Published online Jan 21, 2010. doi: 10.3748/wjg.v16.i3.348
Revised: November 16, 2009
Accepted: November 23, 2009
Published online: January 21, 2010
AIM: To investigate the effect of transjugular intrahepatic porto-systemic shunt (TIPS) on malnutrition in portal hypertensive cirrhotic patients.
METHODS: Twenty-one patients with liver cirrhosis and clinical indications for TIPS insertion were investigated before and 1, 4, 12, 52 wk after TIPS. For each patient we assayed body composition parameters [dry lean mass, fat mass, total body water (TBW)], routine liver and kidney function tests, and free fatty acids (FFA). Glucose and insulin were measured for the calculation of the homeostasis model assessment insulin resistance (HOMA-IR); liver function was measured by the galactose elimination capacity (GEC); the severity of liver disease was graded by model for end-stage liver disease (MELD).
RESULTS: Porto-systemic gradient decreased after TIPS (6.0 ± 2.1 mmHg vs 15.8 ± 4.8 mmHg, P < 0.001). Patients were divided in two groups according to initial body mass index. After TIPS, normal weight patients had an increase in dry lean mass (from 10.9 ± 5.9 kg to 12.7 ± 5.6 kg, P = 0.031) and TBW (from 34.5 ± 7.6 L to 40.2 ± 10.8 L, P = 0.007), as well as insulin (from 88.9 ± 49.2 pmol/L to 164.7 ± 107.0 pmol/L, P = 0.009) and HOMA-IR (from 3.36% ± 2.18% to 6.18% ± 4.82%, P = 0.023). In overweight patients only FFA increased significantly (from 0.59 ± 0.24 mmol/L to 0.93 ± 0.34 mmol/L, P = 0.023).
CONCLUSION: TIPS procedure is effective in lowering portal pressure in patients with portal hypertension and improves body composition without significant changes in metabolic parameters.
- Citation: Montomoli J, Holland-Fischer P, Bianchi G, Grønbæk H, Vilstrup H, Marchesini G, Zoli M. Body composition changes after transjugular intrahepatic portosystemic shunt in patients with cirrhosis. World J Gastroenterol 2010; 16(3): 348-353
- URL: https://www.wjgnet.com/1007-9327/full/v16/i3/348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i3.348
Portal hypertension and malnutrition are two important complications of cirrhosis, both affecting prognosis and risk of death[1-3]. Portal hypertension is present in 60% of cirrhotic patients[4]; the existence of a portal-systemic shunt modifies both fasting and post-prandial metabolism, decreasing the hepatic first-pass effect of nutrients, which become more available for peripheral tissues. Also hormone levels, like insulin, share a similar defect. The lack of a significant first-pass removal by the liver makes insulin flood the systemic circulation and promotes insulin resistance, which is further aggravated by the production of inflammatory molecules [tumor necrosis factor-α (TNF-α), lipopolysaccharide (LPS), etc.] which have an anti-insulin effect and contribute to systemic inflammation[5]. The final event of the above alterations is a progressive deterioration of liver function, also resulting in poor nutritional status. Malnutrition, in turn, is a risk factor for disease progression, and is associated with the development of complications[3], with a high risk of death[6], as well as increased morbidity and mortality after transplantation[7].
Several studies demonstrated that transjugular intrahepatic porto-systemic shunt (TIPS) using polytetrafluorethylene (PTFE)-covered stents are the best rescue therapy for failures of medical and endoscopic treatment of portal hypertension[8-10].
The effects of TIPS on metabolism and body composition are not well defined. Allard et al[11] and Plauth et al[12] found that TIPS insertion in malnourished patients with cirrhosis and hypermetabolism resulted in improved body composition. No studies, however, have clearly defined the relation between TIPS-induced metabolic changes and changes in nutritional status, and/or the underlying mechanism. In particular, no studies addressed the potential impact of pre-TIPS overweight and obesity and associated insulin resistance on the post-TIPS nutritional status and insulin levels.
We aimed to test the effects of TIPS insertion on nutritional status and insulin levels in a group of subjects with cirrhosis and complications of portal hypertension.
Twenty-six patients with liver cirrhosis, consecutively undergoing an elective TIPS procedure were included (Table 1). Three patients did not complete the baseline examination protocol due to variceal bleeding requiring intensive care and immediate TIPS insertion. Of the remaining 23 patients (15 males and 8 females), one received an orthotopic liver transplant after TIPS, and one died during follow-up. Three patients missed one or more follow-up evaluations; therefore, only 18 patients had all the evaluations planned by protocol. The diagnosis of cirrhosis was assessed on the basis of biochemical, clinical, and ultrasonographic findings and confirmed by liver biopsy in 8 cases. Among the 21 patients who completed the follow-up, etiology was as follows: alcohol-related, 15; autoimmune, 3; primary sclerosing cholangitis, 1; HCV-related, 1; cryptogenic, 1. Abstinence from alcohol was a goal during the study, and to our knowledge all but one adhered to that policy. All patients signed a written informed consent to take part in the study in accordance with the Helsinki II Declaration. The study was approved by the Research Ethics Committee of Aarhus County.
| Variable | Basal Pre-TIPS | After 52 wk | P |
| HVPG (mmHg) | 15.8 ± 4.8 | 6.0 ± 2.1 | < 0.001 |
| Bilirubin (mg/dL) | 1.26 ± 1.11 | 2.85 ± 2.29 | 0.017 |
| Creatinine (mg/dL) | 1.02 ± 0.56 | 1.14 ± 0.61 | 0.18 |
| Albumin (g/L) | 31.3 ± 7.7 | 33.0 ± 7.7 | 0.586 |
| GEC (mmol/min) | 1.79 ± 0.48 | 1.61 ± 0.41 | 0.081 |
| MELD (score) (n = 18)1 | 11 (6) | 13 (7) | 0.804 |
| Blood glucose (mmol/L) | 5.8 ± 1.5 | 6.3 ± 1.6 | 0.345 |
| Plasma insulin (pmol/L) | 128.9 ± 84.0 | 182.9 ± 100.6 | 0.024 |
| HOMA-β | 269 ± 409 | 233 ± 108 | 0.700 |
| HOMA-IR | 5.04 ± 4.07 | 7.82 ± 5.89 | 0.028 |
| BMI (kg/m2) | 26.2 ± 5.8 | 27.4 ± 5.6 | 0.172 |
| FFA (mmol/L) | 0.54 ± 0.23 | 0.89 ± 0.44 | 0.013 |
| Creatinine (mg/dL) | 0.98 ± 0.52 | 0.83 ± 0.34 | 0.053 |
| Fat mass (%) | 29.4 ± 7.7 | 27.8 ± 8.7 | 0.278 |
| Fat mass (kg) | 23.4 ± 10.5 | 22.9 ± 10.0 | 0.812 |
| Dry lean mass (kg) | 14.0 ± 5.8 | 14.8 ± 5.0 | 0.115 |
| TBW (L) | 32.3 ± 9.6 | 43.3 ± 10.3 | 0.017 |
This was an observational prospective study. TIPS patients were studied approximately 2 wk before TIPS insertion, and were regularly re-evaluated at follow-up visits 1, 4, 12, and 52 wk after the procedure. All examinations were carried out after overnight fasting.
Indications for TIPS insertion were refractory ascites (12 patients), secondary prevention of variceal bleeding (seven patients) or both (two patients). None had active variceal bleeding at the time of TIPS insertion. The TIPS procedure was carried out using covered stents according to the method described by Rössle et al[13]. After insertion, a clinical and ultrasonographic control of the shunt was performed after 24 h, 4 wk, and then, at 12-wk intervals during the first year. Ascites was totally removed by paracentesis before TIPS insertion.
Each patient had a dietetic investigation and a 7-d diary report before TIPS insertion. During the study, food intake remained unchanged both for quantity and type of nutrients.
Bio-impedance analysis (Quadscan 4000, Bodystat Ltd., Isle of Man, UK) was used to estimate body composition. The predictive equations were taken from Kushner et al[14] and Lautz et al[15]. Bio-impedance analysis was chosen because, despite some limitations in patients with ascites (not present in our patients both before and after TIPS) it is a bedside tool for the determination of body composition in cirrhotic patients with/without ascites[16]. Dry lean body mass was calculated as body weight - fat mass - total body water (TBW). Dry lean mass was preferred to lean mass to reduce the possible interference of changes in TBW due to the fluctuating presence of ascites. According to Tsiaousi et al[17], we considered as malnourished all patients with a body mass index (BMI) lower than 23.
The galactose elimination capacity (GEC) was used to measure quantitatively metabolic liver function, from blood concentration decay curves corrected for urinary excretion, as described by Tygstrup[18]. The clinical status was assessed according to the model for end-stage liver disease score (MELD)[19].
Glucose, creatinine, bilirubin and prothrombin time were routinely assayed by automatic analyzer on fresh serum/plasma samples. Free fatty acids (FFA) were determined with a colorimetric method using a commercial kit (Wako Chemicals, Neuss, Germany). Blood aliquots for insulin concentrations were stored at -80°C and measured by a two-site immunospecific insulin enzyme-linked immunosorbent assay[20].
Basal insulin secretion and sensitivity were assessed by means of the homeostasis model assessment (HOMA)[21,22]. Secretion was estimated using the Beta index (HOMA-β), while peripheral sensitivity was measured by HOMA-IR, with the following equations: HOMA-β = 20 × fasting plasma insulin (mU/L)/[fasting plasma glucose (mmol/L) - 3.5]; HOMA-IR = fasting plasma insulin (mU/L) × fasting plasma glucose (mmol/L)/22.5 .
Data analysis was performed using STATA 10 statistical software (StataCorp LP, Texas, USA). Results are given as mean ± SD. Changes from baseline were explored by analysis of variance (ANOVA) for repeated measurements. Changes from baseline to end-of-observation and between groups were also tested by parametric and non-parametric paired and unpaired methods, whenever appropriate. Due to non-systematic factors, blood samples were missing from 8 examinations in 6 patients. For the statistical analyses these missing values were replaced by the mean of adjacent values. A P-value < 0.05 was considered significant in a two-tailed test.
TIPS was well tolerated by all patients, and there were no procedure-related complications. TIPS insertion produced a reduction in hepatic venous pressure gradient (P < 0.001) (Table 1). Three patients needed a stent revision during follow-up. Transient hepatic encephalopathy was observed in three patients and was reversed by diet and oral disaccharides without the need for shunt reduction.
At the end of the study period, a non-significant increase in BMI was observed in the whole population (Table 1). Patients were divided in two groups according to their BMI at enrolment [BMI ≤ 25 kg/m2, normal/underweight (NW), n = 12; BMI > 25, overweight/obesity (OW), n = 9]. A few NW cases (n = 7; 58%) showed clinical evidence of malnutrition, but only 3 had a BMI below the lower cut-off of normality (18.5 kg/m2). Analysis of variance (ANOVA) for repeated measurements did not show differences in the time trend of BMI after TIPS between the two groups. Also the non-parametric analysis of 52-wk changes failed to detect differences in BMI changes over time.
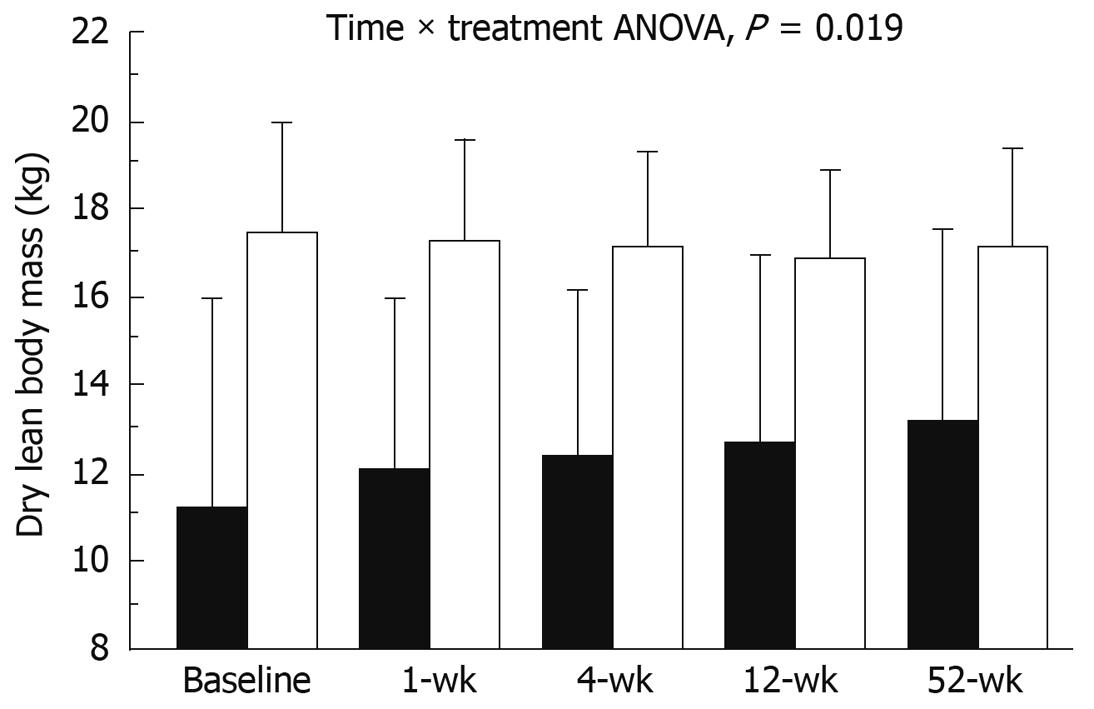
Dry lean mass increased in NW patients by 14.5% (from 10.9 ± 5.9 kg to 12.7 ± 5.6 kg, P = 0.031), but did not change in OW patients; the trend was significant on ANOVA test for repeated measures (P = 0.002) (Figure 1). The increase in dry lean body mass in normal weight patients paralleled a significant increase in TBW of 14.3%, (from 34.5 ± 7.6 L to 40.2 ± 10.8 L, P = 0.007). Fat mass, both as absolute value or as percentage of body weight, did not vary significantly in the 2 groups (ANOVA, P = 0.815).
Regarding liver function, GEC did not vary over time in TIPS-treated patients; similarly MELD increased non-significantly by over 2 points during the observation period (Table 1). These changes were largely due to increased MELD values in NW patients, while no changes were observed in the OW group. Similarly, GEC remained stable in NW patients while in the OW group there was a trend toward a significant deterioration (Table 2).
| Variable | Basal Pre-TIPS | After 52 wk | P | |
| HVPG (mmHg) | NW | 16.4 ± 5.0 | 6.3 ± 2.3 | < 0.001 |
| OW | 15.0 ± 4.7 | 5.7 ± 2.1 | < 0.001 | |
| Albumin (g/L) | NW | 30.8 ± 8.4 | 32.6 ± 8.1 | 0.871 |
| OW | 31.9 ± 7.1 | 33.4 ± 7.6 | 0.581 | |
| Bilirubin (mg/dL) | NW | 1.20 ± 0.47 | 3.21 ± 2.82 | 0.061 |
| OW | 1.71 ± 0.81 | 2.44 ± 1.61 | 0.164 | |
| GEC (mmol/min) | NW | 1.62 ± 0.36 | 1.57 ± 0.47 | 0.623 |
| OW | 1.96 ± 0.55 | 1.65 ± 0.36 | 0.088 | |
| MELD (score) (n = 9) | NW | 11 (5) | 13 (4) | 0.727 |
| OW | 11 (6) | 12 (8) | 0.289 | |
| Blood glucose (mmol/L) | NW | 5.7 ± 1.5 | 5.7 ± 0.8 | 0.941 |
| OW | 5.9 ± 1.5 | 6.8 ± 1.9 | 0.271 | |
| Insulin (pmol/L) | NW | 88.9 ± 49.2 | 164.7 ± 106.9 | 0.009 |
| OW | 171.4 ± 94.4 | 203.0 ± 94.9 | 0.466 | |
| HOMA-β | NW | 152 ± 88 | 253 ± 118 | 0.042 |
| OW | 413 ± 588 | 209 ± 95 | 0.316 | |
| HOMA-IR | NW | 3.36 ± 2.18 | 6.18 ± 4.82 | 0.023 |
| OW | 6.91 ± 4.94 | 9.64 ± 6.70 | 0.262 | |
| BMI (kg/m2) | NW | 21.4 ± 2.6 | 24.1 ± 4.0 | 0.220 |
| OW | 30.9 ± 3.7 | 30.8 ± 5.1 | 0.944 | |
| FFA (mmol/L) | NW | 0.49 ± 0.22 | 0.86 ± 0.53 | 0.124 |
| OW | 0.59 ± 0.24 | 0.93 ± 0.34 | 0.023 | |
| Creatinine (mg/dL) | NW | 0.84 ± 0.43 | 0.76 ± 0.39 | 0.149 |
| OW | 1.17 ± 0.59 | 0.92 ± 0.26 | 0.157 | |
| Fat mass (%) | NW | 27.6 ± 7.2 | 25.3 ± 8.3 | 0.334 |
| OW | 31.5 ± 7.9 | 30.5 ± 8.7 | 0.638 | |
| Fat mass (kg) | NW | 16.9 ± 3.7 | 17.5 ± 6.7 | 0.782 |
| OW | 30.5 ± 11.0 | 28.8 ± 9.9 | 0.649 | |
| Dry lean mass (kg) | NW | 10.9 ± 5.9 | 12.7 ± 5.6 | 0.031 |
| OW | 17.4 ± 3.3 | 17.1 ± 2.9 | 0.557 | |
| TBW (L) | NW | 34.5 ± 7.6 | 40.2 ± 10.8 | 0.007 |
| OW | 44.6 ± 8.9 | 46.6 ± 9.2 | 0.459 |
Plasma insulin and HOMA-IR significantly increased in TIPS-treated cirrhotic patients without any change in fasting glucose (Table 1). The changes were mainly limited to normal weight patients who also had a significant increase in HOMA-β (Table 2). However, no significant differences were demonstrated by repeated-measures ANOVA.
After TIPS insertion, changes in FFA plasma levels (Table 1) were exclusively observed in OW patients (Table 2).
No systematic changes in serum creatinine were observed (Tables 1 and 2), but patients with mild renal failure (creatinine levels ≥ 1.2 mg/dL, n = 7) slightly improved their values (from 1.75 ± 0.37 mg/dL to 1.45 ± 0.43 mg/dL, P = 0.23).
The main finding of this study is that dry lean body mass significantly increases in NW patients with cirrhosis after TIPS insertion. Advanced cirrhosis is associated with reduced lean mass[23]; a significant protein-calorie malnutrition is present in at least 30% of patients with cirrhosis[24]. Both parameters appear to be related to the severity of the liver disease[25]. Considering that a large fraction of our NW patients showed signs of malnutrition at baseline, TIPS appears to modify the natural history of malnutrition. This finding agrees with the results obtained by Plauth et al[12] and Allard et al[11]. Both reported an increase in dry lean mass but many questions on the metabolic mechanisms involved remain unresolved[26]. The significant increase in dry lean mass of about 1.84 kg in the year after TIPS insertion is paralleled by an increase in TBW that, in the absence of ascites and/or regional edemas, is indicative of muscle tissue formation[27]. Our results are in keeping with a very recent preliminary report by Camci et al[28], on six TIPS-treated malnourished cirrhotic patients.
This beneficial effect of TIPS on lean body mass was not observed in overweight or obese subjects at baseline. The reasons for this different metabolic response are not easy to determine. NW cases were characterized by lower insulin resistance at baseline, but also lower insulin and lower insulin secretion. In these normal- or under-weight individuals, the additional metabolic and hormonal abnormalities already in the group with excess body weight do not increase the cirrhosis-related insulin resistance[29]. Muscle tissue takes up 80%-85% of glucose infused during hyperinsulinemic euglycemic clamp[30,31]. After TIPS placement, these cases were characterized by a significant increase in serum insulin, without changes in blood glucose levels, and consequently by increased HOMA-IR and HOMA-β, and stable liver function. Under these conditions, the improved hemodynamic state, in the presence of a normal intake of nutrients, could promote insulin production and insulin action, favoring the improved nutritional state. This mechanism might not be operative in the OW group, with a much higher degree of insulin-resistance than NW cases, and where insulin did not increase further after TIPS placement. In these patients, the TIPS procedure was followed by a remarkable reduction of HOMA-β, and the decreased insulin production could not sustain lean body mass formation.
After TIPS the plasma levels of FFA increased significantly only in the OW group. In agreement with the hypothesis of Yki-Järvinen et al[32-34], the increase in FFA levels is likely to stimulate hepatic gluconeogenesis and competition with glucose in muscle metabolism. These metabolic changes could further promote insulin resistance.
Reduced portal hypertension might be an additional factor playing a role in the improved metabolic and nutritional status. Portal hypertension increases the permeability of enteric mucosa that promotes intestinal bacterial translocation and the systemic diffusion of LPS and other pro-inflammatory molecules, ultimately producing interleukin-1β, interleukin-6 and TNF-α. These molecules promote insulin-resistance and have an anti-insulin, catabolic effect, leading to protein mass wasting. Any mechanism mediated by anti-insulin molecules might play a differing effect in relation to BMI and to the levels of insulin resistance, much higher in obesity. Obesity per se is a chronic inflammatory state that sustains insulin-resistance[35]; in the presence of obesity the multiple factors sustaining insulin resistance could not be totally removed by TIPS; in contrast, in subjects with poor nutritional status the less severe insulin resistance might be removed by TIPS, thus explaining improved nutritional status. Larger studies are needed to explore the potential benefits of TIPS on long-term survival of underweight patients with cirrhosis, at higher risk of morbidity and mortality according to several previous studies[36].
Finally, the presence of insulin-resistance might be related to advanced liver disease and this condition could justify the absence of any improvement in OW patients with liver cirrhosis. We used GEC, an estimate of the functional liver mass with a prognostic value in the medium term interval[37], and there was no overall change in GEC over time, however, in OW patients a trend towards a decrease in GEC was observed. During the study, food intake remained unchanged both for quantity and type of nutrients. Further, it is our policy that alcohol has to be withdrawn in patients before TIPS insertion, as alcohol itself increases portal hypertension and may thus be involved in both the risk of variceal bleeding and ascites formation. During follow up, alcohol abstinence is important and this policy is reflected in the high number of alcohol abstainers in the present study. We therefore suggest that changes in body composition do not reflect a change in food and/or alcohol intake but are an effect of TIPS and of the reduction in portal pressure per se.
Finally, the study confirmed the effectiveness of TIPS on portal hypertension with a reduction in Hepatic venous pressure gradient (HVPG) to a value of 6 mmHg, lower than the 12 mmHg threshold value of increased risk for variceal bleeding, re-bleeding and mortality[1,4]. The absence of re-bleeding episodes and the disappearance of ascites confirm this hypothesis. Furthermore, patients with functional renal failure at baseline improved their creatinine levels at follow-up. Considering that lean mass increased, this value does not reflect a worsening of malnutrition[38] but is due to a positive effect of TIPS on the hemodynamic state[39].
In conclusion, the TIPS procedure is effective in lowering portal pressure in patients with portal hypertension and improves body composition without significant changes in metabolic parameters.
Malnutrition in portal hypertensive cirrhotic patients increases the risk and the severity of clinical complications. Transjugular intrahepatic porto-systemic shunt (TIPS) is a well established therapy for complications of portal hypertension in cirrhotic patients. However, the effect of TIPS on malnutrition is unclear.
The article provides insight into the beneficial effects of TIPS in regard to improvement in nutritional status. Malnutrition in liver diseases is an important area of research as it is an independent factor resulting in increased morbidity and mortality. Furthermore, the article addresses the effects of shunting of hormones/peptides etc. especially insulin, that relate to the possible mechanisms behind insulin resistance in patients with liver cirrhosis.
Related papers have focussed on improvement in body composition without addressing the pre-TIPS nutritional status. With a simple yet comprehensive separation into normal weight and overweight subjects, the results suggest additional nutritional benefits in the group of malnourished patients from TIPS insertion (see next section).
TIPS treatment of the complications of portal hypertension seems to improve nutritional status in liver cirrhosis, especially in patients suffering from malnutrition. Ultimately, malnutrition may provide an additional reason/indication for TIPS insertion in patients with liver cirrhosis.
The TIPS procedure is a minimally invasive procedure used in patients with liver cirrhosis to reduce portal hypertension and thereby ameliorating complications of portal hypertension. Using a catheter technique via the right jugular vein, a stent is placed within the liver connecting the portal vein and the hepatic vein and thus reducing portal hypertension.
This is an interesting and novel study showing an amelioration of nutritional parameters after TIPS especially in lean cirrhotic patients. An improvement in the body composition (nutritional status) of liver cirrhosis patients after TIPS implementation has been studied previously. And this study is well conducted and long term follow up data are provided.
| 1. | Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding--unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764-1772. |
| 2. | Garcia-Tsao G, D'Amico G, Abraldes JG, Schepis F, Merli M, Kim WR, Christensen E. Predictive models in portal hypertension. Portal Hypertension IV. Proceedings of the Fourth Baveno International Consensus Workshop on Methodology of Diagnosis and Treatment. Oxford, UK: Blackwell Sciences 2006; 47-100. |
| 3. | Plauth M, Merli M, Kondrup J, Weimann A, Ferenci P, Müller MJ. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55. |
| 4. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. |
| 5. | Aller MA, Arias JL, Cruz A, Arias J. Inflammation: a way to understanding the evolution of portal hypertension. Theor Biol Med Model. 2007;4:44. |
| 6. | Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis PINC (Policentrica Italiana Nutrizione Cirrosi). Hepatology. 1996;23:1041-1046. |
| 7. | Pikul J, Sharpe MD, Lowndes R, Ghent CN. Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation. 1994;57:469-472. |
| 8. | Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469-475. |
| 9. | Harrison J, McKiernan J, Neuberger JM. A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transpl Int. 1997;10:369-374. |
| 10. | Henderson JM, Boyer TD, Kutner MH, Rikkers L, Jeffers L, Abu-Elmagd K. DSRS vs TIPS for refractory variceal bleeding: a prospective randomized trial. Hepatology. 2004;40:725A. |
| 11. | Allard JP, Chau J, Sandokji K, Blendis LM, Wong F. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol. 2001;96:2442-2447. |
| 12. | Plauth M, Schütz T, Buckendahl DP, Kreymann G, Pirlich M, Grüngreiff S, Romaniuk P, Ertl S, Weiss ML, Lochs H. Weight gain after transjugular intrahepatic portosystemic shunt is associated with improvement in body composition in malnourished patients with cirrhosis and hypermetabolism. J Hepatol. 2004;40:228-233. |
| 13. | Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165-171. |
| 14. | Kushner RF, Schoeller DA. Estimation of total body water by bioelectrical impedance analysis. Am J Clin Nutr. 1986;44:417-424. |
| 15. | Lautz HU, Selberg O, Körber J, Bürger M, Müller MJ. Protein-calorie malnutrition in liver cirrhosis. Clin Investig. 1992;70:478-486. |
| 16. | Pirlich M, Schütz T, Spachos T, Ertl S, Weiss ML, Lochs H, Plauth M. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology. 2000;32:1208-1215. |
| 17. | Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol. 2008;23:527-533. |
| 18. | Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl. 1966;18:118-125. |
| 19. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. |
| 20. | Andersen L, Dinesen B, Jørgensen PN, Poulsen F, Røder ME. Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem. 1993;39:578-82. |
| 21. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. |
| 22. | Pei D, Hung YJ, Chen HD, Hsiao CF, Fu CC, Yang TC, Lian WC, Fang SC, Hsu WL, Kuo SW. The insulin sensitivity, glucose effectiveness and acute insulin response to glucose load of non-obese adolescent type 2 diabetes. Diabetes Res Clin Pract. 2004;66:253-261. |
| 23. | Nolte W, Hartmann H, Ramadori G. Glucose metabolism and liver cirrhosis. Exp Clin Endocrinol Diabetes. 1995;103:63-74. |
| 24. | Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. Nutritional status in cirrhosis. J Hepatol. 1994;21:317-325. |
| 25. | Crawford DH, Shepherd RW, Halliday JW, Cooksley GW, Golding SD, Cheng WS, Powell LW. Body composition in nonalcoholic cirrhosis: the effect of disease etiology and severity on nutritional compartments. Gastroenterology. 1994;106:1611-1617. |
| 26. | Holland-Fischer P, Vilstrup H, Frystyk J, Nielsen DT, Flyvbjerg A, Grønbaek H. The IGF system after insertion of a transjugular intrahepatic porto-systemic shunt in patients with liver cirrhosis. Eur J Endocrinol. 2009;160:957-963. |
| 27. | Mukherjee A, Adams JE, Smethurst L, Shalet SM. Interdependence of lean body mass and total body water, but not quality of life measures, during low dose GH replacement in GH-deficient adults. Eur J Endocrinol. 2005;153:661-668. |
| 28. | Camci C, Gurakar A, Kanoski M, Sharma S, Kanagala R, Monlux R, Wright H, Jabbour N. Nutritional effects of transjugular intrahepatic portosystemic shunt--an often neglected benefit "A preliminary report". J Okla State Med Assoc. 2009;102:10-11. |
| 29. | Iversen J, Vilstrup H, Tygstrup N. Kinetics of glucose metabolism in relation to insulin concentrations in patients with alcoholic cirrhosis and in healthy persons. Gastroenterology. 1984;87:1138-1143. |
| 30. | DeFronzo RA, Binder C, Wahren J, Felig P, Ferrannini E, Faber OK. Sensitivity of insulin secretion to feedback inhibition by hyperinsulinaemia. Acta Endocrinol (Copenh). 1981;98:81-86. |
| 31. | Petrides AS, DeFronzo RA. Glucose and insulin metabolism in cirrhosis. J Hepatol. 1989;8:107-114. |
| 32. | Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709-E1715. |
| 33. | Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. |
| 35. | Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654-1659. |
| 36. | Merli M, Nicolini G, Angeloni S, Riggio O. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002;18:978-986. |
| 37. | Marchesini G, Fabbri A, Bugianesi E, Bianchi GP, Marchi E, Zoli M, Pisi E. Analysis of the deterioration rates of liver function in cirrhosis, based on galactose elimination capacity. Liver. 1990;10:65-71. |
| 38. | Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, Heilberg IP. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348-354. |
| 39. | Michl P, Gülberg V, Bilzer M, Waggershauser T, Reiser M, Gerbes AL. Transjugular intrahepatic portosystemic shunt for cirrhosis and ascites: Effects in patients with organic or functional renal failure. Scand J Gastroenterol. 2000;35:654-658. |
Peer reviewers: Dr. Paolo Del Poggio, Hepatology Unit, Department of Internal Medicine, Treviglio Hospital, Piazza Ospedale 1, Treviglio Bg 24047, Italy; Giuseppe Montalto, Professor, Clinical Medicine and Emerging Diseases, University of Palermo, via del Vespro, 141, Palermo 90100, Italy; Tomoharu Yoshizumi, MD, PhD, Department of Surgery, Saiseikai Fukuoka General Hospital, 1-3-46, Tenjin, Chuou-ku, Fukuoka, 810-0001, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP