Published online Aug 21, 2010. doi: 10.3748/wjg.v16.i31.3905
Revised: February 22, 2010
Accepted: February 28, 2010
Published online: August 21, 2010
AIM: To study the significance of cap-fitted colonoscopy in improving cecal intubation time and polyp detection rate.
METHODS: This study was a prospective randomized controlled trial conducted from March 2008 to February 2009 in a tertiary referral hospital at Sydney. The primary end point was cecal intubation time and the secondary endpoint was polyp detection rate. Consecutive cases of total colonoscopy over a 1-year period were recruited. Randomization into either standard colonoscopy (SC) or cap-assisted colonoscopy (CAC) was performed after consent was obtained. For cases randomized to CAC, one of the three sizes of cap was used: D-201-15004 (with a diameter of 15.3 mm), D-201-14304 (14.6 mm) and D-201-12704 (13.0 mm). All of these caps were produced by Olympus Medical Systems, Japan. Independent predictors for faster cecal time and better polyp detection rate were also determined from this study.
RESULTS: There were 200 cases in each group. There was no significant difference in terms of demographic characteristics between the two groups. CAC, when compared to the SC group, had no significant difference in terms of cecal intubation rate (96.0% vs 97.0%, P = 0.40) and time (9.94 ± 7.05 min vs 10.34 ± 6.82 min, P = 0.21), or polyp detection rate (32.8% vs 31.3%, P = 0.75). On the subgroup analysis, there was no significant difference in terms of cecal intubation time by trainees (88.1% vs 84.8%, P = 0.40), ileal intubation rate (82.5% vs 79.0%, P = 0.38) or total colonoscopy time (23.24 ± 13.95 min vs 22.56 ± 9.94 min, P = 0.88). On multivariate analysis, the independent determinants of faster cecal time were consultant-performed procedures (P < 0.001), male patients (P < 0.001), non-usage of hyoscine (P < 0.001) and better bowel preparation (P = 0.01). The determinants of better polyp detection rate were older age (P < 0.001), no history of previous abdominal surgery (P = 0.04), patients not having esophagogastroduodenoscopy in the same setting (P = 0.003), trainee-performed procedures (P = 0.01), usage of hyoscine (P = 0.01) and procedures performed for polyp follow-up (P = 0.01). The limitations of the study were that it was a single-center experience, no blinding was possible, and there were a large number of endoscopists.
CONCLUSION: CAC did not significantly different from SC in term of cecal intubation time and polyp detection rate.
- Citation: Tee HP, Corte C, Al-Ghamdi H, Prakoso E, Darke J, Chettiar R, Rahman W, Davison S, Griffin SP, Selby WS, Kaffes AJ. Prospective randomized controlled trial evaluating cap-assisted colonoscopy vs standard colonoscopy. World J Gastroenterol 2010; 16(31): 3905-3910
- URL: https://www.wjgnet.com/1007-9327/full/v16/i31/3905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i31.3905
Colonoscopic examination has been used in clinical practice for approximately 40 years. Despite the fact that colonoscopy is widely available and is performed by many experienced colonoscopists, there are concerns about the quality of colonoscopy as measured by several technical endpoints such as rate of failed cecal intubation and polyp miss rate. A large population-based study[1] has revealed that 13.1% of colonoscopies failed to reach the cecum. In addition, one large review of back-to-back colonoscopies has shown polyp miss rates of 24%[2] for adenoma. Numerous innovations, as described by Rex[3], have been studied to improve these two key issues, with some showing promise.
One potentially promising technique is cap-assisted colonoscopy (CAC). A transparent cap (or hood) is a simple plastic device that can be attached to the tip of a colonoscope before performing the colonoscopy. Several randomized trials from Japan[4-8] have reported mixed results regarding improved cecal intubation times and polyp detection rates. A recent large study from Hong Kong[9] has shown improved time to cecum intubation but a reduced polyp detection rate. To date, as far as we are aware, there is no large randomized study that has used the cap in a western population, in whom the colorectal cancer (CRC) incidence is known to be higher than Asian population[10]. We conducted a randomized controlled trial to investigate the usefulness of CAC in a western population at Royal Prince Alfred Hospital, Sydney.
This was a prospective randomized controlled trial conducted in a tertiary referral hospital from March 2008 to February 2009. All patients who were referred to our endoscopy service for colonoscopy were invited to participate in the study. All were aged 18 years or older. Exclusion criteria included prior colonic resection, pregnancy, severe comorbidity and acute surgical conditions such as severe colitis, toxic megacolon, ischemic colitis, tertiary referral for endomucosal resection, acute gastrointestinal bleeding, or inability to provide consent, such as dementia. This prospective study was approved by Sydney South West Area Health Service Ethics Review Committee. The trial was registered with ClinicalTrial.gov with the registration identification number NCT00930462.
The preparation for the procedure included a clear fluid diet the day before the procedure, routine bowel preparation with either a sodium picosulfate-based (Picoprep, Pharmatel Fresenius Kabi Pty Ltd., Hornby, Australia), or sodium-phosphate-based (Fleet, Ferring Pharmaceuticals, Gordon, Australia) preparation, and a fasting period of 8 h. Colonoscopy was performed in either the hospital endoscopy unit or a private outpatient endoscopy center associated with our hospital. Procedures were performed by a team of five consultant gastroenterologists and 10 trainees. All procedures were performed under conscious or deep sedation with a combination of intravenous midazolam (Pfizer, Bentley, Australia), fentanyl (Mayne Pharma Ltd, Mulgrave, Australia), and propofol (Fresofol 1%; Pharmatel Fresenius Kabi Pty Ltd.) administered by the assistant or attending anesthetist. As an antispasmodic, hyoscine butylbromide was administered as appropriate. Colonoscopes used were CF-Q160AL, CF-Q180AL, PCF-160AL and PCF-180AL (Olympus Optical Co., Tokyo, Japan).
Three sizes of cap were used: D-201-15004 (with a diameter of 15.3 mm, used for CF-Q180AL), D-201-14304 (14.6 mm, used for CF-Q160AL) and D-201-12704 (13.0 mm, used for both PCF-160AL and PCF-180AL). All of these caps were produced by Olympus Medical Systems (Tokyo, Japan). The cap was placed so that 4 mm was beyond the tip of the colonoscope (Figure 1). Even though the rim of the cap was visible on the monitor, the visual field was not limited as the endoscopist was able to see through the transparent cap.
Once informed consent was obtained, patients were randomized according to a computer-generated randomization protocol to standard colonoscopy without the cap (SC) or CAC. Patients were blinded to the allocation. The colonoscopes were assigned in no specific order and were used according to availability after cleaning. Data were collected during and after the colonoscopic examinations on procedure times, polyps, complications and other parameters.
The procedure was defined as successful if the colonoscope reached the cecum, confirmed by either visualization of the appendicular orifice or the ileocecal valve. Trainee success was only recorded if the cecum was reached without help from a consultant. Terminal ileal intubation was attempted in all cases. The quality of bowel preparation was graded either as good (no or small volume of clear liquid, easily removed), satisfactory (moderate to large volume of liquid stool, removable with suction), or poor (presence of semi-solid stool that could not be cleared or washed away).
Polyps were removed in standard fashion and sent for pathology. The size of the polyps was determined by the endoscopist performing the polypectomy. Advanced lesions were defined as lesions > 10 mm, with high grade dysplasia, or villous in nature. If a diminutive polyp was encountered on insertion and the endoscopist wished to remove this during the insertion phase of the procedure, the time taken for this was subtracted from the insertion time but included in the total procedure time.
The primary endpoint of this study was cecal intubation time. The secondary endpoint was polyp detection rate. Other endpoints included ileal intubation rate, total colonoscopy time, trainee success rate and complication rate.
The study also determined the independent predictors for faster cecal time and better polyp detection rate.
The study sample size was calculated to be 200 in each arm to detect a difference with a power of 0.8 and an α of 0.05 by two-tailed testing based on historical data[5], for the primary endpoint of cecal intubation time available at the time the study was designed. All statistical analyses were performed using SPSS for Windows version 12.0 (Chicago, IL, USA). The Mann-Whitney test was used for continuous variables that appeared to have a skewed distribution, and the χ2 or Fisher’s exact test for categorical variables. Statistical significance was defined as P < 0.05 (two-tailed).
Cecal intubation time and polyp detection rate were log transformed to approximate normality prior to fitting a multiple linear regression model using stepwise variable selection. This model was used to identify only the independent predictors of these parameters.
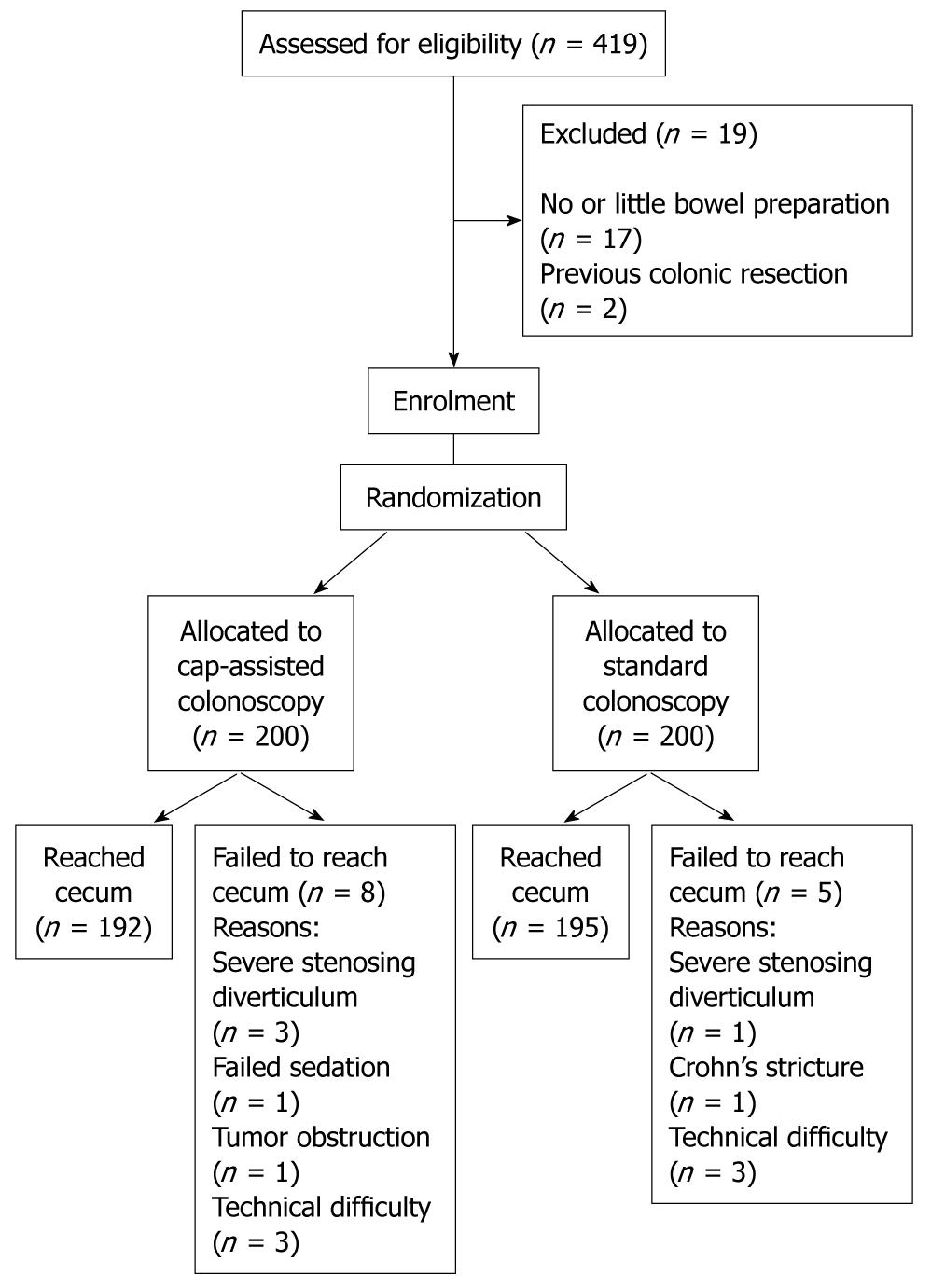
Between March 2008 and February 2009, a total of 400 patients were recruited, with 200 each in the CAC and SC groups. A flow diagram of the enrollment is shown in Figure 2. Mean age was 52 years, and 49% of the subjects were male. The characteristics of the two groups are shown in Table 1, and no significant differences were found between them. Trainees performed a total of 310 (77.5%) procedures in this study.
| CAC (n = 200) | SC (n = 200) | P value | |
| Sex (male) | 102 (51.0) | 88 (44.0) | 0.16 |
| Age (yr) (mean ± SD) | 53.8 ± 15.1 | 53.6 ± 14.8 | 0.89 |
| Previous difficult colonoscopy | 19 (9.5) | 15 (7.5) | 0.65 |
| Known diverticular disease | 8 (0.04) | 11 (0.06) | 0.74 |
| Previous pelvic surgery | 25 (12.5) | 26 (13.0) | 0.80 |
| Gastroscopy at the same time | 74 (37.0) | 89 (44.5) | 0.17 |
| Indications | |||
| CRC screening | 38 (19.0) | 35 (17.5) | |
| Rectal bleeding | 37 (18.5) | 32 (16.0) | |
| Abdominal pain | 32 (16.0) | 33 (16.5) | |
| Polyp follow up | 30 (15.0) | 27 (13.5) | |
| Change in bowel habit | 18 (9.0) | 28 (14.0) | |
| Others | 45 (22.5) | 45 (22.5) | 0.58 |
| Endoscopist | |||
| Specialist | 41 (20.5) | 49 (24.5) | |
| Trainee | 159 (79.5) | 151 (75.5) | 0.34 |
| Bowel preparation | |||
| Good | 107 (54.0) | 130 (65.7) | |
| Satisfactory | 71 (35.9) | 53 (26.8) | |
| Poor | 20 (10.1) | 15 (7.6) | 0.10 |
| Scopes used | |||
| P160/P180 | 137 (66.5) | 143 (70.5) | |
| A160/A180 | 63 (32.0) | 57 (28.5) | 0.61 |
Cecal intubation was achieved in 387 (96.8%) of the 400 colonoscopic examinations. The success rate of cecal intubation was 192/200 (96.0%) in the CAC group and 195/200 (97.5%) in the SC group (P = 0.40). When analyzing the data on procedures by trainees only, the unassisted cecal intubation rate was 268/310 (86.5%), with no significant difference between CAC (140/159, 88.1%) and SC (128/151, 84.8%), and P value was 0.40 (Table 2). Forty-two procedures performed by trainees required assistance from consultants, and among these, 32 (76.2%) were successful in reaching the cecum.
| CAC | SC | P value | |
| Cecal intubation rate | 192/200 (96.0) | 195/200 (97.0) | 0.401 |
| Cecal intubation rate by trainees | 140/159 (88.1) | 128/151 (84.8) | 0.401 |
| Ileal intubation rate | 165/200 (82.5) | 158/200 (79.0) | 0.381 |
| Ileal intubation rate by trainees | 126/159 (79.2) | 115/151 (76.2) | 0.511 |
| Cecal time (min) (mean ± SD) (n = 387) | 9.94 ± 7.05 | 10.34 ± 6.82 | 0.212 |
| Trainee cecal time (min) (mean ± SD) (n = 268) | 10.72 ± 6.75 | 9.66 ± 4.86 | 0.642 |
| Total colonoscopy time (min) (mean ± SD) (n = 387) | 23.24 ± 13.95 | 22.56 ± 9.94 | 0.862 |
| Trainee total colonoscopy time (min) (mean ± SD) (n = 268) | 25.97 ± 1.22 | 23.70 ± 0.72 | 0.472 |
Of the 13 cases of failed cecal intubation, four were due to severe sigmoid stenosing diverticular disease (three CAC, and one SC); one each for failed sedation (CAC), Crohn’s stricture (SC) and tumor obstruction (CAC); and the other six cases were due to technical difficulties that included irreducible colonic loops (three CAC, and three SC).
Overall ileal intubation rate was 323/400 (80.8%). There was no significant difference between CAC and SC with regards to ileal intubation rate, either overall (CAC 82.5% vs SC 79.0%, P = 0.38), or for procedures performed by trainees only (CAC 79.2% vs SC 76.2%, P = 0.51) (Table 2).
The mean (± SD) time to reach the cecum was 9.9 ± 7.1 min in the CAC group and 10.4 ± 6.8 min in the SC group (P = 0.19). Mean total colonoscopy times were 23.3 ± 14.0 min in the CAC group and 22.6 ± 10.0 in the SC group (P = 0.86). When confined to procedures performed by trainees unassisted by consultants, the mean time to reach the cecum was 10.7 ± 6.75 min in the CAC group and 9.66 ± 4.86 min in the SC group (P = 0.64) (Table 2).
On univariate analysis, factors found to be significantly associated with faster time to the cecum were: male sex, younger age, colonoscopy by a consultant, good or satisfactory bowel preparation, and non-use of hyoscine. Multiple linear regression confirmed each of these as independent predictors, apart from young age (Table 3).
Among the 387 subjects who had successful cecal intubation, colorectal polyps were detected in 123 (31.8%) of them (63 in the CAC and 61 in the SC group, P = 0.75). CAC and SC detected polyps in 32.8% and 31.3% of their subjects, respectively. When confined to subjects aged 50 years and above, CAC detected polyps in 43.2% and SC in 35.9% (P = 0.24) (Table 4). The total number of polyps detected was 254, and this also did not differ between the two groups (147 in CAC and 107 in SC, P = 0.59). There was no difference between the two groups in terms of the number of polyps of 5 mm or less (P = 0.45), adenoma number (P = 0.26), or advanced adenoma (P = 0.52) (Table 4). On univariate analysis, factors found to be significantly associated with detection of polyp were: male sex, older age, polyp follow-up as the indication, patients who had colonoscopy alone (vs patients having combined gastroscopy and colonoscopy), non-use of hyoscine, and colonoscopy performed by a trainee. On multivariate analysis, the independent predictors were older age, polyp follow-up as the indication, no history of prior abdominal surgery, patients having colonoscopy alone, trainee-performed colonoscopy, and the use of hyoscine (Table 5).
| CAC | SC | P value | |
| Subjects with polyps (n = 387) | 63/192 (32.8) | 61/195 (31.3) | 0.751 |
| Subjects aged ≥ 50 yr with polyps (n = 246) | 51/118 (43.2) | 46/128 (35.9) | 0.241 |
| Total number of polyps | 147 | 107 | 0.592 |
| Total number of polyps with size ≤ 5 mm | 121 | 84 | 0.452 |
| Total number of adenomas | 75 | 55 | 0.262 |
| Total number of advanced adenomas | 23 | 14 | 0.522 |
| Independent predictors | OR | 95% CI | P value |
| Age | 1.450 | 1.213-1.734 | < 0.001 |
| Previous abdominal surgery | 0.559 | 0.322-0.971 | 0.039 |
| Having EGD on the same setting | 0.451 | 0.266-0.765 | 0.003 |
| Consultant | 0.460 | 0.250-0.845 | 0.012 |
| Use of hyoscine | 2.877 | 1.374-6.026 | 0.005 |
| Indication: polyp follow-up | 2.722 | 1.342-5.523 | 0.006 |
There was no complication associated with the use of the cap. There were two cases of post-polypectomy bleeding, one each in the SC and CAC groups, although these cases were minor and did not require transfusion.
Despite the fact that polypectomy prevents 76%-90% of CRC when compared to the expected incidence[11], data from interval CRCs (CRCs diagnosed between the time of a negative screening colonoscopy to that of next recommended colonoscopy) raise important questions about how effective colonoscopy is as a screening practice[12]. Possible reasons for these interval cancers include failed colonoscopy and missed lesions. A large population-based study[1] has revealed that 13.1% of colonoscopy failed to reach the cecum. Rex et al[2] have published data on adenoma miss rates based on a large back-to-back colonoscopy series, and estimated that the rate was 17%-48%. Important advances in colonoscopy have been suggested to improve the outcome of colonoscopy. These include improving colonoscopy techniques (optimizing withdrawal time[13-15], chromoendoscopy and bowel preparation quality[16,17]) and technologies (wide-angle colonoscopy in 2003[18], narrow band imaging in 2004[19], and fluorescence confocal endomicroscopy[20].
Caps have been used previously in endoscopic procedures, for instance, in mucosal resection and double balloon enteroscopy. Their use in colonoscopy has been studied in the past with regards to cecal intubation and polyp detection. Our study did not show any benefit of CAC for our primary endpoint of time to cecal intubation Even though all six previous randomized trials of CAC have been faster with the cap, only four were significantly faster. In our study, CAC had a shorter cecal intubation time although this was not statistically significant. One possible explanation is that studies with cecal intubation times below 10 min are inclined to have no significance between the two study arms, with the exception of the study of Lee et al[9]. This illustrates a point whereby the benefit of the cap is small in those procedures where cecal intubation times are already short. The short cecal intubation time and high cecal success rate in our study probably left little room for improvement with the cap. The overall cecal intubation rate was 96.8%, this high success rate was probably due to the fact that our center is a tertiary teaching hospital.
Factors that predicted faster colonoscopy in our multivariate analysis (Table 3) were as expected and well-described in the literature[21-23], except for the use of hyoscine. We reserved hyoscine for difficult insertions, which suggests that we selected patients with difficult colonoscopy, hence the prolonged cecal intubation time in that group.
We also showed no significant improvement of the polyp detection rate in the CAC group. This was almost certainly due to a small sample size because this study was powered to look at cecal intubation time rather than polyp detection rate. Furthermore, our study cohort had a mean age of 52 years (vs 62.0-66.4 in Asian studies[5-8]), older only than the patients in the study of Lee et al[9]. Our low polyp detection rate at 31.8%, despite the fact that the study was carried out on a western population, was likely a consequence of this younger patient population. The youngest population studied to date was in Hong Kong[9], which demonstrated significantly lower polyp detection in the CAC group. Our study does not support the use of a cap for polyp detection, however, the question of any benefit with the cap has not been sufficiently answered. Further studies, particularly in a western population, are required. With the known polyp miss rates at about 24%[2], it is likely that large numbers will need to be recruited in prospective randomized studies to show any benefit of this intervention.
In conclusion, there was no statistically significant difference between CAC and SC with regards to cecal intubation success, time and polyp detection rate.
Despite 40 years of advances in colonoscopic practices, there are a significant number of incomplete procedures and polyp miss rate. This study looked at the use of cap-assisted colonoscopy (CAC) in improving these endpoints.
Colonoscopy is the most important screening tool for cancer of the colon. Many new developments are aimed at improving the quality of colonoscopy. These include improving colonoscopy techniques (optimizing withdrawal time, chromoendoscopy and bowel preparation quality) and improved technologies (wide-angle colonoscopy in 2003, narrow band imaging in 2004, and fluorescence confocal endomicroscope in 2005).
CAC is a relatively simple and inexpensive tool that is used to improve the usefulness of colonoscopy. Several Asian centers have performed trials on CAC, with conflicting results, and we aim to add more information on the usefulness of this device.
This article provides important data on the usefulness of CAC especially in a Western population.
This is an interesting study that investigated the use of CAC in a day-to-day setting of a busy tertiary endoscopy unit.
| 1. | Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132:2297-2303. |
| 2. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. |
| 3. | Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866-2877. |
| 4. | Matsushita M, Hajiro K, Okazaki K, Takakuwa H, Tominaga M. Efficacy of total colonoscopy with a transparent cap in comparison with colonoscopy without the cap. Endoscopy. 1998;30:444-447. |
| 5. | Kondo S, Yamaji Y, Watabe H, Yamada A, Sugimoto T, Ohta M, Ogura K, Okamoto M, Yoshida H, Kawabe T. A randomized controlled trial evaluating the usefulness of a transparent hood attached to the tip of the colonoscope. Am J Gastroenterol. 2007;102:75-81. |
| 6. | Horiuchi A, Nakayama Y. Improved colorectal adenoma detection with a transparent retractable extension device. Am J Gastroenterol. 2008;103:341-345. |
| 7. | Shida T, Katsuura Y, Teramoto O, Kaiho M, Takano S, Yoshidome H, Miyazaki M. Transparent hood attached to the colonoscope: does it really work for all types of colonoscopes? Surg Endosc. 2008;22:2654-2658. |
| 8. | Harada Y, Hirasawa D, Fujita N, Noda Y, Kobayashi G, Ishida K, Yonechi M, Ito K, Suzuki T, Sugawara T. Impact of a transparent hood on the performance of total colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2009;69:637-644. |
| 9. | Lee YT, Lai LH, Hui AJ, Wong VW, Ching JY, Wong GL, Wu JC, Chan HL, Leung WK, Lau JY. Efficacy of cap-assisted colonoscopy in comparison with regular colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2009;104:41-46. |
| 10. | Rim SH, Seeff L, Ahmed F, King JB, Coughlin SS. Colorectal cancer incidence in the United States, 1999-2004 : an updated analysis of data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1967-1976. |
| 11. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. |
| 12. | Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, Burke CA, Snover DC, Bresalier RS, McKeown-Eyssen G. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34-41. |
| 13. | Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533-2541. |
| 14. | Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091-1098. |
| 15. | Simmons DT, Harewood GC, Baron TH, Petersen BT, Wang KK, Boyd-Enders F, Ott BJ. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24:965-971. |
| 16. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. |
| 17. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. |
| 18. | Rex DK, Chadalawada V, Helper DJ. Wide angle colonoscopy with a prototype instrument: impact on miss rates and efficiency as determined by back-to-back colonoscopies. Am J Gastroenterol. 2003;98:2000-2005. |
| 19. | Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094-1098. |
| 20. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. |
| 21. | Anderson JC, Messina CR, Cohn W, Gottfried E, Ingber S, Bernstein G, Coman E, Polito J. Factors predictive of difficult colonoscopy. Gastrointest Endosc. 2001;54:558-562. |
| 22. | Bernstein C, Thorn M, Monsees K, Spell R, O’Connor JB. A prospective study of factors that determine cecal intubation time at colonoscopy. Gastrointest Endosc. 2005;61:72-75. |
| 23. | Arcovedo R, Larsen C, Reyes HS. Patient factors associated with a faster insertion of the colonoscope. Surg Endosc. 2007;21:885-888. |
Peer reviewers: Dr. Vui Heng Chong, Gastroenterology and Hepatology Unit, Department of Medicine, Raja Isteri Pengiran Anak Saleha Hospital, Bandar Seri Begawan BA 1710, Brunei Darussalam; Keiji Hirata, MD, Surgery 1, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8555, Japan; Ian C Roberts-Thomson, Professor, Department of Gastroenterology and Hepatology, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South 5011, Australia
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH