Published online Jul 28, 2010. doi: 10.3748/wjg.v16.i28.3499
Revised: February 16, 2010
Accepted: February 23, 2010
Published online: July 28, 2010
AIM: To examine the relevance of hypoxia inducible factor (HIF-1) and nitric oxide (NO) on the preservation of fatty liver against cold ischemia-reperfusion injury (IRI).
METHODS: We used an isolated perfused rat liver model and we evaluated HIF-1α in steatotic and non-steatotic livers preserved for 24 h at 4°C in University of Wisconsin and IGL-1 solutions, and then subjected to 2 h of normothermic reperfusion. After normoxic reperfusion, liver enzymes, bile production, bromosulfophthalein clearance, as well as HIF-1α and NO [endothelial NO synthase (eNOS) activity and nitrites/nitrates] were also measured. Other factors associated with the higher susceptibility of steatotic livers to IRI, such as mitochondrial damage and vascular resistance were evaluated.
RESULTS: A significant increase in HIF-1α was found in steatotic and non-steatotic livers preserved in IGL-1 after cold storage. Livers preserved in IGL-1 showed a significant attenuation of liver injury and improvement in liver function parameters. These benefits were enhanced by the addition of trimetazidine (an anti-ischemic drug), which induces NO and eNOS activation, to IGL-1 solution. In normoxic reperfusion, the presence of NO favors HIF-1α accumulation, promoting also the activation of other cytoprotective genes, such as heme-oxygenase-1.
CONCLUSION: We found evidence for the role of the HIF-1α/NO system in fatty liver preservation, especially when IGL-1 solution is used.
- Citation: Zaouali MA, Mosbah IB, Boncompagni E, Abdennebi HB, Mitjavila MT, Bartrons R, Freitas I, Rimola A, Roselló-Catafau J. Hypoxia inducible factor-1α accumulation in steatotic liver preservation: Role of nitric oxide. World J Gastroenterol 2010; 16(28): 3499-3509
- URL: https://www.wjgnet.com/1007-9327/full/v16/i28/3499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i28.3499
During the last decade, the dramatic shortage of organs has obliged physicians to consider the transplantation of liver grafts with moderate steatosis[1-3]. Liver steatosis results from the abnormal accumulation of fat in the cytoplasm of hepatocytes. This causes alterations in the microcirculation with a higher risk of graft dysfunction or primary nonfunction. Steatotic liver grafts thus show poor tolerance to ischemia-reperfusion injury (IRI) associated with transplantation[3-5]. The composition of preservation solutions is critical for the quality of liver grafts during cold ischemia. Although University Wisconsin solution (UW) is the gold standard for abdominal organs, its use has been questioned because it contains hydroxyl-ethyl starch (HES), and a high concentration of K+ ions[6-9]. It is has been reported that HES could be responsible for red blood cell aggregation[10-12].
Recently, IGL-1 solution has been proposed as an effective alternative to UW for steatotic liver preservation[12]. It has a lower concentration of K+ ions and contains polyethylene glycol as osmotic support instead of HES. The benefits of IGL-1 are due in part to its capacity to increase the levels of nitric oxide (NO)[12], which protects the liver against IRI[13], and thus mitigates the alterations to hepatic microcirculation[12].
Cold ischemia graft preservation is characterized by a lack of oxygen supply to the liver. In these conditions, hypoxia inducible factor 1 (HIF-1) regulates the adaptive response of the organ to the changes in oxygenation induced during preservation[14]. HIF-1 is a heterodimer formed by α and β subunits which are constitutively expressed. The β subunit is independent of O2, whereas the protein stability of the α subunit depends on the cellular levels of O2[15]. Under normoxic conditions, the α subunit is degraded by a complex process involving the prolyl-hydroxylases, the Von Hippel Landau protein and the 26 S proteasomes[15,16]. It is well established that NO impairs normoxic degradation of HIF-1α by inhibition of HIF-1 prolyl-hydroxylases and contributes to its stabilization[17].
HIF-1α confers protection against IRI by activating others genes such as heme oxygenase-1 (HO-1)[18-20] which plays an important cytoprotective role in liver graft preservation against cold IRI[21,22].
Recently, it has been demonstrated that anti ischemic drugs such as trimetazidine (TMZ) protect heart mesenchymal stem cells against hypoxic insult by increasing HIF-1α expression[23]. Similar results were reported by Jayle et al[14] in TMZ-treated pig kidneys subjected to warm IRI. In addition, the benefits of TMZ seem to be associated with its capacity to generate NO[24] and the activation of endothelial NO synthase (eNOS) when it was used as an additive in UW solution[25].
As HIF-1α is involved in cell survival during IRI, we examined how hypoxic adaptations can be exploited to protect fatty livers in preservation solutions. We hypothesized that the HIF-1α accumulation during cold ischemia would be hepato-protective in normoxic reperfusion as a result of the NO-stabilizing action induced by IGL-1.
To test this hypothesis, we added TMZ to IGL-1 solution, and examined the effects of various protocols on the preservation of livers excised from rats. We found that the addition of TMZ to IGL-1 solution has a synergistic effect on NO generation and thus favors HIF-1α accumulation during normothermic reperfusion. Preserved HIF-1α levels contribute to the increase in the over-expression of HO-1 in fatty liver grafts.
The results reported here reveal the importance of the HIF-1α/NO system in the prevention of liver cold IRI, especially when moderate steatosis is present.
Homozygous obese (Ob) Zucker rats and heterozygous lean (Ln) Zucker rats (reference group), aged 16-18 wk, were obtained from Iffa-Credo (L’Abresle, France)[12,25].
An isolated perfused rat liver model was used to evaluate hepatic function separate from the influence of other organ systems, undefined plasma constituents, and neural/hormonal effects. Hepatic architecture, microcirculation, and bile production are preserved in this experimental model[26,27]. All procedures were performed under isoflurane inhalation anesthesia. This study adhered to European Union regulations (Directive 86/609/CEE) for animal experiments.
The surgical technique was performed as described elsewhere[12,28]. After cannulation of the common bile duct, the portal vein was isolated and the splenic and gastroduodenal veins were ligated. All animals were randomly distributed into groups as described below.
Protocol I: induction of HIF-1α in steatotic livers after 24 h of cold ischemia in IGL-1 preservation solution: In order to evaluate the potential generation of HIF-1α during liver graft storage (24 h, 4°C) in IGL-1 preservation solution, and the benefits of the addition of TMZ, the following experiments were carried out: (1) Cont 1: Control livers from 16 Zucker rats (8 Ln and 8 Ob) were flushed with Ringer’s lactate solution immediately after laparatomy via the portal vein without cold storage; (2) UW: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 h in UW solution; (3) IGL-1: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 h in IGL-1 solution; and (4) IGL-1 + TMZ: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 h in IGL-1 with TMZ at a concentration of 10-6 mol/L, as previously described[25,28].
Aliquots of the effluent flush corresponding to groups 1, 2, 3 and 4 were sampled for measurements of cumulative transaminases. Liver tissue samples were used for HIF-1α activity determination after prolonged ischemia.
Protocol II: effect of NO on HIF-1α stabilization and HO-1 induction after cold IRI: To examine the role of NO in stabilizing HIF-1α formed during cold ischemia, and the subsequent effect on HO-1 generation, fatty livers were subjected to 2 h-normoxic reperfusion, in the following groups: (5) Cont 2: Control livers from 16 Zucker rats (8 Ln and 8 Ob) were flushed with Ringer’s lactate and immediately perfused ex vivo without ischemic preservation; (6) UW: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 h in UW solution and then inserted into an isolated perfused rat liver system for 2 h; (7) IGL-1: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved in IGL-1 solution and then inserted into an isolated perfused rat liver system for 2 h; (8) IGL-1 + TMZ: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 h in IGL-1 with the addition of 10-6 mol/L TMZ and then inserted into an isolated perfused rat liver system for 2 h[25,28]; and (9) IGL-1 + TMZ + NAME: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 h in IGL-1 with the addition of 10-6 mol/L TMZ and Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), an inhibitor of NO synthesis, at a dose of 60 μg/g liver before cold ischemia, and then inserted into an isolated perfused rat liver system for 2 h[28].
In order to account for the period of rewarming during surgical implantation in vivo[28], after 24 h of cold preservation livers from groups 5, 6, 7, 8 and 9 (Protocol II) were exposed to 22°C for 30 min prior to reperfusion. Livers were then connected via the portal vein to a recirculating perfusion system for 120 min at 37°C[12,25]. Time 0 was the point at which the portal catheter was connected to the circuit. As previously reported[12,28], during the first 15 min of perfusion (initial equilibration period), the flow was progressively increased in order to stabilize the portal pressure at 12 mmHg (Pression Monitor BP-1; Pression Instruments, Sarasota, FL, USA). The flow was controlled using a peristaltic pump (Minipuls 3, Gilson, France)[12,25]. The reperfusion liquid consisted of a cell culture medium (William’s medium E; Bio Whitaker, Barcelona, Spain) with a Krebs-Heinseleit-like electrolyte composition enriched with 5% albumin osmotic support. The buffer was continuously ventilated with 95% O2 and 5% CO2 gas mixture. The buffer was subsequently passed through a heat exchanger (37°C) and a bubble trap prior to entering the liver[25]. During 120 min of normothermic reperfusion, the effluent was collected at 30-min intervals to measure liver transaminases. After the initial equilibration period of 15 min, flow rate and vascular resistance were assessed continuously throughout the reperfusion. Bile output, hepatic clearance [expressed as percentage of sulfobromophthalein (BSP) in bile samples], HIF-1α activity, and NOS proteins were evaluated at 120 min of reperfusion.
Liver injury: Hepatic injury was evaluated according to transaminase levels using a commercial kit from Boehringer Mannheim (Munich, Germany).
Liver function: Liver function was assessed by measuring bile production[12,25]. Bile was collected through the cannulated bile duct and output is reported as μL/g liver.
Hepatic clearance: As with bile output, hepatic clearance was considered as another parameter of hepatic function[27,28]. Thirty minutes after the onset of the perfusion (t30), 1 mg of BSP (Sigma, Spain) was added to the perfusate. The concentration of BSP in bile samples (t120) was measured at 580 nm with a UV-visible spectrometer. Bile BSP excretion was expressed as a percentage of perfusate content (t120 bile/t30 perfusate × 100)[28,29].
Vascular resistance: Liver circulation was assessed by measuring perfusion flow rate and vascular resistance[26,28]. Perfusion flow rate was assessed continuously throughout the reperfusion period and expressed as mL/min × g. Vascular resistance was defined as the ratio of portal venous pressure to flow rate and expressed in mmHg × min × g/mL[26,28].
Glutamate dehydrogenase activity: Glutamate dehydrogenase (GLDH) is a mitochondrial enzyme and was used as an indirect measure of mitochondrial damage[25,28]. GLDH activity was determined in perfusates as described elsewhere[25,28].
Determination of nitrite and nitrate: NO production in liver was determined by tissue accumulation of nitrite and nitrate, as previously reported[25,28].
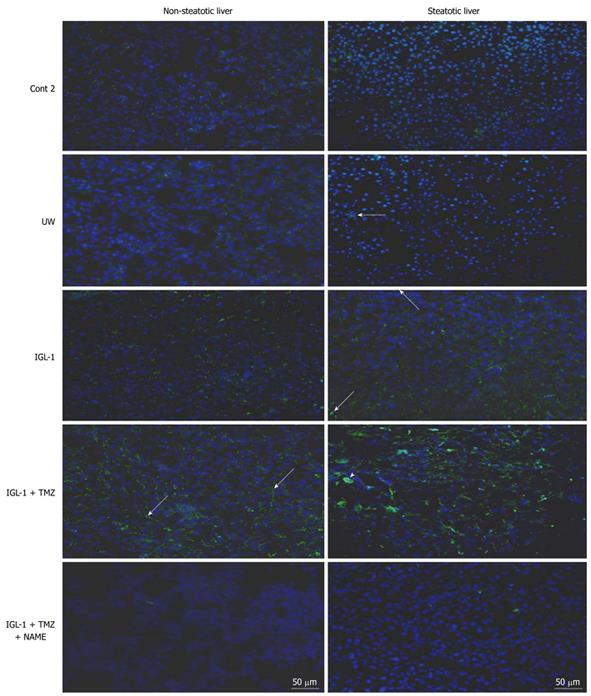
eNOS immunohistochemistry: Frozen liver sections (18 μm thick) were fixed in pre-cooled acetone for 10 min at room temperature. After washing with phosphate-buffered saline (PBS), permeabilization was performed with 0.5% Triton X-100 for 15 min each. Antibody non-specific binding was blocked for 1 h in PBS with 10% normal goat serum, 3% bovine serum albumin, 1.5% NaCl and 0.5% Triton X-100 for 1 h. The primary eNOS antibody (Polyclonal IgG anti-NOS-3, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was applied at a dilution of 1:100 in the blocking solution and incubated for 1 h at room temperature in a moist chamber. After rinsing with PBS, permeabilization was performed again with 0.5% Triton X-100, 3 times for 10 min each. The secondary antibody goat anti-rabbit Alexa488 Fluor labeled (Invitrogen) was applied at a dilution of 1:400 in the blocking solution for 50 min at room temperature. After washing with PBS, the slides were mounted with ProLong Gold antifade (with DAPI) mounting medium, and eNOS immunohistochemistry images were obtained by fluorescence microscope.
Liver tissue was homogenized as previously described[28] and proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Membranes were immunoblotted with antibodies against anti-eNOS (transduction laboratories, Lexington, KY, USA), against anti-HO-1 (Sigma Chemical, St Louis, MO, USA) and β-actin (Sigma Chemical, St. Louis, MO, USA). Signals were detected by enhanced chemiluminescence and quantified by scanning densitometry.
Preparation of nuclear fraction: Nuclear fractions were prepared from frozen liver tissues which were suspended in a hypotonic buffer that contained 10 mmol/L HEPES (pH 7.6), 15 mmol/L KCl, 2 mmol/L MgCl2, 0.1 mmol/L EDTA, 1 mmol/L dithiothreitol, and 0.2% Nonidet P-40 with protease inhibitor (1 mmol/L phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, 1 μg/mL leupeptin and 1 μg/mL pepstatin) and homogenized for 40 s with a polytron homogenizer. After centrifugation at 850 g the supernatant that contained cytoplasmic and membrane protein was collected. Nuclear proteins were extracted at 4°C by gently resuspending the nuclear pellet in hypertonic buffer that contained 25 mmol/L HEPES (pH 7.8), 50 mmol/L KCl, 0.1 mmol/L EDTA, 1 mmol/L dithiothreitol, 10% glycerol, 0.4 mol/L NaCl and protease inhibitor, followed by 30 min incubation at 4°C with occasional vortexing. After centrifugation at 18 000 g for 15 min at 4°C, the supernatant that contained nuclear protein was collected and protein concentration was measured by the Bradford Protein method (Bio-Rad, Hercules, CA, USA).
HIF-1α measurement: HIF-1α translocation to nuclei was assessed by Western blotting and was also quantified by specific binding of HIF-1α to its specific oligonucleotide recognition hypoxia responsive element, using the Trans AM HIF-1α kit (Active Motiv, Carlsbad, CA, USA). Membranes were incubated with an antibody against anti-HIF-1α (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Signals were detected by enhanced chemiluminescence and quantified by scanning densitometry. For the quantitative method, samples were run in duplicate in microtiter plates coated with an oligonucleotide for HIF-1α following manufacturer’s instructions. Results are expressed as μg HIF-1α/mg protein of nuclei extract[30,31].
Data are expressed as mean ± SE, and were compared statistically by variance analysis, followed by the Student-Newman-Keuls test (Graph Pad Prism software). P < 0.05 was considered significant.
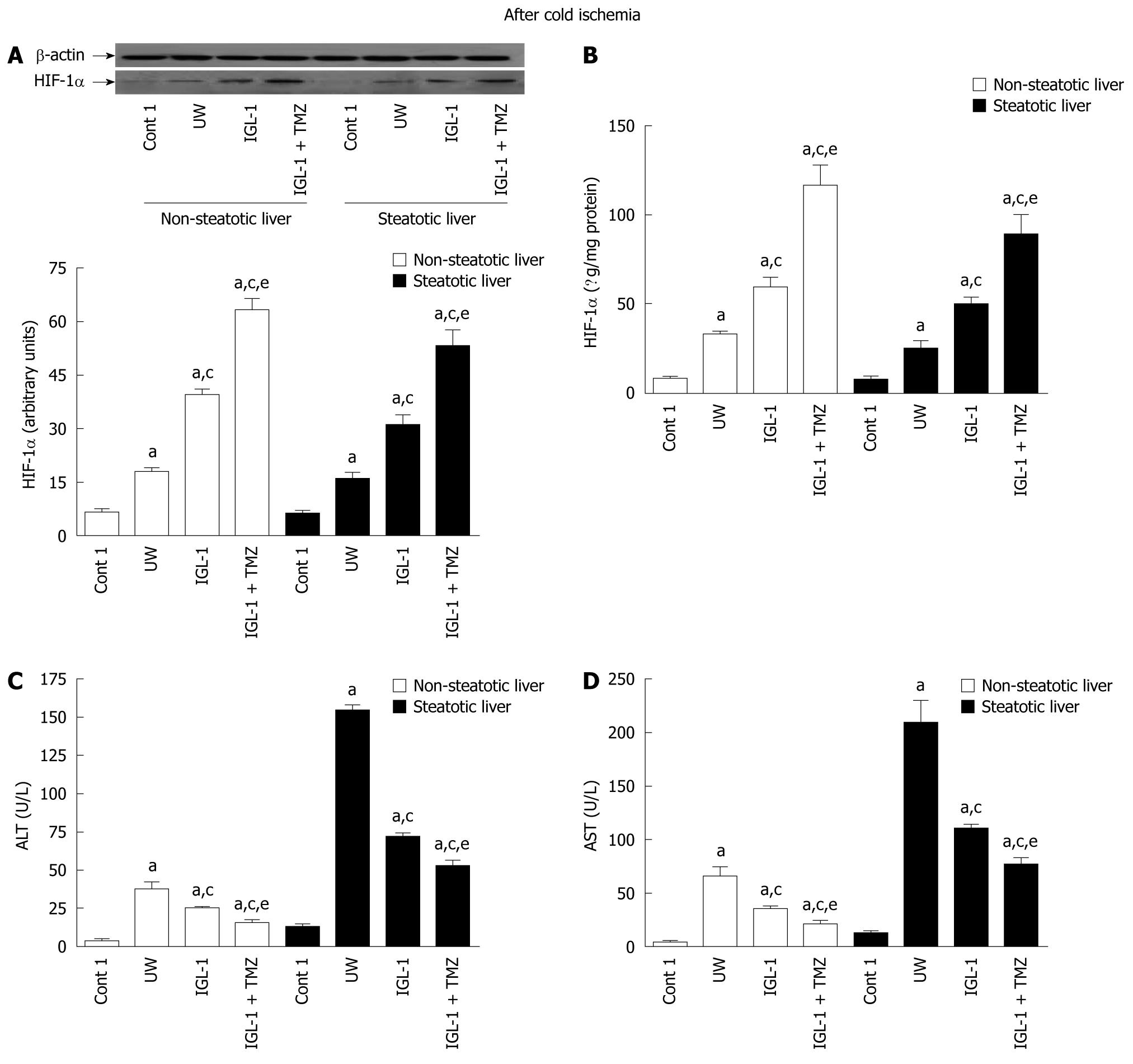
As indicated in Figure 1A and B, cumulative HIF-1α levels were determined in non-steatotic and steatotic livers preserved in IGL-1 + TMZ solutions and compared to levels observed in IGL-1 and UW alone. HIF-1α accumulation was significantly increased by the addition of TMZ to IGL-1 solution in both types of liver. Although steatotic livers showed slightly lower HIF-1α levels than non-steatotic livers, these differences were not significant (Figure 1A and B).
Liver ischemic damage was assessed by alanine transaminase (ALT) and aspartate transaminase (AST) levels after 24 h of cold storage as shown in Figure 1C and D. A significant reduction in release of ALT and AST occurred in non-steatotic and steatotic livers preserved in IGL-1 + TMZ when compared to IGL-1 and UW, respectively. This reduction correlated with the significant accumulation of HIF-1α observed for the IGL-1 + TMZ and IGL-1 solutions (Figure 1A and B).
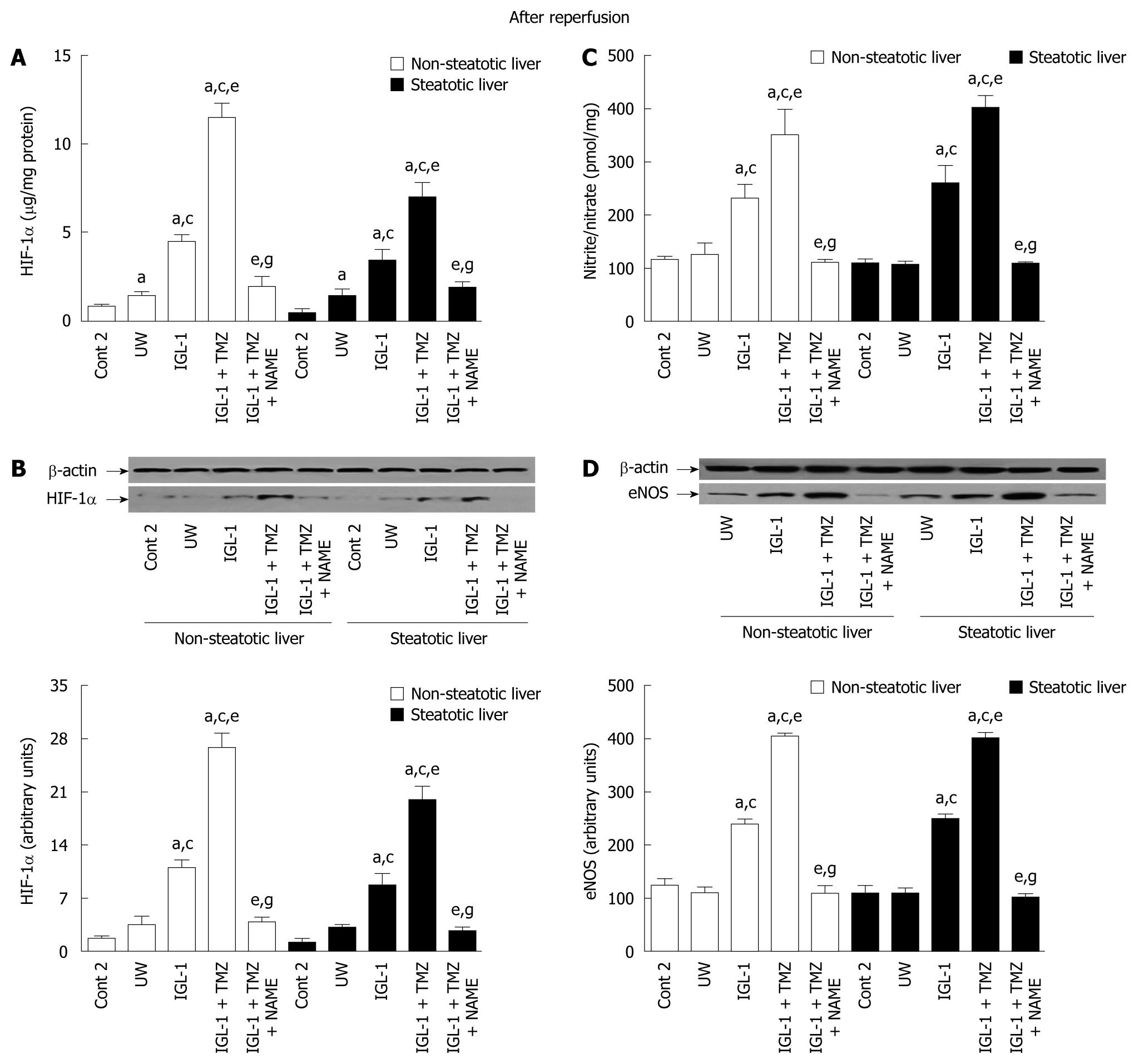
In order to evaluate whether HIF-1α could be stabilized by NO after hepatic reperfusion, we measured HIF-1α accumulation in steatotic and non-steatotic livers preserved in UW, IGL-1 and IGL-1 + TMZ solutions (24 h, 4°C) and then subjected to 2 h normoxic reperfusion (37°C). NO generation was inhibited by the addition of L-NAME to IGL-1 + TMZ solution (IGL-1 + TMZ + NAME).
Figure 2 shows the HIF-1α levels after 2 h of normothermic reperfusion in steatotic and non-steatotic livers preserved in IGL-1, IGL-1 + TMZ and UW solutions, respectively. HIF-1α levels were higher in the non-steatotic and steatotic livers stored in IGL-1 + TMZ solution than in those stored in IGL-1 and UW respectively. In all cases, the greatest increases were for the non-steatotic and steatotic livers preserved in IGL-1 enriched with TMZ (IGL + TMZ), and were significantly prevented by L-NAME (Figure 2A and B).
eNOS activity and nitrite/nitrate levels were higher in the steatotic and non-steatotic livers preserved in IGL-1 + TMZ when compared to UW and IGL-1 alone (Figure 2C, D and Figure 3). This enhanced eNOS activity and nitrite/nitrate levels were more evident when TMZ was added to IGL-1. In contrast, the addition of L-NAME to IGL-1 + TMZ solution reduced both.
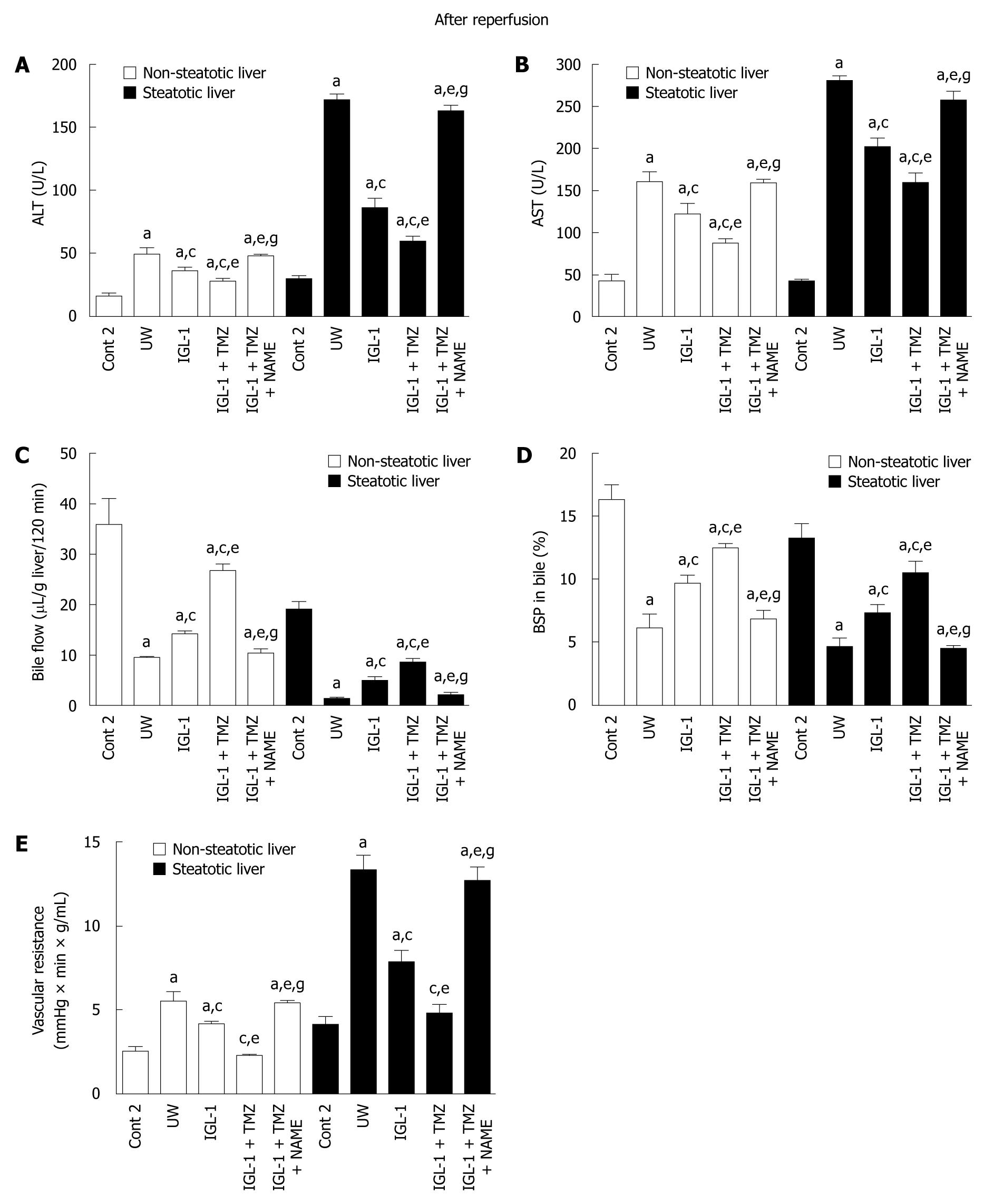
In order to evaluate the benefits of HIF-1α and NO in livers preserved in IGL-1 and IGL-1 + TMZ after reperfusion, we evaluated liver injury (AST/ALT) and function (bile production, vascular resistance), as indicated in Figure 4.
At 2 h reperfusion release of ALT and AST was significantly lower in non-steatotic and steatotic livers preserved in IGL-1 + TMZ solution than in IGL-1 and UW solutions (Figure 4A and B). In contrast, the inhibition of NO by L-NAME showed significantly increased in AST/ALT release, in both kinds of liver. In all cases, the AST/ALT profiles were consistent with those obtained for HIF-1α accumulation during reperfusion (Figure 2).
TMZ also had beneficial effects on bile production and %BSP clearance in bile (Figure 4C and D). It also promoted a significant reduction in vascular resistance (Figure 4E), when compared to IGL-1 and UW solutions. The greatest reduction in vascular resistance occurred when TMZ was added to IGL-1. This finding is consistent with highest increases in eNOS activity and nitrite/nitrate levels as shown in Figures 2 and 3.
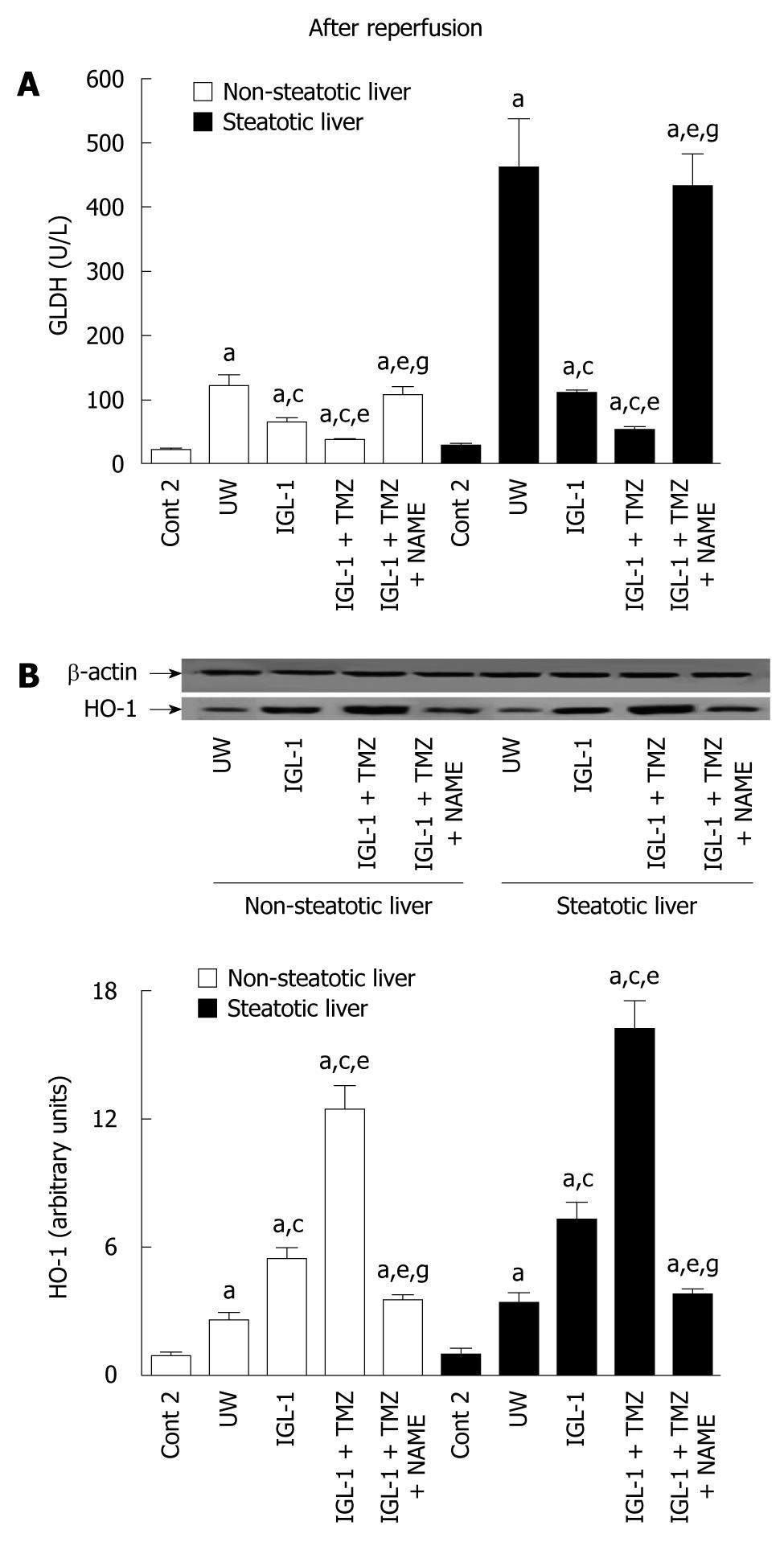
Mitochondrial damage was evaluated by measuring GLDH activity levels in perfusate at the end of the reperfusion period. Steatotic livers preserved in UW solution showed greater GLDH activity than did non-steatotic livers (Figure 5A). Livers stored in IGL-1 as well as in IGL-1 + TMZ solution reduced GLDH levels in both type of livers (Figure 5).
Finally, we examined the effect of HIF-1α accumulation during liver reperfusion on the induction of other cytoprotective agents, such as HO-1. HO-1 expression was exacerbated in both kinds of liver preserved in IGL-1 + TMZ solution when compared to IGL-1 and UW alone (Figure 5B). As with HIF-1α, HO-1 was inhibited by the presence of L-NAME in both livers. The HO-1 pattern profiles were similar to those obtained for HIF-1α (Figure 2).
HIF-1 is the main factor involved in the regulation of transcriptional responses to hypoxia[32,33]. HIF-1α levels accumulate and trigger an increase in expression of genes involved in glycolysis, glucose metabolism, mitochondrial function, cell survival and resistance to oxidative stress[20], in response to oxygen deprivation, and during organ cold storage. For this reason, we have explored new pharmacological strategies to promote the highest accumulation of cytoprotective factors such as HIF-1α to protect fatty livers against cold ischemia and reperfusion injury.
Here, we demonstrate for the first time that significant activation of HIF-1α occurs after 24 h of cold storage in both types of liver when TMZ is added to IGL-1 solution. This improves liver protection, as evidenced by the reduction in transaminase levels. This is of particular interest in clinical practice, since numerous pharmacological strategies that are effective in non-steatotic livers may not be useful in the presence of steatosis[34,35]. These results agree with the protective effects observed when TMZ was added to UW preservation solution[14,25].
Significant HIF-1α accumulation was also found after 2 h of liver reperfusion. This is consistent with the finding that mesenchymal stem cells pre-treated with TMZ were protected against H2O2 induced loss of cellular viability and membrane damage, with a concomitant increases in HIF-1α[23]. Similar observations were reported by Jayle et al[14] when pig kidneys were subjected to warm ischemia reperfusion.
Cumulative HIF-1α levels contributed to liver protection but they were abolished by the addition of L-NAME, an inhibitor of NO synthesis. This demonstrates by the first time in fatty liver preservation, the relevance of NO in HIF-1α accumulation during normoxic liver reperfusion.
NO is an intracellular and/or intercellular and diffusible mediator whose role on hepatic IRI is controversial[28,36-38]. Overall, the important factors in determining the beneficial vs harmful effects of NO are the amount, duration, and site of NO production[39,40]. A low concentration eNOS-derived NO serves to maximize blood perfusion, promote cell survival and protect the liver against IRI[41]. However, a sustained presence of iNOS-derived NO might become detrimental by increasing toxic reactive oxygen species leading to liver injury[37,40-42]. The results reported here show that the NO benefits were derived mainly from eNOS and that they did not originate from iNOS activation (data not shown).
NO vasodilator properties prevent the alterations of microcirculation observed during liver reperfusion, which are exacerbated in the presence of steatosis[12,28]. The addition of NO donors to UW solution preserves livers against cold ischemic injury[13]. Thus, the benefits of the use of IGL-1 solution for fatty liver preservation are associated with the capacity of generating NO[12]. However, the relevance of NO and it relationship with HIF-1α accumulation for increasing fatty liver graft protection against cold IRI is demonstrated here for the first time.
In recent years, there has been increasing interest in the relationship between HIF-1α and NO. NO profoundly affects the HIF-1α signaling pathway[43,44]. In normoxia, NO inhibits the prolyl-hydroxylases responsible for HIF-1α degradation, thus contributing to its stability[17].
Our results demonstrate that HIF-1α also accumulate during reperfusion in the presence of NO in liver grafts preserved in IGL-1 + TMZ or IGL-1 alone. Beneficial effects on HIF-1α accumulation are confirmed by the increased constitutive eNOS activity and nitrite/nitrate levels in liver grafts preserved in both solutions. In addition, these data confirm the synergism between IGL-1 solution and TMZ, responsible for the increased NO production by the activation of constitutive eNOS[24,28]. NO levels generated from the synergistic action of TMZ in IGL-1 solution are determinant of effective HIF-1α accumulation and subsequent protection of the liver graft against IRI. This differs from the effects observed when TMZ was added to UW, where the induction of eNOS activity was lower (data not shown).
Previous studies from our group showed that bile production is higher when livers are preserved in IGL-1than when they are preserved in UW[12]. Similarly, TMZ increased bile secretion when added to UW solution[25]. Our results show a synergistic action when TMZ is added to IGL-1 solution, which enhances the increase in bile production.
Fat accumulation in the cytoplasm of the hepatocytes is associated with an increase in cell volume, which may result in the partial or complete obstruction of the hepatic sinusoidal space[45,46]. Our results demonstrate that NO generated from the synergistic action mentioned above contributes to the reduction in vascular resistance. In fact NO inhibition by L-NAME increased vascular resistance, confirming the role of NO in fatty liver preservation.
Fatty degeneration, which induces a series of ultrastructural and biochemical alterations in mitochondria[47,48] may render these organelles intrinsically more susceptible to I/R injury. Mitochondrial injury is a common pathway of cell necrosis and apoptosis in IRI. Recent studies have shown that activation of HIF-1α prevents mitochondrial injury after mouse liver ischemia reperfusion[49]. Our results indicate that the HIF-1α accumulation after normoxic reperfusion prevented mitochondrial injury.
Finally, we measured the expression of HO-1 after 2 h of normothermic reperfusion, as a potential gene target induced by HIF-1α activation[18,19]. HO-1 is activated during cellular stress: its over-expression is a requisite for increasing organ cytoprotection against IRI, especially when steatosis is present[20,21,45,50].
We confirmed increased HO-1 expression in livers preserved in IGL-1 and IGL-1 + TMZ solutions. This is in line with the better protection against reperfusion injury in livers stored in IGL-1 + TMZ solution than in IGL-1 or UW alone. The abolition of HIF-1α stabilization by NO inhibition with L-NAME reversed HO-1 levels and increased liver injury after reperfusion.
In conclusion, we report by the first time the importance of NO in fatty liver reperfusion in stabilizing HIF-1α which is generated during liver cold storage.HIF-1α accumulation determines, in part, the increase in HO-1, a cytoprotective factor which improves the outcome of grafts after transplantation.
Steatosis is a major risk against cold ischemia-reperfusion injury (IRI). Hypoxia inducible factor-1α (HIF-1α) is a cytoprotective factor generated in response to lack of oxygen, as occurs during cold storage of liver grafts in preservation solutions. IGL-1 solution has been proposed as an effective alternative to University Wisconsin for steatotic liver preservation. The benefits of IGL-1 solution are due in part to its capacity to increase the levels of nitric oxide (NO).
NO impairs normoxic degradation of HIF-1α by inhibition of prolyl-hydroxylases and contributes to its stabilization. HIF-1α confers protection against IRI by activating genes such as heme oxygenase-1 which plays an important cytoprotective role in liver graft preservation against cold IRI. In this study, the authors focused on the importance of HIF-1α and NO in fatty liver preservation.
The authors provide evidence that the enrichment of IGL-1 solution with trimetazidine (an anti-ischemic drug) increases NO generation and prevents HIF-1α degradation during steatotic liver graft reperfusion.
The use of modified IGL-1 solutions should be a useful strategy for increasing steatotic liver graft preservation through HIF-1α accumulation in normothermic reperfusion.
In essence, the paper demonstrates that the HIF/NO system is important in fatty liver preservation following ischemia reperfusion injury. The study is well designed and the interpretation of the data is adequate.
| 1. | Imber CJ, St Peter SD, Handa A, Friend PJ. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8:415-423. |
| 2. | Crowley H, Lewis WD, Gordon F, Jenkins R, Khettry U. Steatosis in donor and transplant liver biopsies. Hum Pathol. 2000;31:1209-1213. |
| 3. | Alfany-Fernandez I, Casillas-Ramirez A, Bintanel-Morcillo M, Brosnihan KB, Ferrario CM, Serafin A, Rimola A, Rodés J, Roselló-Catafau J, Peralta C. Therapeutic targets in liver transplantation: angiotensin II in nonsteatotic grafts and angiotensin-(1-7) in steatotic grafts. Am J Transplant. 2009;9:439-451. |
| 4. | Fernández L, Carrasco-Chaumel E, Serafín A, Xaus C, Grande L, Rimola A, Roselló-Catafau J, Peralta C. Is ischemic preconditioning a useful strategy in steatotic liver transplantation? Am J Transplant. 2004;4:888-899. |
| 5. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. |
| 6. | Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673-676. |
| 7. | Mühlbacher F, Langer F, Mittermayer C. Preservation solutions for transplantation. Transplant Proc. 1999;31:2069-2070. |
| 8. | Straatsburg IH, Abrahamse SL, Song SW, Hartman RJ, Van Gulik TM. Evaluation of rat liver apoptotic and necrotic cell death after cold storage using UW, HTK, and Celsior. Transplantation. 2002;74:458-464. |
| 9. | Morariu AM, Vd Plaats A, V Oeveren W, 'T Hart NA, Leuvenink HG, Graaff R, Ploeg RJ, Rakhorst G. Hyperaggregating effect of hydroxyethyl starch components and University of Wisconsin solution on human red blood cells: a risk of impaired graft perfusion in organ procurement? Transplantation. 2003;76:37-43. |
| 10. | van der Plaats A, 't Hart NA, Morariu AM, Verkerke GJ, Leuvenink HG, Ploeg RJ, Rakhorst G. Effect of University of Wisconsin organ-preservation solution on haemorheology. Transpl Int. 2004;17:227-233. |
| 11. | Mosbah IB, Franco-Gou R, Abdennebi HB, Hernandez R, Escolar G, Saidane D, Rosello-Catafau J, Peralta C. Effects of polyethylene glycol and hydroxyethyl starch in University of Wisconsin preservation solution on human red blood cell aggregation and viscosity. Transplant Proc. 2006;38:1229-1235. |
| 12. | Ben Mosbah I, Roselló-Catafau J, Franco-Gou R, Abdennebi HB, Saidane D, Ramella-Virieux S, Boillot O, Peralta C. Preservation of steatotic livers in IGL-1 solution. Liver Transpl. 2006;12:1215-1223. |
| 13. | Quintana AB, Rodriguez JV, Scandizzi AL, Guibert EE. The benefit of adding sodium nitroprusside (NPNa) or S-nitrosoglutathion (GSNO) to the University of Wisconsin solution (UW) to prevent morphological alterations during cold preservation/reperfusion of rat livers. Ann Hepatol. 2003;2:84-91. |
| 14. | Jayle C, Favreau F, Zhang K, Doucet C, Goujon JM, Hebrard W, Carretier M, Eugene M, Mauco G, Tillement JP. Comparison of protective effects of trimetazidine against experimental warm ischemia of different durations: early and long-term effects in a pig kidney model. Am J Physiol Renal Physiol. 2007;292:F1082-F1093. |
| 15. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. |
| 16. | Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642-22647. |
| 17. | Metzen E, Zhou J, Jelkmann W, Fandrey J, Brüne B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470-3481. |
| 18. | Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA 3rd, Kukreja RC. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542-H548. |
| 19. | Dawn B, Bolli R. HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? Am J Physiol Heart Circ Physiol. 2005;289:H522-H524. |
| 20. | Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ. 2008;15:686-690. |
| 21. | Redaelli CA, Tian YH, Schaffner T, Ledermann M, Baer HU, Dufour JF. Extended preservation of rat liver graft by induction of heme oxygenase-1. Hepatology. 2002;35:1082-1092. |
| 22. | Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121-128. |
| 23. | Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther. 2009;329:543-550. |
| 24. | Di Napoli P, Chierchia S, Taccardi AA, Grilli A, Felaco M, De Caterina R, Barsotti A. Trimetazidine improves post-ischemic recovery by preserving endothelial nitric oxide synthase expression in isolated working rat hearts. Nitric Oxide. 2007;16:228-236. |
| 25. | Ben Mosbah I, Casillas-Ramírez A, Xaus C, Serafín A, Roselló-Catafau J, Peralta C. Trimetazidine: is it a promising drug for use in steatotic grafts? World J Gastroenterol. 2006;12:908-914. |
| 26. | Ahmed I, Attia MS, Ahmad N, Lodge JP, Potts DJ. Use of isolated perfused rat liver model for testing liver preservation solutions. Transplant Proc. 2001;33:3709-3711. |
| 27. | Defamie V, Laurens M, Patrono D, Devel L, Brault A, Saint-Paul MC, Yiotakis A, Barbry P, Gugenheim J, Crenesse D. Matrix metalloproteinase inhibition protects rat livers from prolonged cold ischemia-warm reperfusion injury. Hepatology. 2008;47:177-185. |
| 28. | Ben Mosbah I, Massip-Salcedo M, Fernández-Monteiro I, Xaus C, Bartrons R, Boillot O, Roselló-Catafau J, Peralta C. Addition of adenosine monophosphate-activated protein kinase activators to University of Wisconsin solution: a way of protecting rat steatotic livers. Liver Transpl. 2007;13:410-425. |
| 29. | Minor T, Yamaguchi T, Isselhard W. Effects of taurine on liver preservation in UW solution with consecutive ischemic rewarming in the isolated perfused rat liver. Transpl Int. 1995;8:174-179. |
| 30. | Amador A, Grande L, Martí J, Deulofeu R, Miquel R, Solá A, Rodriguez-Laiz G, Ferrer J, Fondevila C, Charco R. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180-2189. |
| 31. | Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci USA. 2006;103:5379-5384. |
| 32. | Shi LB, Huang JH, Han BS. Hypoxia inducible factor-1alpha mediates protective effects of ischemic preconditioning on ECV-304 endothelial cells. World J Gastroenterol. 2007;13:2369-2373. |
| 34. | Serafín A, Roselló-Catafau J, Prats N, Xaus C, Gelpí E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587-601. |
| 35. | Selzner M, Rüdiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280-1288. |
| 36. | Mittal MK, Gupta TK, Lee FY, Sieber CC, Groszmann RJ. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Physiol. 1994;267:G416-G422. |
| 37. | Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337-345. |
| 38. | Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22:46-55. |
| 39. | Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: friend, foe, or just passerby? Ann N Y Acad Sci. 2002;962:275-295. |
| 40. | Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18-31. |
| 41. | Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H2980-H2986. |
| 42. | Abe Y, Hines I, Zibari G, Grisham MB. Hepatocellular protection by nitric oxide or nitrite in ischemia and reperfusion injury. Arch Biochem Biophys. 2009;484:232-237. |
| 43. | Sumbayev VV, Yasinska IM. Peroxynitrite as an alternative donor of oxygen in HIF-1alpha proline hydroxylation under low oxygen availability. Free Radic Res. 2006;40:631-635. |
| 44. | Brüne B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007;75:275-282. |
| 45. | Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076-1082. |
| 46. | Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105-113. |
| 47. | Rashid A, Wu TC, Huang CC, Chen CH, Lin HZ, Yang SQ, Lee FY, Diehl AM. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. Hepatology. 1999;29:1131-1138. |
| 48. | Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol. 1999;31:430-434. |
| 49. | Zhong Z, Ramshesh VK, Rehman H, Currin RT, Sridharan V, Theruvath TP, Kim I, Wright GL, Lemasters JJ. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2008;295:G823-G832. |
| 50. | Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631-1639. |
Peer reviewers: Willemijntje A Hoogerwerf, MD, Assistant Professor, Internal Medicine, Division of Gstroenterology, University of Michigan, 2215 Fuller Road, Ann Arbor, MI 48105, United States; Damiao Carlos Moraes Santos, DCM, PhD, Department of Quality Control (deque), Bio-Manguinhos - FIOCRUZ, Avenida Brazil, 4365, Manguinhos - Rio de Janeiro, 21045-900, Brazil
S- Editor Wang YR L- Editor Cant MR E- Editor Zheng XM