified otherwise, were supplied by Sigma-Aldrich (Munich, Germany). Primary antibodies were used in this study as summarized in Table 1. Secondary peroxidase-conjugated antibodies (EnVision Kit) were purchased from DAKO Diagnostica, Germany. Secondary species-specific fluorescence conjugated antibodies (Alexa Fluor 488, Alexa Fluor 555) were obtained from Molecular Probes (Goettingen, Germany).
| Antibody/detected antigen | Species | Manufacturer | Cat. No. | Dilution |
| DPPIV (dipeptidyl peptidase IV = CD26) | Mouse monoclonal IgG2a | BD pharmingen | 559 639 | 1:100 |
| CD45 (leukocyte common antigen) | Mouse monoclonal IgG1 | BD pharmingen | 554 875 | 1:20 |
| CD49f (integrin α6) | Mouse monoclonal IgG1 | Serotec | MCA 2034 | 1:500 |
| Desmin (hepatic stellate cells) | Rabbit | Lab vision | RB-9014 | 1:500 |
| Connexin 32 = CX32 (gap junction protein) | Rabbit | Sigma-aldrich | C3595 | 1:5000 |
| HSE (hepatic sinusoidal endothelial cells) | Mouse monoclonal IgG2a | IBL | 10 078 | 1:500 |
| CD31 (PECAM-1 = platelet endothelial cell adhesion molecule-1) | Mouse monoclonal IgG1 | BD pharmingen | 55 025 | 1:1000 |
Hepatocytes and NPCs were isolated in a 2-step in situ collagenase digestion of the liver[8]. The hepatocytes were segregated using gradual centrifugation steps at 35 g for 10 and 5 min and processed separately. Freshly isolated hepatocytes (purification grade approx 98%) displaying a vitality of greater than 90% (tested with trypan blue exclusion) and cell attachment greater than 70% proved to be sufficient for further transplantation experiments. NPCs in the supernatant were centrifuged for 7 min at 400 g and resuspended in 20 mL phosphate-buffered saline (PBS). The suspension was further processed by a 2-step Percoll gradient centrifugation (25% and 50%) for 10 min at 1000 g aimed at enriching the endothelial cell content. The cells of the interface were washed for 10 min at 200 g and resuspended in 800 μL PBS for transplantation. Cytospins were performed to characterize the transplanted cells with immunohistochemistry.
As recipients, a strain of dipeptidyl peptidase IV (DPPIV)-deficient Fisher 344 rats was established in the animal care facility of the University Medical Centre Goettingen, Germany. Syngeneic donor Fisher 344 rats were purchased from Charles River, Germany. All animal breeding, care, and experimentation procedures were in accordance with German national legislation on animal protection. All procedures were performed under constant sevoflurane/oxygen inhalation. Buprenorphine (0.1 mg/kg body weight) was applied intraperitoneally during anesthesia, and was repeated subcutaneously 8-12 h later.
Recipient livers of rats were preconditioned with external beam, computed tomography-based partial liver IR (25 Gy) of the right liver lobules (2/3 of hepatic mass) 4 d prior to 1/3 partial hepatectomy (PH) as described previously[4]. For transplantation experiments, the spleen was mobilized and the cell suspension was slowly injected over 3 min into the parenchyma, from where they are known to migrate via the portal vein into the recipient liver (all residual liver lobules). In experimental group 1, rats were transplanted with 20 × 106 NPCs and additionally received 12 × 106 hepatocytes. Experimental group 2 was only transplanted with NPC preparations. Control animals were transplanted with hepatocytes only.
Rats were sacrificed for tissue analysis after 1 wk and after the long-term interval of 16 wk following transplantation. Tissue samples from each liver lobe were excised and snap frozen in 2-methylbutane at -80°C. Cryosections of 5 μm thickness were fixed in ice-cold acetone for 10 min.
Cytospins of cell preparations (3 × 104 cells were centrifuged onto a glass slide at 28 g for 5 min) or cryosected tissues were immunostained for the first antigen (incubation with the first primary antibody [anti-CD49f, anti-CD45, anti-desmin, anti-CX32, anti-platelet endothelial cell adhesion molecule (PECAM) or anti-hepatic sinusoidal endothelial (HSE) marker], using Alexa 488-conjugated goat anti-mouse IgG or anti-rabbit for fluorescence detection [1:400, 1 h at room temperature (RT)] and then further processed with the second immunostaining protocol. After rehydration in Tris buffer, specimens were blocked and subsequently incubated with the second primary antibody (anti-DPPIV), rinsed with Tris buffer and exposed to the second Alexa Fluor 568 goat anti-mouse IgG2a (1:400, 1 h at RT). Slides were finally covered with Vectashield® mounting medium with DAPI (1 μL/mL) (Vector Laboratories, UK) to visualize the cell nuclei. Negative controls were used for each antibody by omitting the primary antibody from the protocol. Multiple immunofluorescence-labeled specimens were serially excited and observed with the TEXAS Red-, FITC- and UV-filter sets on an inverted confocal microscope (LEICA DM IRE2, Bensheim, Germany). Pictures of each filter set were digitally merged using image layering software (Leica FW 4000, Version 1.1). The labeling index was expressed as a percentage of positive cells counted.
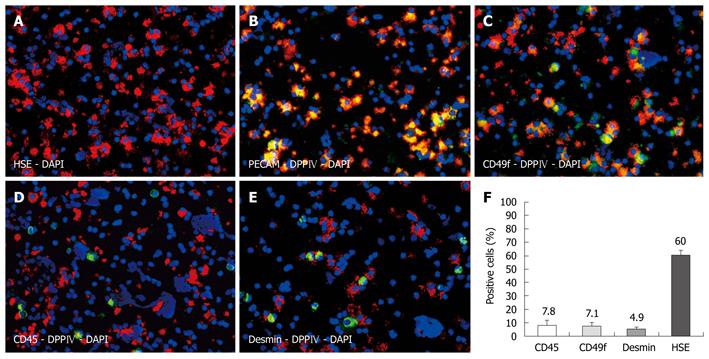
Immunofluorescence co-localization studies were performed on cytospins of freshly isolated NPC preparations enriched with LSECs (Figure 1A), of which 80% were immunoreactive for DPPIV. The majority of all cells displayed the hepatic sinusoidal endothelial marker HSE (60%). For technical reasons, the anti-HSE could not be co-stained with DPPIV, as both antibodies are mouse monoclonal of the same subtype (IgG2a). We therefore used anti-PECAM to co-localize the endothelial marker with the donor-specific antigen in sections of transplanted livers. Indeed, when examining cytospins of NPC preparations, all PECAM-positive cells co-expressed donor specific DPPIV (Figure 1B). Biliary epithelial cells expressing CD49f represented the second largest fraction (7.1%), and these cells were colocalized with DPPIV too (Figure 1C). Furthermore, we assessed co-staining with CD45 and desmin to identify other NPCs. We detected CD45-positive cells (7.8%), representing hematopoietic cells, and were mostly negative for the donor cell antigen (apart from very few activated T lymphocytes) (Figure 1D). Desmin-positive cells (4.9%) did not display DPPIV either (Figure 1E). Figure 1F depicts the labeling indices as stated. We could not detect any cytokeratin 18-positive cells in the NPC preparations, indicating there was no contamination with hepatocytes (data not shown).
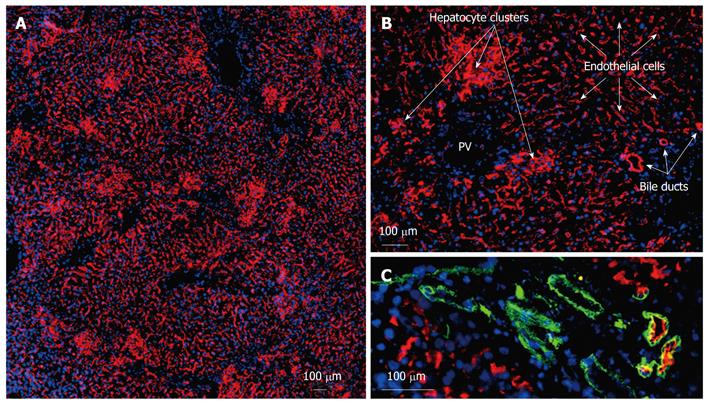
NPC preparations and hepatocytes from donor wild-type rats (DPPIV-positive) (experimental group 1) were transplanted into DPPIV-deficient recipients following the repopulation stimulus of partial IR and PH. Immunofluorescence co-localization studies assessed the extent and quality of liver repopulation after 1 and 16 wk. One week following transplantation, single DPPIV-positive cells and small clusters were detectable in the host liver parenchyma (data not shown). However, after 16 wk, extensive repopulation by donor cells and descendents was documented in the transplanted livers of all groups. On gross examination, the repopulated areas appeared histologically identical to normal, unharmed liver tissue. Morphological evaluation revealed 3 different types of donor-derived cell [Figure 2A (overview) and more detailed in Figure 2B]. Firstly, well-defined clusters of donor cells displaying DPPIV in a canalicular (garland-like) pattern were found in close proximity to the portal areas. These compact clusters appeared to comprise mature hepatocytes with the characteristic enzyme expression of DPPIV in the basolateral membranes. Their size varied and ranged from 40-50 to several hundred cells in diameter. Cells in these clusters co-expressed the hepatocyte differentiation markers cytokeratin 18, connexin 32 (CX32) (gap junction protein enabling intercellular communication) and cytochrome p450 subtype 2B1, revealing intact metabolic function (data not shown). Secondly, large areas of DPPIV-positive donor cells were arranged in a string-like pattern and emerged from the portal veins. They covered a maximum 90% of the section plane and expressed the donor specific antigen as it is known from LSECs (longitudinal cells expressing DPPIV in the cytoplasm as well in the membranes). Thirdly, formation of duct-like structures expressing DPPIV could be detected. These donor-derived cells were mostly found in association with the bile duct system of the recipient liver and co-stained with an antibody detecting the biliary epithelial CD49f (Figure 2C). The red fluorescent DPPIV on the apical side partially overlapped with the green cytoplasmatic staining of CD49f resulting in a yellow ring. These duct-like structures were also found as individual formations outside the donor hepatocyte clusters, mostly but not always encircled by endothelial donor cells.
In experimental group 2 (transplantation of NPC preparations only), the extent of repopulation was similar, apart from the fact that no clusters of hepatocytes could be detected in these host livers. Subtotal repopulation by DPPIV-positive endothelial cells could be visualized in these animals and donor-derived bile duct structures were as frequent as in group 1, being scattered throughout the parenchyma but generally in close proximity to the portal triads.
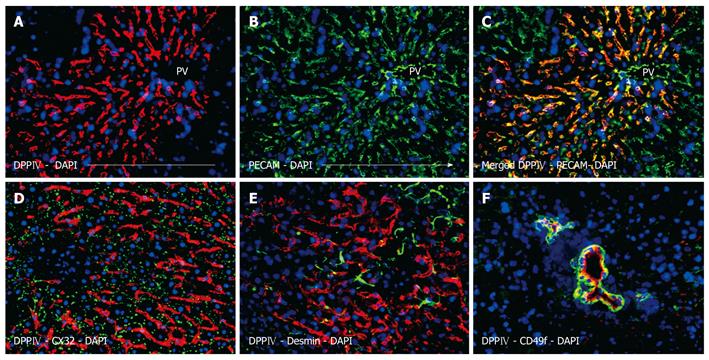
Subsequent analysis revealed the phenotypic characteristics of the transplanted cells and their descendents. The pan-endothelial marker PECAM (CD31) is most commonly used to detect LSECs in situ. In the present study, this marker was also employed to co-localize LSECs with donor specific DPPIV. Co-staining of DPPIV and PECAM resulted in a yellow-orange overlay, suggesting the endothelial phenotype of the string-like repopulation areas (Figure 3A-C). The merged figure clearly illustrates that both antigens were present in the squamous layer of cells that lined the interior surface of the sinusoids. It has to be pointed out that there was no donor-specific DPPIV co-expression in the endothelial cells of the portal vessels.
When the repopulating LSECs were double-labeled for DPPIV and CX32, the punctuated canalicular pattern seen in hepatocytes was not apparent (Figure 3D). Additionally, the LSECs were negative for desmin, a marker of hepatic stellate cells (Ito cells) (Figure 3E). Indeed, desmin-positive host cells could be clearly visualized in between the repopulating donor endothelial cells but also in the surrounding tissue. In both cases, the distribution pattern was not different from healthy liver. Furthermore, there was no evidence of CD45 co-expressing donor blood cells in transplanted livers (data not shown). Figure 3F shows DPPIV-positive ductular cells co-expressing CD49f. In this picture, the donor biliary epithelial cells form duct-like structures of different sizes, which is representative of the overall repopulation of this kind. Some ductules only have a narrow diameter, whereas others form large ducts consisting of some dozen surrounding cells. On examination of serial sections, it appears that the duct formations possess a 3-dimensional structure of communicating tubes.
Hepatocyte transplantation has been used in many animal models. Preconditioning of the host liver prior to cell transfer is regularly used to enhance proliferation of the transplanted cells, up to a near total repopulation of the host liver by donor hepatocytes and their descendents. However, little is known as to whether the replacement of endogenous NPCs may be facilitated in terms of a preparative regimen for cell therapy.
Hepatic IR is an established stimulus for priming the host liver for hepatocyte repopulation purposes in preclinical models[4,9]. Moreover, IR is already being considered for human application in the upper abdomen for the high dose treatment of gastrointestinal and hepatic primary malignancies or metastases[10-13]. IR can be therapeutically targeted to a whole organ or to small portions, but photons always affect the variety of cells present. The liver comprises different cell types, of which the majority represents hepatocytes, making up approximately 80% of hepatic cells[14]. The NPC fraction (20%) comprises endothelial cells, Kupffer cells, stellate cells, epithelial cells of bile ducts, and some neural cells. However, when considering therapeutic strategies, the endothelium and bile duct cells are known to be highly susceptible to injuries following microenvironmental changes (e.g. caused by ischemia/reperfusion, endotoxemia, tumor growth, angiogenesis or the response to cytotoxic treatment)[15-17]. Radiation also induces oxidative stress through the mitochondria-dependent generation of reactive oxygen species and is therefore another potent candidate that may harm NPCs of the liver[18,19]. Considering liver IR as a preparative regimen with clinical prospects, we wanted to demonstrate whether the suggested “multi-cell” damage may be compensated for by subsequent transplantation of liver cell suspensions explicitly containing NPCs solely or in addition to hepatocytes. We used a reliable transplantation model (hepatocytes isolated from wild type Fisher 344 rats were transplanted into DPPIV-negative hosts) to assess the extent and quality of liver repopulation by donor cells[20].
As proof of concept, our study demonstrated that the preparative regimen of IR and PH led to recipient liver repopulation by sinusoidal endothelial and biliary cells. Replacement of the endothelium was extensive, covering up to 90% of the section plane. Bile duct-forming structures could often be detected in the neighborhood of endogenous bile ducts, but biliary cells derived from donors were also seen as individual ductular arrangements in the recipient parenchyma. It has to be pointed out that both types of cell engraftment (endothelial and biliary) were not necessarily bound to additional hepatocyte transplantation. In the experimental group of NPC transplantation only, engraftment and repopulation occurred to the same level and extent as following transplantation in addition to hepatocytes. In the latter group, repopulation by all 3 cell types was rarely seen separated from each other, but more frequently at the same site.
Our results are in line with recent literature reporting that hepatic IR is a highly desirable preparative stimulus[9,21,22]. However, IR in those studies was always performed as an invasive procedure (by laparotomy) with doses of 15-50 Gy administered to the whole liver. We focused on a more clinically acceptable approach of non-invasive external beam IR administered to selected lobules of the liver. The workgroup of Guha also demonstrated the outstanding role of the hepatic sinusoidal endothelial barrier as a key player supporting subsequent donor hepatocyte engraftment[9]. They found that IR caused a transient disruption of the endothelial cell lining with exfoliation facilitating the subsequent passage of donor hepatocytes. Indeed, this report confirms our previously published results revealing the significant damage to LECs following IR [prolonged detection of double strand breaks (phosphorylated histone H2AX)][5]. These observations encouraged us to perform the present transplantation study elucidating the feasibility of NPC replacement in irradiated liver.
There are a few reports in the literature confirming the engraftment of NPCs in preconditioned liver. Brilliant and co-workers used injections of mitomycin C to demonstrate that bile ducts and endothelial cells could be generated within 4 wk following transplantation[23]. This substance is commonly known as a cytotoxin widely used as an effective anti-cancer agent, e.g. to treat bladder carcinoma or gastrointestinal tumors[24,25]. Mitomycin C and PH offered a rapid protocol to assess the engraftment efficiency of fetal liver as well as adult liver isolates. However, the preconditioning protocol resulted in a high morbidity (up to 100%) which could be reduced, though not totally eradicated by the additional application of antibiotics (gentamycin). In our study, both morbidity and mortality in all groups prior to and following hepatocyte transplantation remained low overall (1%-2%). The cases of mortality examined were determined to be caused by individual narcotic or surgical complications than to the preconditioning by IR.
The workgroup of Gupta reported using a murine knockout transplantation model in which the liver endothelium was repopulated sufficiently following the administration of the genotoxic pyrrolizidine alkaloid monocrotaline[26]. They demonstrated that transplanted LECs proliferated and reconstituted 9% of the host liver NPCs after 3 mo, thereby correcting the bleeding phenotype of NOD/SCID hemophilia A mice by elevating the Factor VIII activities to over 10% (13 of 15 animals). This study clearly demonstrated the functional correction of a genetic defect as an excellent example for the feasibility of targeted cell therapy. Once again, the preparative agent monocrotaline could not be specifically targeted at the liver. When applied systemically, monocrotaline is known to cause clinically relevant injuries to the endothelium of the lung, resulting in pulmonary hypertension which may obviate its human application[27].
It is well known that high dose IR of the liver is hindered by the induction of radiation induced liver disease (RILD) and more severely by fatal veno-occlusive disease, a non-thrombotic obliteration of the lumina of small intrahepatic veins initially triggered by endothelial injury leading to the deposition of fibrin-related aggregates in the subendothelial zone[28]. These aggregates, and the intramural entrapment of fluid and cellular debris, progressively occlude the hepatic venous flow and generate intrahepatic hypertension. Owing to the risk of RILD, whole liver IR doses exceeding 30 Gy have to be generally avoided in humans[13]. However, we may address the question as to whether endothelial reconstitution following autologous transplantation may allow for the higher IR doses necessary in the local treatment of advanced intrahepatic tumors and multi-lobular metastases.
Endothelial cell reconstitution may also be used to reduce the host immune reaction in liver transplantation[29]. Taking into account that endothelial cells play a pivotal role in both acute and chronic rejection, the transplant immunogenicity could be significantly reduced by endothelial chimerism. A possible strategy would be to generate a chimeric liver, in which damaged endothelial cells are replaced by host cells. Two different mechanisms may be considered: firstly, endothelial damage can result from ischemia/reperfusion injury sui generis in whole organ transplantation from cadavers, or secondly may be generated by limited dose IR of the donor liver organ. The workgroup of Murase already demonstrated that circulating endogenous bone marrow-derived cells routinely contributed to LSEC repopulation between 1% and 5% in a rat model of naïve orthotopic liver transplantation[30]. Further experimentation might elucidate whether the rate of engraftment is enhanced by exogenously delivered endothelial cells or progenitors[31,32] or by additional IR.
In the present report, we show for the first time that the preconditioning stimulus of liver IR and PH triggers the repopulation of hepatocytes, LSECs and bile duct cells. We may conclude from our results that this “multi-cell” repopulation compensates for the liver tissue damage following IR. The extensive engraftment of endothelial cells in particular offers a variety of new therapeutic concepts concerning high dose IR of the liver, immunological cell chimerism in liver allografts, and the treatment of genetic disorders based upon endothelial cells (e.g. lack of coagulation factors such as Factor VIII and von Willebrand Factor).
Hepatocyte transplantation is regarded as a promising option to correct acute liver failure and hereditary metabolic liver disease. However, the liver not only constitutes mature hepatocytes, but also a multitude of non-parenchymal cells. So far, little is known concerning the supportive role of non-parenchymal cells in cell therapy studies and whether their engraftment and subsequent proliferation in the host parenchyma may be triggered.
Liver repopulation is based on the preferential proliferation of engrafted donor cells in response to some mitogenic stimulus. The aim of this study was to investigate whether irradiation, known to suppress endogenous cell proliferation, and partial hepatectomy as the powerful mitogenic stimulus could prepare the host liver not only for hepatocyte but also for non-parenchymal cell transplantation. Both pretreatment techniques may be considered as suitable in the clinical setting and may be targeted to the implantation site, thereby limiting side effects.
The precondition stimulus of liver irradiation and partial hepatectomy prompted the engraftment of hepatocytes, liver sinusoidal cells and bile duct cells. This “multi-cell” repopulation may not only compensate for irradiation-induced liver damage, thus enabling new therapeutic concepts such as high dose radiotherapy, but also generate immunologically relevant cell chimerism and finally facilitate the correction of genetic disorders based upon endothelial cells. This would broaden the therapeutic potential of liver cell therapy.
As a proof of concept, the study demonstrated that transplanted non-parenchymal cells repopulate the host liver by forming sinusoidal endothelium as well as bile duct-like structures.
This is a well-conducted experimental study that deserves early publication in the journal.
| 1. | Fitzpatrick E, Mitry RR, Dhawan A. Human hepatocyte transplantation: state of the art. J Intern Med. 2009;266:339-357. |
| 2. | Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319-329. |
| 3. | Joseph B, Kumaran V, Berishvili E, Bhargava KK, Palestro CJ, Gupta S. Monocrotaline promotes transplanted cell engraftment and advances liver repopulation in rats via liver conditioning. Hepatology. 2006;44:1411-1420. |
| 4. | Christiansen H, Koenig S, Krause P, Hermann RM, Rave-Frank M, Proehl T, Becker H, Hess CF, Schmidberger H. External-beam radiotherapy as preparative regimen for hepatocyte transplantation after partial hepatectomy. Int J Radiat Oncol Biol Phys. 2006;65:509-516. |
| 5. | Koenig S, Krause P, Schmidt TK, Rave-Fraenk M, Rothe H, Hermann RM, Becker H, Hess CF, Christiansen H. Irradiation as preparative regimen for hepatocyte transplantation causes prolonged cell cycle block. Int J Radiat Biol. 2008;84:285-298. |
| 6. | Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33:1200-1208. |
| 7. | Pober JS. Activation and injury of endothelial cells by cytokines. Pathol Biol (Paris). 1998;46:159-163. |
| 8. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. |
| 9. | Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, Yannam GR, Enke C, Solberg TD, Adelson AB. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258-267. |
| 10. | Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572-1578. |
| 11. | Mornex F, Girard N, Beziat C, Kubas A, Khodri M, Trepo C, Merle P. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies--mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152-1158. |
| 12. | Hazard L, Tward JD, Szabo A, Shrieve DC. Radiation therapy is associated with improved survival in patients with pancreatic adenocarcinoma: results of a study from the Surveillance, Epidemiology, and End Results (SEER) registry data. Cancer. 2007;110:2191-2201. |
| 13. | Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, Cummings B, Ringash J, Tse RV, Knox JJ. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585-1591. |
| 14. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. |
| 15. | Dewhirst MW. Relationships between cycling hypoxia, HIF-1, angiogenesis and oxidative stress. Radiat Res. 2009;172:653-665. |
| 16. | Lissidini G, Piccinni G, Portincasa P, Grattagliano I, Gurrado A, Testini M. Surgically-induced bile duct injury is followed by early hepatic oxidative stress. A preliminary experimental study in rats. Hepatogastroenterology. 2009;56:602-605. |
| 17. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. |
| 18. | Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801. |
| 19. | Rabbani ZN, Mi J, Zhang Y, Delong M, Jackson IL, Fleckenstein K, Salahuddin FK, Zhang X, Clary B, Anscher MS. Hypoxia inducible factor 1alpha signaling in fractionated radiation-induced lung injury: role of oxidative stress and tissue hypoxia. Radiat Res. 2010;173:165-174. |
| 20. | Koenig S, Stoesser C, Krause P, Becker H, Markus PM. Liver repopulation after hepatocellular transplantation: integration and interaction of transplanted hepatocytes in the host. Cell Transplant. 2005;14:31-40. |
| 21. | Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, Sokhi R, Roy-Chowdhury N, Tanaka KE, Vikram B. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999;59:5871-5874. |
| 22. | Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, Roy-Chowdhury N, Vikram B, Roy-Chowdhury J. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology. 2002;36:354-362. |
| 23. | Brilliant KE, Mills DR, Callanan HM, Hixson DC. Engraftment of syngeneic and allogeneic endothelial cells, hepatocytes and cholangiocytes into partially hepatectomized rats previously treated with mitomycin C. Transplantation. 2009;88:486-495. |
| 24. | Shelley MD, Mason MD, Kynaston H. Intravesical therapy for superficial bladder cancer: a systematic review of randomised trials and meta-analyses. Cancer Treat Rev. 2010;36:195-205. |
| 25. | Hofheinz RD, Beyer U, Al-Batran SE, Hartmann JT. Mitomycin C in the treatment of gastrointestinal tumours: recent data and perspectives. Onkologie. 2008;31:271-281. |
| 26. | Follenzi A, Benten D, Novikoff P, Faulkner L, Raut S, Gupta S. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest. 2008;118:935-945. |
| 27. | Huang J, Wolk JH, Gewitz MH, Mathew R. Progressive endothelial cell damage in an inflammatory model of pulmonary hypertension. Exp Lung Res. 2010;36:57-66. |
| 28. | Carreras E, Grañena A, Rozman C. Hepatic veno-occlusive disease after bone marrow transplant. Blood Rev. 1993;7:43-51. |
| 29. | Rifle G, Mousson C, Hervé P. Endothelial cells in organ transplantation: Friends or foes? Transplantation. 2006;82:S4-S5. |
| 30. | Stolz DB, Ross MA, Ikeda A, Tomiyama K, Kaizu T, Geller DA, Murase N. Sinusoidal endothelial cell repopulation following ischemia/reperfusion injury in rat liver transplantation. Hepatology. 2007;46:1464-1475. |
| 31. | Fujii H, Hirose T, Oe S, Yasuchika K, Azuma H, Fujikawa T, Nagao M, Yamaoka Y. Contribution of bone marrow cells to liver regeneration after partial hepatectomy in mice. J Hepatol. 2002;36:653-659. |
| 32. | Kienstra KA, Jackson KA, Hirschi KK. Injury mechanism dictates contribution of bone marrow-derived cells to murine hepatic vascular regeneration. Pediatr Res. 2008;63:131-136. |
Peer reviewers: Fausto Catena, MD, PhD, Department of General, Emergency and Transplant Surgery, St Orsola-Malpighi University Hospital, Via Massarenti 9, Bologna 40139, Italy; Julio Mayol, MD, PhD, Department of Digestive surgery, Hospital Clinico San Carlos, Martin-Lagos S/n, Madrid 28040, Spain
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH