INTRODUCTION
Pancreatic cancer, the fourth leading cause of cancer-related mortality in the United States[1], portends a median survival of 6 mo and a 5-year survival of less than 5%[1-4]. Operative resection is indicated in less than 20% of patients and only increases median 5-year survival to 12%[5,6]. Given these grim statistics and paucity of therapeutic options, this disease represents an arena in which the development of novel therapies is greatly needed.
Since the 1980s, innovative, minimally invasive transcatheter intraarterial techniques to treat malignancies, particularly in the liver, have been developed. These techniques, including bland-, chemo-, and radio-embolization, have been shown to improve survival and/or induce imaging responses while being well tolerated by patients[7,8]. Despite some reports demonstrating preliminary success in the treatment of advanced pancreatic cancer with the transcatheter intraarterial infusion of chemotherapy directly to the pancreas[9-12], few studies have assessed local pancreas-directed therapies.
Before catheter-directed clinical therapies can be developed, it would be useful to develop functional imaging targets in a pre-clinical animal model of pancreatic cancer. Functional imaging is important because conventional anatomic magnetic resonance imaging (MRI) has a low sensitivity to detect pancreatic cancer and separate tumor from adjacent uninvolved pancreatic tissue[13,14]. In addition, even in instances where the tumor can be readily visualized with conventional MRI, therapies might have an effect on tumor function but no effect on overall tumor size. Functional targets could include the assessment of changes in water mobility (diffusion) and tumor perfusion using MRI.
The mouse is the most commonly used animal model of pancreatic cancer[15]. However, catheter-based therapies require an animal model large enough to permit catheterization of mesenteric vessels and tumor sizes large enough that can be visible using clinical MRI scanners. The VX2 model of cancer in rabbits is established in translational studies of uterine[16], liver[17,18], lung[19,20], brain[21,22], and renal neoplasms[23,24]. We have recently established this cancer model in the pancreas[25]. The purpose of this study is to test the hypotheses that (1) diffusion weighted (DW); and (2) perfusion MRI can be used to assess regional differences in tumor function in this rabbit model of pancreatic cancer. If feasible, these functional MRI parameters could potentially be used as surrogate functional endpoints when testing future novel transcatheter pancreatic therapies.
MATERIALS AND METHODS
VX2 animal model
Our Animal Care and Use Committee approved this study. Three VX2 tumors were implanted by an attending surgical oncologist and grown in the pancreata of 6 New Zealand White rabbits using the chunk implantation method[26]. At 2 wk following pancreatic VX2 implantation, MRI scans were obtained using a 1.5-T scanner (Espree; Siemens Medical Solutions, Erlangen, Germany). Rabbits were imaged in the supine position with a single-channel head coil. We obtained T2-weighted turbo spin echo images with the following parameters: 4210/86 (repetition time ms/echo time ms), 3 mm thick sections, 180 mm × 121 mm field of view, 256 × 172 matrix, 150° flip angle, 205 Hz per pixel bandwidth, and two signals acquired. Tumor growth was considered positive when tumor was identified in axial and coronal imaging planes by two independent attending interventional radiologists.
Digital subtraction angiography and catheter placement
We performed X-ray digital subtraction angiography (DSA) using a C-arm unit (Powermobil; Siemens Medical Solutions, Erlangen, Germany) 3 wk after VX2 tumor implantation. The femoral artery was accessed through a surgical cut-down and catheterized with a 22-gauge angiocatheter (Terumo Medical, Somerset, NJ, USA). A 2-F catheter (Cook, Bloomington, IN, USA) was advanced over a 0.014-inch diameter guide wire (Terumo Medical, Tokyo, Japan). We then selectively catheterized the gastroduodenal artery. We performed X-ray DSA of the celiac artery and gastroduodenal artery by using hand injections of iohexol (Omnipaque 350; Amersham Health, Princeton, NJ, USA). Once catheter placement was complete, a 2-0 silk suture in the rabbit’s groin was used to hold the catheter in place while the rabbit was transferred to the adjacent MRI suite.
MRI following catheterization
MRI after catheter placement was performed using a Siemens 1.5-T Espree clinical MR scanner. To depict tumor anatomy, we first acquired two-dimensional turbo spin-echo T2-weighted images with the above described scan parameters. DW-MRI was performed with single-shot spin-echo echo planar imaging with the following scan parameters: repetition time/echo time, 4000/93 ms; slice thickness 3 mm; bandwidth, 1185 Hz/pixel; partial Fourier factor, 6/8; nonselective fat saturation; twice refocused spin-echo diffusion weighting to reduce eddy current-induced distortion with b values of 0, 50 and 500 s/mm2. DW-MRI measures changes in the mobility of water as a means of differentiating viable and highly cellular regions from acellular or necrotic regions of tumors. The mobility of water is measured using the apparent diffusion coefficient (ADC). ADC maps, which showed water mobility measurements corresponding to separate spatial locations, were reconstructed from each series of DW images. In these ADC maps, signal intensity directly correlates with water mobility. Using T1-weighted contrast agent-enhanced images as a reference, we drew regions of interest to calculate mean tumor ADC values. Regions of interest were also drawn to compare the ADC values for the necrotic core and the viable outer ring typically present in VX2 tumors.
For tumor perfusion imaging, we used transcatheter intraarterial perfusion (TRIP)-MRI[27], an innovative first-pass perfusion technique employing direct catheter-based intraarterial injections of contrast medium. This technique can be used to detect intra-procedural changes in perfusion to targeted tumor cells and surrounding parenchyma[28]. For TRIP-MRI, we used a three-dimensional spoiled gradient echo sequence with the following parameters: repetition time/echo time, 5/1.6 ms; 15° flip angle; contiguous axial slices of 3 mm thickness; eight partitions; 200 mm × 100 mm field of view; 128 × 64 matrix; and 660 Hz/pixel bandwidth. This TRIP-MRI sequence is a real-time three-dimensional MR fluoroscopy technique[29] that rapidly and continuously images the entire tumor during transcatheter contrast medium injection. TRIP-MRI scans were obtained during hand injections of 2 mL 20% gadopentetate dimeglumine solution (Magnevist; Berlex, Wayne, NJ, USA) over 5 s via a catheter previously placed during DSA. Each contrast medium injection was immediately followed by a 4-mL saline solution flush injected over 5 s. For each TRIP-MRI scan, the entire pancreas area including tumor(s) was continuously sampled at 1.6-s intervals for 100 s.
Before and after TRIP-MRI, we obtained anatomic images with two-dimensional T1-weighted gradient-echo MRI. The T1-weighted scan parameters were as follows: repetition time/echo time, 193/6 ms; average of 2; flip angle of 80°; bandwidth of 475 Hz/pixel; slice thickness of 3; 256 × 160 matrix; and field of view of 180 mm × 113 mm.
With the baseline R10 map, a longitudinal relaxation rate R1 map time series was derived from the signal intensity ratio between the baseline image and each TRIP-MRI series with nonlinear curve fitting. Increases in R1 after injection are proportional to increases in contrast agent concentration. For each R1 map time series, we calculated the first-pass area under the curve for each voxel, thereby producing spatially resolved perfusion maps for each TRIP-MRI scan. Regions of interest were drawn to measure mean tumor area under the curve. Each TRIP-MRI area under the curve (AUC) measurement served as a semi-quantitative index of tumor perfusion. Tumor regions of interest were placed in peripheral hypervascular regions to avoid the necrotic core.
Necropsy and histopathology
Once the MRI was completed, each rabbit was sacrificed with intravenous administration of 150-200 mg/kg sodium pentobarbital (Euthasol; Delmarva Laboratories, Midlothian, VA, USA). Each rabbit tumor was harvested for pathologic confirmation of VX2 tumor growth and location in pancreatic tissue. Pancreatic tumors were inspected grossly for anatomic consistency with MR images. Tumor samples, including surrounding tissue, were then removed, embedded in paraffin, and mounted on glass slides. These 4-μm-thick slices were stained with hematoxylin and eosin. An attending surgical pathologist performed histopathologic analysis using a Zeiss Axioskop Confocal (Germany) microscope and Zeiss Plan (NEOFLUAR 2.5 ×) objective lens. Imaging was performed with a Cambridge Research and Instrumentation model N-MSI-420-FL camera and Cri Nuance Multispectral Imaging System version 1.6 software.
Statistical analysis
MR and X-ray DSA images were viewed in conjunction with one another. All MR images were analyzed on a computer workstation (Argus; Siemens Medical Solutions). We compared mean differences between necrotic tumor core and viable tumor periphery for DW-MRI (ADC value) and TRIP-MRI (AUC) using a 2-tailed paired t-test, with α = 0.05. Mean values were reported ± 1SD. All statistical tests were performed using InStat version 3.06 for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
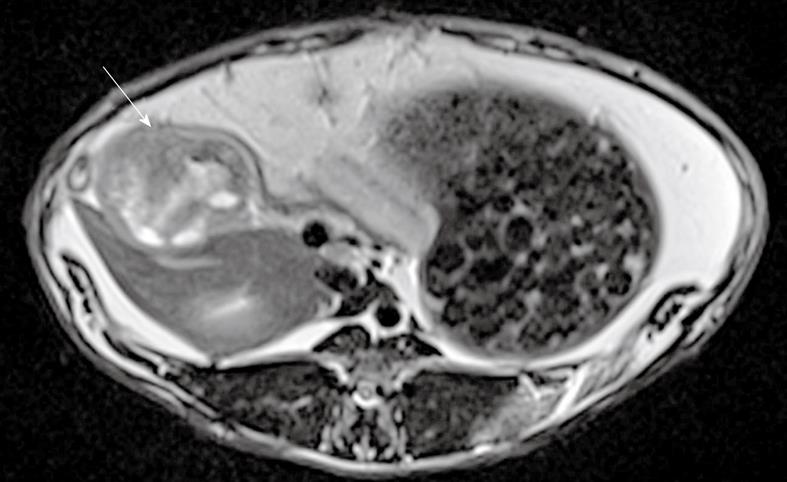
Eleven tumors were successfully grown in 6 rabbits, as confirmed by necropsy (Figure 1). Tumors in all 6 rabbits were successfully depicted using anatomic and DW-MRI. Anatomic T2-weighted images showed increased signal intensity within the tumors compared to surrounding tissues (Figure 2).
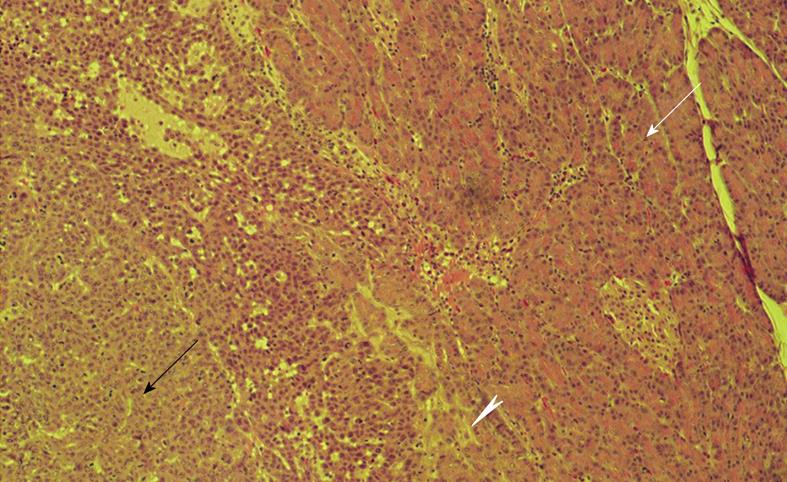
Figure 1 Histopathologic image obtained at necropsy.
Border between viable VX2 tumor cells (black arrow) and healthy pancreatic tissue (white arrow) with irritated pancreatitis-like tissue at the tumor-pancreas interface (arrowhead). Note islet of Langerhans within healthy pancreatic tissue. Hematoxylin-eosin stain; original magnification × 25.
Figure 2 T2-weighted turbo spin echo magnetic resonance imaging showing pancreatic tumor (arrow) in axial view.
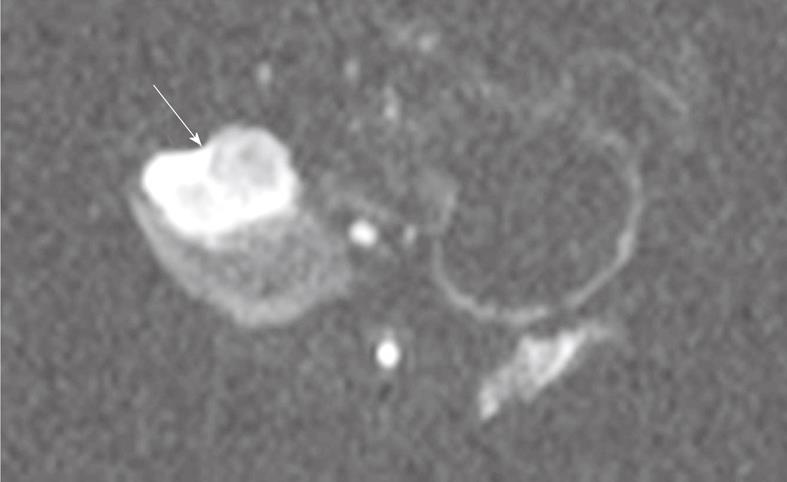
With DW-MRI, tumors showed regions of low water mobility within the viable periphery, as signified by increased signal intensity (SI) at higher b values (Figure 3). This finding suggests increased cellularity compared to the tumor core and surrounding tissues. The mean ADC value was higher in the necrotic tumor core (2.1 ± 0.3 mm2/s) than in the viable tumor periphery (1.4 ± 0.5 mm2/s) (P < 0.05).
Figure 3 Single shot spin-echo planar diffusion weighted MRI (b = 500 s/mm2) showing pancreatic tumor (arrow) in axial view.
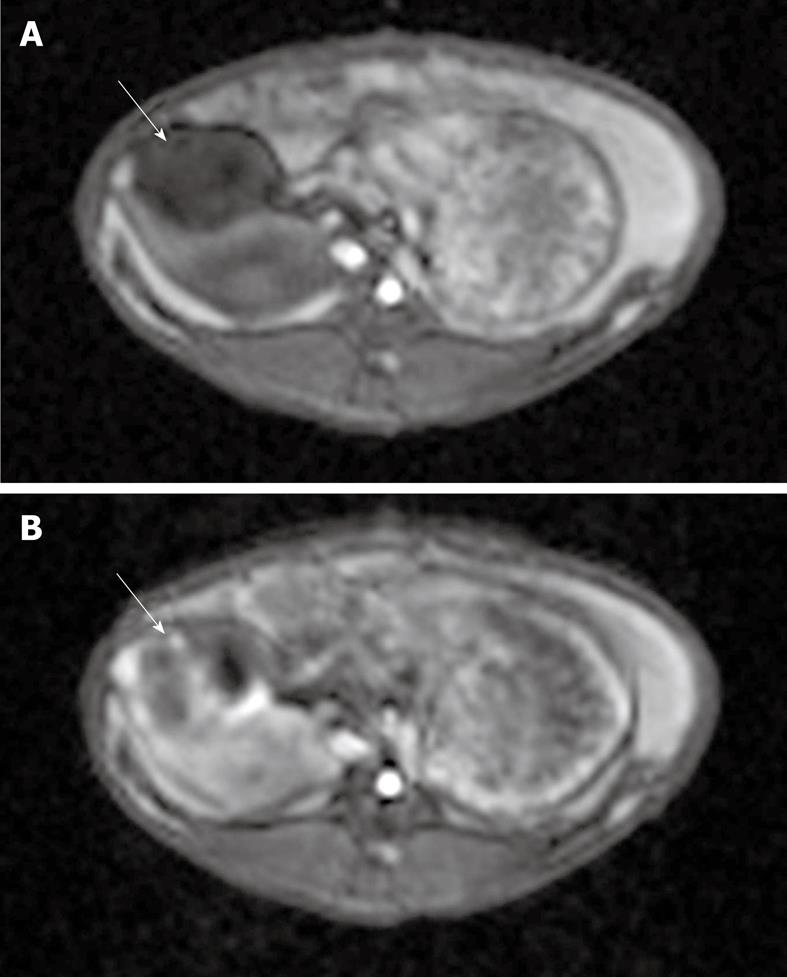
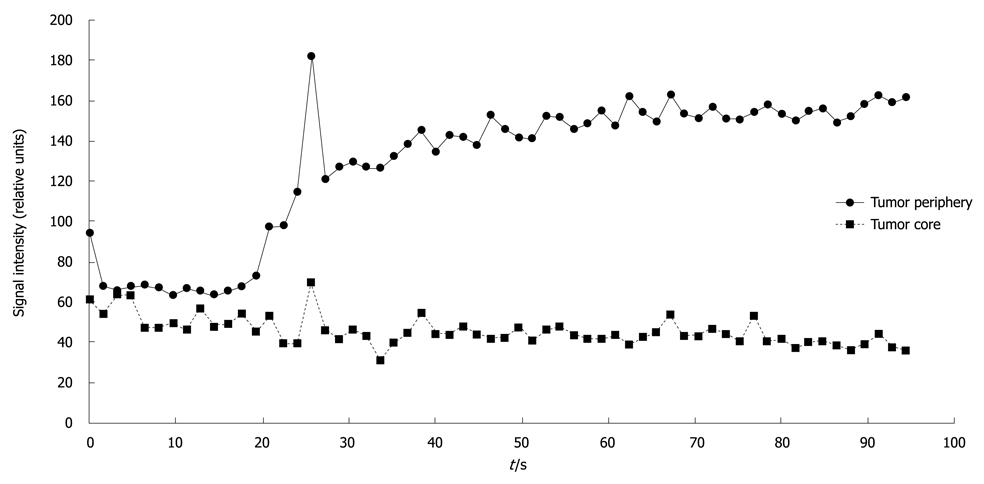
TRIP-MRI was successful in 5 of 6 rabbits. One rabbit could not be catheterized for DSA due to severe vasospasm. TRIP-MRI scans showed tumor perfusion to the peripheral viable portions of the tumor, with lack of perfusion to the central necrotic portions of the tumor (Figure 4). Mean AUC was significantly higher in the periphery (110 ± 47 relative units) than in the central core (66 ± 31 relative units) (P < 0.00002) (Figure 5).
Figure 4 T1-weighted gradient-echo magnetic resonance [taken before (A) and after (B) TRIP imaging occurred].
Each image shows a VX2 tumor (solid arrows) located in the pancreas. Note the areas of increased perfusion to the viable tumor periphery in image B.
Figure 5 Signal intensity vs time curve for one pancreatic tumor, depicting higher signal intensity in the viable tumor periphery than in the necrotic tumor core.
When comparing DW- and TRIP-MRI, regions of relatively low water mobility correlated with relatively greater tissue perfusion.
DISCUSSION
The results of this study confirm the hypothesis that functional MRI can be used to differentiate necrotic from viable tumor cells in an animal model of pancreatic cancer. Regions of necrosis were delineated from viable tumor cells by larger ADC values on DW-MRI and reduced perfusion values with TRIP-MRI. These results are relevant because the differentiation between malignant and uninvolved or adequately treated pancreatic tissue is difficult with conventional anatomic MRI.
In several preliminary clinical studies enrolling 8-26 patients, DW-MRI has been shown to be able to detect pancreatic carcinoma and differentiate neoplasm from confluent pancreatitis[30-32]. Viable tumor cells are highly cellular and have intact cell membranes. This restricts the motion of water molecules and results in a decrease in the ADC value. Conversely, cellular necrosis causes increased membrane permeability, allowing free diffusion of water molecules and a marked increase in the ADC value. DW-MRI has also been shown to be able to accurately predict the degree of tumor necrosis in hepatocellular carcinoma following transcatheter liver-directed therapy[33-36]. Our current study demonstrates that DW-MRI can be used to differentiate necrotic from viable cells in this animal model of pancreatic cancer.
TRIP-MRI uses catheter-directed intraarterial injections of gadolinium. This technique is distinct from dynamic contrast-enhanced (DCE)-MRI, which uses intravenous injections of gadolinium. TRIP-MRI has several advantages over DCE-MRI including the capacity for dramatically lower volumes of contrast agents and the ability to perform serial injections without needing to wait > 1 h for injected contrast agent to wash-out. A pre-clinical study in the VX2 rabbit liver tumor model has validated the utility of TRIP-MRI to monitor iterative changes in liver tumor perfusion during embolization[27]. This technique has been translated clinically to measure intra-procedural changes in perfusion to targeted tumors during the treatment of patients with hepatocellular carcinoma with chemoembolization[28]. Because of its ability to enhance tissue distal to the catheter tip, TRIP-MRI can also verify that tumors will be adequately targeted by localized catheter-based techniques prior to their injection. This study demonstrates that TRIP-MRI can be used to detect differences in perfusion to necrotic and viable pancreatic tumor cells.
The development of localized therapies for unresectable pancreatic cancer represents a new avenue for research. As recently demonstrated by Olive et al[37], a stromal desmoplastic reaction encapsulating pancreatic tumors greatly impedes systemic therapies and thus localized therapies that are able to greatly increase the local concentration of therapeutics may offer benefit in overcoming this fibrotic capsule. Preliminary studies have reported on transcatheter intraarterial infusion of chemotherapy for advanced pancreatic cancer[9-11], which may provide improved response to therapy[12]. The functional MRI parameters described in this animal model of pancreatic cancer could potentially be used as surrogate functional endpoints to test such transcatheter pancreatic therapies. The animal model and functional MRI techniques described in this paper could also be used to assess novel localized therapies intended to target some of the molecular mechanisms underlying pancreatic cancer, including a mutant p53 tumor suppressor gene[38] or other inhibitors of apoptosis, such as survivin[39,40].
Our study had several important limitations. First, the molecular mechanisms that govern pancreatic ductal adenocarcinoma and VX2-based tumors differ. VX2 is a squamous cell carcinoma that does not have the alterations in the multiple molecular pathways seen with true pancreatic cancer[41]. Our model, however, does mimic well the position and blood supply of a human pancreatic tumor which makes it well suited to the translational development of localized therapies. Second, the smaller diameter of rabbit pancreatic arteries precluded direct catheterization of the pancreaticoduodenal, dorsal pancreatic, great pancreatic, and caudal pancreatic arteries. These arteries are also extremely difficult to selectively catheterize in patients. Additionally, TRIP-MRI verified that perfusion to viable pancreatic tumor cells could be achieved via catheterization of the rabbit gastroduodenal artery. Third, although functional imaging in animals is not entirely novel, previous studies have focused on rodents instead of rabbits[42], and have primarily employed high field strength magnets[43]. Using a 1.5-T scanner, we believe, allows our findings to be more readily translated into future clinical studies. Finally, we did not assess the effect of therapy in this animal model. Such assessment will be the subject of future studies.
In conclusion, functional MRI parameters can be used to differentiate necrotic from viable tumor cells in an animal model of pancreatic cancer. Regions of necrosis are delineated by larger ADC values on DW-MRI and reduced perfusion values with TRIP-MRI. In a disease that so desperately needs a more effective treatment, these functional MRI parameters could potentially be used as surrogate functional endpoints when testing future novel local transcatheter pancreatic therapies.
COMMENTS
Background
Pancreatic ductal adenocarcinoma (commonly referred to as pancreatic cancer) carries the worst prognosis of any cancer, thus novel therapies must be developed to treat this disease. Because evaluation of these new therapies may be difficult, imaging modalities to assess therapeutic efficacy based on tumor necrosis are desirable.
Research frontiers
Functional magnetic resonance imaging (MRI) modalities are quickly emerging as new imaging methods with the potential to provide more information about structures in the body than traditional anatomic MRI. In this study, the authors demonstrate that the use of two specific types of functional MRI: diffusion-weighted MRI (DW-MRI) to measure tissue diffusion; and transcatheter intraarterial perfusion MRI (TRIP-MRI) to measure perfusion, could be used to differentiate tumor cells that are dead from those that are still alive.
Innovations and breakthroughs
This study is the first to combine cutting edge imaging modalities (DW-MRI and TRIP-MRI) performed using a clinical 1.5T MRI scanner with a recently created rabbit model of pancreatic cancer which allows for intraarterial catheterization.
Applications
By characterizing this model of pancreatic cancer using functional MRI, this study may allow for more effective development of much-needed novel therapeutics for the treatment of this disease.
Terminology
DW-MRI and TRIP-MRI are types of MRI called “functional” MRI because they provide information about tissue function in addition to anatomical information provided by traditional MRI. DW-MRI is used to acquire a value called the apparent diffusion coefficient which is a relative measure of water diffusion within a tissue. TRIP-MRI is used to acquire a measure of relative perfusion which represents the amount of blood flow to a tissue.
Peer review
The manuscript is well written and methodology is accurately described. The manuscript applied function MRI in pancreas tumor model and results showed that MR-DWI and TRIP-MRI can distinguish the necrotic tissue form living tissue of pancreas tumor model. This gives us more confidence on the potential of function MRI in the therapy response evaluation. Limitations of the study are considered by the authors themselves and substantiated in the discussion.
Peer reviewers: Dr. Mark De Ridder, MD, PhD, Professor, Department of Radiotherapy, UZ Brussels, Vrije Universiteit Brussels, Laarbeeklaan 101, B-1090 Brussels, Belgium; Xiao-Peng Zhang, Professor, Department of Radiology, Peking University School of Oncology, Beijing Cancer Hospital and Institute, No. 52 Haidian District, Beijing 100142, China
S- Editor Wang JL L- Editor Webster JR E- Editor Ma WH