Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2931
Revised: April 18, 2010
Accepted: April 25, 2010
Published online: June 21, 2010
AIM: To investigate the impact of postoperative antiviral treatment on tumor recurrence and survival of patients with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection-related primary hepatocellular carcinoma (HCC) after curative therapy.
METHODS: We performed a meta-analysis of randomized and non-randomized control trials from electronic search and manual search. The fixed effect model of Mantel-Haenszel method and the random effect model of Der Simonian and Laird method were used for homogeneous and heterogeneous studies, respectively. Seven HCV-related studies, three HBV-related studies and three studies on HBV or HCV-related HCC were identified.
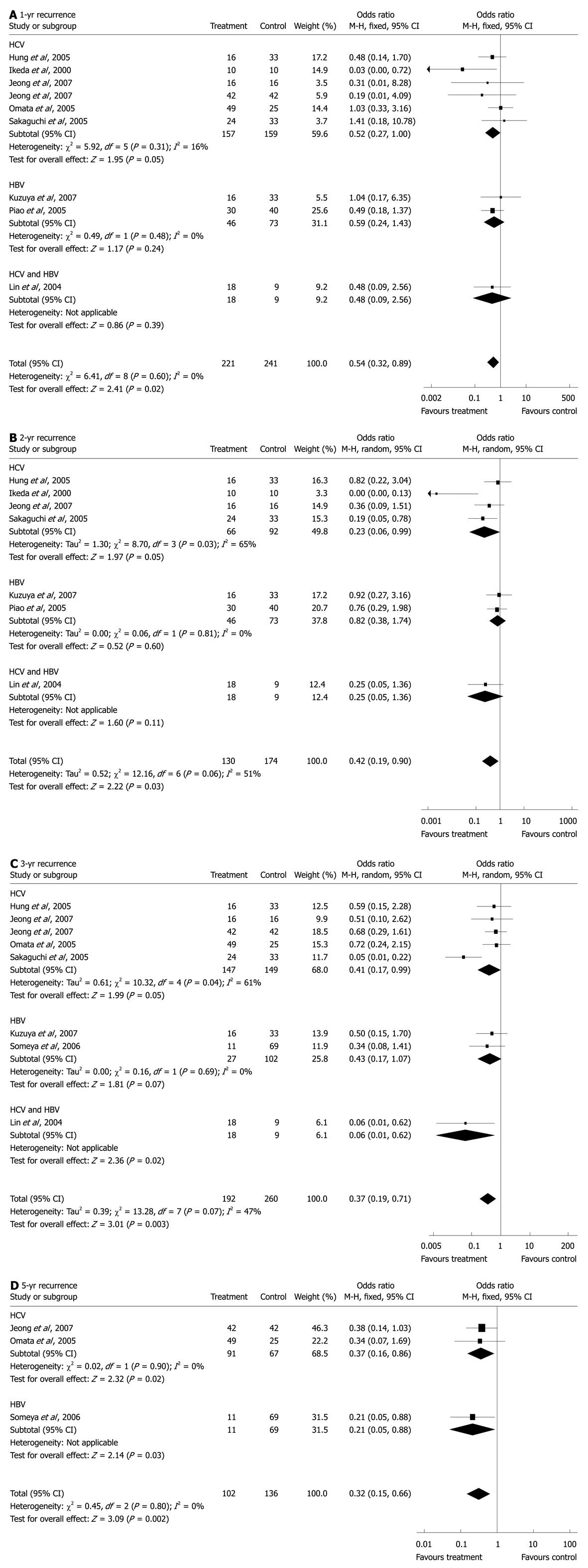
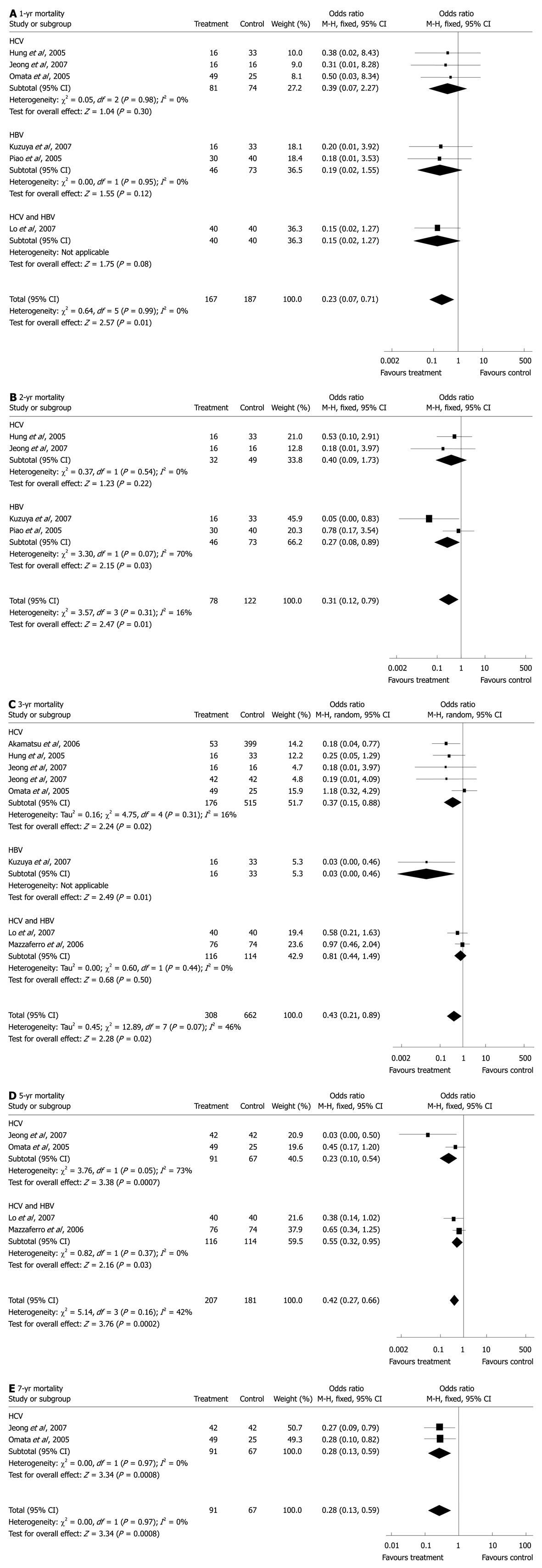
RESULTS: A total of 1224 patients were included in this analysis. The estimated odds ratios (OR) for the 1-, 2-, 3- and 5-year recurrence were 0.54 [15.4% vs 24.1%, 95% confidence interval (CI): 0.32-0.89, P = 0.02], 0.42 (36.9% vs 58.0%, 95% CI: 0.19-0.90, P = 0.03), 0.37 (47.9% vs 63.8%, 95% CI: 0.19-0.71, P = 0.003), and 0.32 (66.7% vs 74.3%, 95% CI: 0.15-0.66, P = 0.002), respectively; and the OR for the 1-, 2-, 3-, 5- and 7-year mortality were 0.23 (1.2% vs 9.1%, 95% CI: 0.07-0.71, P = 0.01), 0.31 (6.4% vs 22.1%, 95% CI: 0.12-0.79, P = 0.01), 0.43 (12.7% vs 20.8%, 95% CI: 0.21-0.89, P = 0.02), 0.42 (25.1% vs 42.0%, 95% CI: 0.27-0.66, P = 0.0002) and 0.28 (31.9% vs 52.2%, 95% CI: 0.13-0.59, P = 0.0008).
CONCLUSION: This meta-analysis indicates the postoperative antiviral therapy, interferon in particular, may serve as a favorable alternative to reduce recurrence and mortality in patients with HBV/HCV related HCCs.
- Citation: Miao RY, Zhao HT, Yang HY, Mao YL, Lu X, Zhao Y, Liu CN, Zhong SX, Sang XT, Huang JF. Postoperative adjuvant antiviral therapy for hepatitis B/C virus-related hepatocellular carcinoma: A meta-analysis. World J Gastroenterol 2010; 16(23): 2931-2942
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2931
Hepatocellular carcinoma (HCC) is common worldwide, particularly in Asia where chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is the most common etiology. Generally, hepatectomy, percutaneous ethanol injection (PEI), percutaneous microwave coagulation therapy (PMCT), radiofrequency ablation (RFA) and orthotopic liver transplantation are recognized as curative treatment options for HCC, although liver transplantation can only be offered to a small proportion of patients due to graft availability, selection criteria, and high cost. The long-term prognosis, however, remains guarded because of the high frequency of recurrence, which is also the main cause of death, in addition to concomitant hepatic decompensation.
Interferons (IFNs) are cytokines possessing a variety of biologic properties, including antiviral, immunomodulatory, antiproliferative, antiangiogenic and tumoricidal effects[1]. They are effective in suppressing the replication of HBV/HCV. Response to IFN treatment is associated with improved clinical outcomes and less cirrhosis-related complications. It is also reported that the combination of IFN and certain cytotoxic drugs has resulted in a modest response in patients with inoperable HCC[2]. Certain nucleoside/nucleotide analogues (NAs), such as lamivudine, adefovir, and entecavir, which inhibit the reverse transcriptase activity of viral DNA polymerase, have been demonstrated to be useful for HBV-infected patients, by means of not only suppressing the viral load but also reducing fibrosis in the liver[3,4]. Lamivudine treatment has been reported to consistently reduce HBV replication, and thus might improve the remnant liver function, prevent liver failure and prolong survival[5-7].
Up till now, although several randomized controlled trials (RCTs) and non-randomized controlled trials (NRCTs) have evaluated the efficacy and outcome of antiviral therapies in patients with virus-related HCC after curative treatment, no clear recommendations have been proposed yet. Usually, it is the small sample size of the RCTs that has resulted in a lack of power to detect clinically meaningful differences. In order to test the hypothesis that postoperative antiviral treatment would reduce or postpone recurrence and improve overall survival in patients with chronic HBV/HCV infection-related HCC after curative therapy, we performed a meta-analysis on the basis of published data from both RCTs and NRCTs in the literature.
Two investigators independently assessed the generated papers for relevance. In case of discrepancies found between the two investigators, a third investigator would make the definitive decision for study eligibility and data extraction.
Eligible patients were those who had undergone a curative treatment for primary HCC associated with HBV or HCV. Curative treatment was defined as complete tumor eradication, with no residual tumor visible by computed tomography, or resection of all evident tumor tissues, which included percutaneous tumor ablation [PTA, e.g. percutaneous ethanol injection (PEI), percutaneous acetic acid injection (PAI), radiofrequency ablation (RFA), microwave coagulation therapy (MCT), etc.], surgical treatment (e.g. hepatectomy, ablation during laparotomy, etc.), or their combinations. Antiviral therapy included regularly administered IFN (IFNα, IFNβ, and recombinant IFN) for HBV or HCV, nucleoside/nucleotide analogue such as lamivudine for HBV, and other anti-hepatitis virus medications. Primary outcomes were cumulative survival rate, mortality rate, and cumulative recurrence rate at 1, 2, 3, 5 or 7 years.
Studies with the following situations were excluded: (1) those evaluating antiviral therapies before primary HCC developed or after non-curative treatment or liver transplantation; (2) those evaluating an antiviral therapy in combination with chemotherapy; (3) those with no information on cumulative probability of survival, mortality or recurrence; and (4) those evaluating patients with other subtypes of liver malignancy, e.g. cholangiocellular carcinomas or liver metastases.
We performed an electronic search of Medline, Embase, Cancerlit, and the Cochrane Library covering a period from 1980 to March 2009 to identify full publications or abstracts of literatures using the terms “hepatocellular carcinoma “, “hepatitis “, “recurrence”, “survival”, “antiviral agents”, “interferon”, “lamivudine”, “adefovir”, “entecavir” and “ribavirin”.
In addition, manual search for the abstracts published in major international conferences were also conducted and general reviews and the reference lists from the identified publications were cross-checked to obtain other relevant publications.
We considered articles presenting data from RCTs and NRCTs originally published in English. When the data of a study were included in more than one publication, we excluded studies with duplicated publications on the same group of patients, including those reporting the early stages of study findings with incomplete sample size; only the most recent and complete studies were included in the meta-analysis.
The intention-to-treat method was used. The measure of interest was the OR for cohort studies and the corresponding 95% CI. When ORs were not available in the published article, they were computed from the exposure distributions. For each meta-analysis, χ2 test was used to assess the statistical heterogeneity among studies and results were defined as heterogeneous when P values were less than 0.10. A fixed effect model, the Mantel-Haenszel method, was used for homogeneous studies, and when significant heterogeneity existed, a random effect model, the Der Simonian and Laird method, would be applied (RevMan, version 5; The Cochrane Collaboration, Copenhagen, Denmark). A significance level of 5% was taken as the α risk. Only meta-analyses of antiviral therapies that included two or more studies (RCTs or NRCTs) were performed.
To compute summary OR for different viral etiology, we calculated the study-specific estimates separately for HBV and HCV. Forest plots were given. Publication bias was evaluated using funnel plots and Egger’s test.
One hundred and twenty-four abstracts were found through the search in literature; among them, 17 studies with 22 publications fulfilled the inclusion criteria. Three additional publications from the bibliographies of retrieved studies were also identified. Among them, ten publications[8-17] reported five studies at different time, therefore, only the five most recent articles[8,10,12,16,17] were used for the analysis; five studies[12,17-20] did not report the number of death or recurrence at 1, 2, 3, 5 or 7 years or provide data separately for the treatment and control groups and were thereby excluded; one study[21] enrolled not only patients who received postoperative antiviral therapy but also those who were preoperatively treated, another study[10] included patients who received palliative treatment before antiviral therapy, both of which did not provide separate data on survival or recurrence for different treatment approaches and were therefore excluded. The final analysis included seven studies of HCV-associated HCC with IFN therapy[8,22-27], three studies of HBV-associated HCC, two with lamivudine[28,29] and one with IFN therapy[30], and three studies of HBV/HCV-associated HCC with IFN therapy[16,31,32] (Table 1).
| RCT/NRCT | Medium follow-up time (treatment, control) (mo) | HBV/HCV | Curative intervention | Regimen | Treatment | Control | Cumulative survival rate at year | Cumulative recurrence rate at year | SVR/non-SVR | Ref. |
| NRCT | 32, 31 | HCV | Hepatectomy, RFA, PEI, PMCT, ablation under LC | IFNα 6 MIU Qd × 2 w + tiw × 22 w | 42 | 42 | 3, 5, 7 | 1, 3, 5, 7 | 29/13 | [22] |
| NRCT | 37, 45 | HCV | Hepatectomy, RFA, PEI, PMCT, ablation under LC | IFNα 3 MIU tiw × 48 w | 16 | 16 | 1, 2, 3, 4 | 1, 2, 3 | 2/14 | [23] |
| NRCT | NA | HCV | Hepatectomy, RFA, PEI, PMCT | IFNα 3-6 MIU tiw × 24-48 w | 53 | 399 | 3, 6, 9 | NA | 17/36 | [24] |
| NRCT | < 36 | HCV | PEI, RFA, PMCT | IFNα-2b 3-5 MIU biw + ribavirin 1000-1200 mg Qd × 24-48 w | 16 | 33 | 1, 2, 3 | 1, 2, 3 | 10/6 | [25] |
| NRCT | 28, 23 | HCV | RFA | IFNα-2b 3 MIU biw × as long as possible | 24 | 33 | NA | 1, 2, 3 | 1/23 | [26] |
| RCT | 85 ± 16 | HCV | PEI | IFN 6 MIU tiw × 48 w | 49 | 25 | 1, 3, 5, 7 | 1, 3, 5 | 14/35 | [8] |
| RCT | 25.0 (2.0-34.6) | HCV | Hepatectomy, PEI | IFNβ 6 MIU biw × 36 m | 10 | 10 | NA | 1, 2 | 0/10 | [27] |
| NRCT | 38.0 (9.2-78.2), | HBV | Hepatectomy, RFA | lamivudine 100 mg Qd × as long as possible | 16 | 33 | 1, 2, 3 | 1, 2, 3 | NA | [28] |
| 32.6 (0.5-75.7) | ||||||||||
| NRCT | 53 | HBV | Hepatectomy, ablation | IFNα biw/tiw ×≥ 24 w | 11 | 69 | NA | 3, 5, 10 | NA | [30] |
| NRCT | 31 | HBV | Hepatectomy, ablation | lamivudine 100 mg Qd | 30 | 40 | 1, 2 | 1, 2 | NA | [29] |
| RCT | 30 | HBV 77 | Hepatectomy | IFNα-2b 10 MIU/m2 tiw × 16 w | 40 | 40 | 1, 3, 5 | NA | NA | [16] |
| HCV 2 | ||||||||||
| HBV + HCV 1 | ||||||||||
| RCT | 45 | HCV 80 | Hepatectomy | IFNα 3 MIU tiw × 48 w | 76 | 74 | 3, 5 | NA | 2/74 | [31] |
| HBV + HCV 70 | ||||||||||
| RCT | 27 (4-53) | HBV 16 | PAI | IFNα 3 MIU tiw × 24 w/ IFNα 3 MIU Qd × 10 d/m × 6 m + 10 d/3 m × 18 m | 18 | 9 | NA | 1, 2, 3, 4 | NA | [32] |
| HCV 13 | ||||||||||
| HBV + HCV 11 |
Among the seven HCV-related studies using IFN, two were RCTs[8,27], the rest five were cohort studies (NRCTs)[22-26]. A total of 768 patients were enrolled, and 210 received IFN treatment. The medium duration of follow-up ranged from 23 to 85 mo. The three HBV-related studies are all NRCTs, with a total of 199 patients included; 11 patients received IFN treatment and 46 received lamivudine. The medium duration of follow-up ranged from 31 to 53 mo. All of the three HBV/HCV-related studies are RCTs with a total of 260 patients, including 93 HBV-infected, 95 HCV-infected, and 72 HBV/HCV-co-infected HCCs; three of them were excluded from the final analysis as they had already experienced one recurrence. Among the rest 257 patients, 134 received IFN therapy. The medium duration of follow-up ranged from 27 to 45 mo.
For 1-year recurrence, pooled data from nine studies[8,22,23,25-29,32] showed significantly more HCC recurrence cases in the control (24.1%) than in the antiviral therapy (15.4%) groups (OR: 0.54, 95% CI: 0.32-0.89, P = 0.02, Figure 1A). No statistical heterogeneity was found (I2 = 0%, P = 0.60). Subgroup analysis of the six HCV-related studies[8,22,25-27,32] revealed significantly fewer HCC recurrences in IFN groups (12.1%) than in the control groups (20.8%) (OR: 0.52, 95% CI: 0.27-1.00, P = 0.05) with no statistical heterogeneity (I2 = 16%, P = 0.31). In the two HBV-associated studies[28,29], which were also the only two lamivudine-treated studies, no statistically significant difference was found between lamivudine (21.7%) and control groups (28.8%) (OR: 0.59, 95% CI: 0.24-1.43, P = 0.24). Subgroup analysis of the seven IFN studies, which combined outcomes of the six HCV-related and 1 HBV/HCV-related HCC studies[31], revealed that compared with control (22.0%), IFN groups had a lower HCC recurrence rate (13.7%) (OR: 0.52, 95% CI: 0.28-0.95, P = 0.03). No statistical heterogeneity was found (I2 = 0%, P = 0.43).
For 2-year recurrence, as statistical heterogeneity (I2 = 51%, P = 0.06) was found, the randomized model was applied to the data from seven studies[23,25-29,32], revealing significantly fewer HCC recurrences in the treatment (36.9%) than the control (58.0%) groups (OR: 0.42, 95% CI: 0.19-0.90, P = 0.03, Figure 1B). Subgroup analysis of the four HCV-related studies[23,25-27] showed significantly more HCC recurrences in the control (63.0%) than in IFN groups (30.3%) (OR: 0.23, 95% CI: 0.06-0.99, P = 0.05) with statistical heterogeneity (I2 = 65%, P = 0.03). In the two HBV studies[28,29], no significant difference was found between lamivudine (47.8%) and control groups (50.7%) (OR: 0.82, 95% CI: 0.38-1.74, P = 0.60). Subgroup analysis of the five IFN studies, which combined outcomes of the four HCV-related and 1 HBV/HCV-related HCC studies[32], revealed that there was significantly fewer HCC recurrences in IFN groups (31.0%) than in control (63.4%) (OR: 0.26, 95% CI: 0.09-0.78, P = 0.02). There existed statistical heterogeneity (I2 = 54%, P = 0.07).
For 3-year recurrence, the randomized model of eight studies[8,22,23,25,26,28,30,32] revealed statistical heterogeneity (I2 = 47%, P = 0.07) and significantly fewer recurrent cases in the treatment (47.9%) than in the control (63.8%) groups (OR: 0.37, 95% CI: 0.19-0.71, P = 0.003, Figure 1C). Subgroup analysis of the five HCV-related studies[8,22,23,25,26] showed significantly fewer recurrences in IFN (52.4%) than in the control groups (69.8%) (OR: 0.41, 95% CI: 0.17-0.99, P = 0.05). Statistical heterogeneity was found among these studies (I2 = 61%, P = 0.04). In the two HBV studies[28,30], there was a trend of fewer recurrences in the treatment groups (33.3%) than in the controls (52.9%) (OR: 0.43, 95% CI: 0.17-1.07, P = 0.07). By combining data from the five HCV-, 1[30] HBV-, and 1[32] HBV/HCV-related studies, subgroup analysis of the seven IFN studies revealed that the recurrence rate in IFN groups (48.9%) was significantly lower than in the control groups (65.2%) (OR: 0.34, 95% CI: 0.16-0.73, P = 0.006). There existed statistical heterogeneity (I2 = 55%, P = 0.04).
For 5-year recurrence, three studies[8,22,30] showed significantly fewer HCC recurrences in IFN (66.7%) than in the control (74.3%) groups (OR: 0.32, 95% CI: 0.15-0.66, P = 0.002) with no statistical heterogeneity (I2 = 0%, P = 0.80, Figure 1D). Subgroup analysis of the two HCV-related HCC studies[8,22] revealed significantly fewer recurrences in IFN groups (71.4%) compared with the control groups (85.1%) (OR: 0.37, 95% CI: 0.16-0.86, P = 0.02). No statistical heterogeneity was found (I2 = 0%, P = 0.90).
For 1-year survival, combined data from six studies[8,16,23,25,28,29] showed significantly more deaths in the control (9.1%) than in the treatment (1.2%) groups (OR: 0.23, 95% CI: 0.07-0.71, P = 0.01), with no statistical heterogeneity (I2 = 0%, P = 0.99, Figure 2A). Subgroup analysis of the three HCV studies[8,23,25] failed to show a significant difference in mortality between IFN (1.2%) and control groups (5.4%) (OR: 0.39, 95% CI: 0.07-2.27, P = 0.30). No statistical heterogeneity was found (I2 = 0%, P = 0.95). In the two HBV studies[28,29], there was no significant difference between lamivudine (0%) and control groups (9.6%) (OR: 0.19, 95% CI: 0.02-1.55, P = 0.12) and no statistical heterogeneity (I2 = 0%, P = 0.98). Subgroup analysis of the four IFN studies which included three HCV-related and 1 HBV/HCV-related studies[16], revealed a significantly higher mortality rate in the control (8.8%) than in IFN groups (1.7%) (OR: 0.25, 95% CI: 0.07-0.96, P = 0.04) with no statistical heterogeneity (I2 = 0%, P = 0.91).
For 2-year survival, pooled data from four studies[23,25,28,29] showed a significantly lower mortality rate in the treatment groups (6.4%) compared with the control groups (22.1%) (OR: 0.31, 95% CI: 0.12-0.79, P = 0.01, Figure 2B), with no statistical heterogeneity (I2 = 16%, P = 0.31). In subgroup analysis, the two studies of HCV-related HCC[23,25] failed to show a significant difference between IFN (6.2%) and control (18.4%) groups (OR: 0.40, 95% CI: 0.09-1.73, P = 0.22), with no statistical heterogeneity (I2 = 0%, P = 0.54). Among the two HBV studies[28,29], the mortality in lamivudine groups (6.5%) was lower than in the control groups (24.7%) (OR: 0.27, 95% CI: 0.08-0.89, P = 0.03).
For 3-year survival, the randomized model, including eight studies[8,16,22-25,28,31], revealed statistical heterogeneity (I2 = 46%, P = 0.07) and a significantly lower mortality rate in the treatment (12.7%) than in the control (20.8%) groups (OR: 0.43, 95% CI: 0.21-0.89, P = 0.02, Figure 2C). Subgroup analysis of the 5 HCV-related studies[8,22-25] showed significantly fewer deaths in IFN groups (7.4%) compared with the control groups (17.5%) (OR: 0.37, 95% CI: 0.15-0.88, P = 0.02), with no statistical heterogeneity (I2 = 16%, P = 0.31). Combined outcomes of the seven IFN studies which included data from the five HCV-related and two HBV/HCV-related studies[16,31], revealed that there were significantly fewer deaths in IFN (13.4%) than in the control (19.1%) groups (OR: 0.54, 95% CI: 0.34-0.84, P = 0.006). No statistical heterogeneity was found (I2 = 22%, P = 0.26).
For 5-year survival, four studies[8,16,22,31] showed significantly fewer deaths in IFN (25.1%) than in the control (42.0%) groups (OR: 0.42, 95% CI: 0.27-0.66, P = 0.0002), with no statistical heterogeneity (I2 = 42%, P = 0.16, Figure 2D). Subgroup analysis of the two HCV-related studies[8,22], using the randomized model, failed to reveal a significant difference between IFN (71.4%) and control (85.1%) (OR: 0.15, 95% CI: 0.01-2.67, P = 0.20) groups with a statistical heterogeneity (I2 = 73%, P = 0.05).
For 7-year survival, combined data from two HCV-related studies[8,22] including 158 patients revealed a significantly lower mortality rate in IFN (31.9%) than in the control (52.2%) groups (OR: 0.28, 95% CI: 0.13-0.59, P = 0.0008), with no statistical heterogeneity (I2 = 0%, P = 0.97, Figure 2E).
The prevention of recurrence constitutes one of the most important challenges to improve the prognosis of patients with HCC after curative therapies. Currently, there is insufficient evidence to reach a consensus on appropriate adjuvant therapies for secondary prevention. In the present meta-analysis, postoperative antiviral therapy as a whole has been shown to reduce HCC recurrence at year 1, 2, 3, and 5. Subgroup analyses have further demonstrated that this effect of reduction remained significant in IFN-treated HCCs as well as pure HCV-associated HCCs.
Generally, recurrences can be differentiated into early or late ones. Early HCC recurrences were assumed to be derived from residues of original HCC after a potentially curative therapy and usually appear within the first 2 years. Late recurrences, which typically occur more than 2 years after the elimination of primary tumors, develop on the basis of underlying liver diseases, resulting from new carcinogenesis[14]. Persistent active hepatitis is common in advanced stages of chronic HBV or HCV infection, including HCC. The prevalence of multicentric carcinogenesis increases during progression to active hepatitis and hepatic fibrosis[33]. Hepatocyte necrosis and inflammation may be closely involved in multifocal recurrence in chronic HBV/HCV-infected patients who had complete resection or ablation of HCC tumors. Hence, it is hypothesized that antiviral therapies would help prevent HCC recurrence by cleaning the carcinogenic soil and eliminating possibilities of novel tumorigenesis through their viral suppression and anti-inflammation action.
There have been studies indicating that IFN therapy for chronic hepatitis C has improved the histological features of hepatic inflammation and fibrosis, induced remission of active hepatitis, and thereby hindered HCC development[34,35]. Similarly, IFN may also suppress multifocal recurrence arising from multicentric carcinogenesis after resection or ablation in the same way. Nonetheless, the results of this meta-analysis have shown that IFN not only reduced late recurrent HCCs but also early ones, especially in patients with pure HCV infection, which indicates that more than one mechanism may actually contribute to the benefit of adjuvant therapy with IFN in lowering recurrent HCCs. Beside immunostimulant, antiviral, antiangiogenic, and anticarcinogenic properties, IFN has been found to have in vitro and in vivo antiproliferative and tumoricidal effects against HCC as well[1,16,36]. Therefore, postoperative IFN therapy might be of advantage to recurrence prevention due to a combination of chemopreventive effects on multicentric carcinogenesis and antiproliferative and tumoricidal effects on residual HCC cells.
Compared with recurrence, although the overall results of antiviral therapies or IFN alone at various years were almost positive in improving survival, subgroup analyses of HCV-related HCCs produced mixed results. For survival at year 1, 2, and 5, current data are insufficient to give out significant results; for survival at year 7, given the condition that only two studies with a total of 158 patients were available for analysis, the significant difference between IFN and control groups should be taken with a grain of salt; only the benefit for 3-year survival was based on a relatively larger sample size, but it also needs further confirmation by even larger studies.
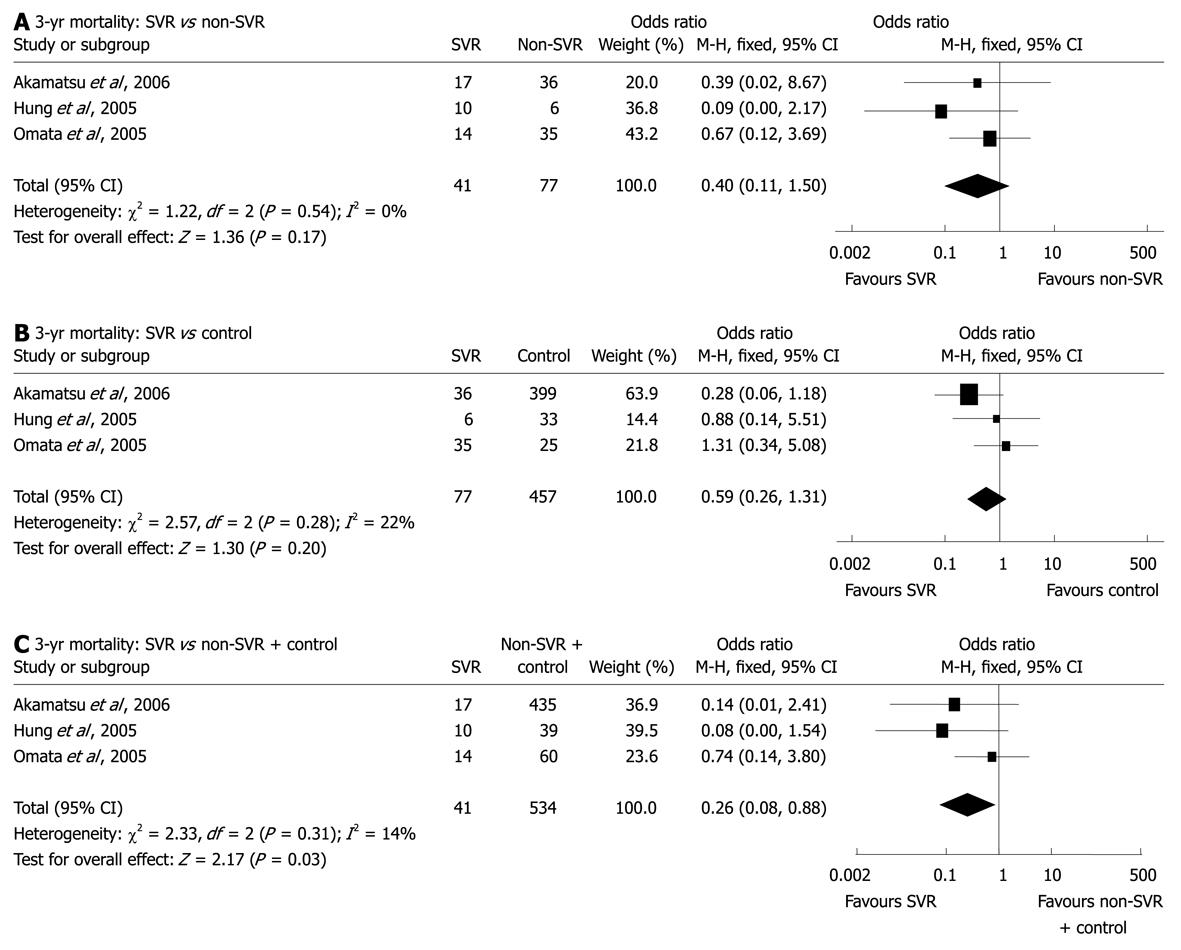
Sustained virological response (SVR) is defined on the basis of the absence of serum HCV RNA at least 6 mo after completion of IFN therapy. All seven studies of pure HCV-related HCCs presented information on the response to IFN therapy (Table 1); however, the effects varied greatly as different IFN formulas with different doses, intervals, and durations were applied in different patient populations. The comparisons between patients with SVR and those without SVR or IFN treatment were performed in four studies; all showed a certain superiority of SVR, either in survival or the first/second HCC recurrence[8,22,24,25]. As among all nine endpoints, i.e. 1, 2, 3 and 5-year recurrence and 1, 2, 3, 5 and 7-year survival, only the data on the 3-year survival of SVR and non-SVR patients were available in two or more studies, a subgroup analysis was able to be performed. Although pooled data from three studies[8,24,25] did not reveal significant difference in the 3-year mortality between SVR patients (2.4%) and non-SVR ones (14.3%) (OR: 0.40, 95% CI: 0.11-1.50, P = 0.17, Figure 3A), or between non-SVR patients and control subjects (18.8%) (OR: 0.59, 95% CI: 0.26-1.31, P = 0.20, Figure 3B), it was shown that the mortality rate in SVR patients was significantly lower than that in non-SVR patients plus control ones (18.2%) (OR: 0.26, 95% CI: 0.08-0.88, P = 0.03, Figure 3C). Therefore, for HCV-related HCC, it may be worth considering whether it is reasonable to take SVR as a target of antiviral therapies in order to improve prognosis. If so, intensified or combined treatment, such as the combination of PEG-IFN and ribavirin, would be indicated as long as the adverse effects are well balanced and patients are tolerant to such treatment.
Regarding HBV-related HCC, analysis on the two lamivudine studies that provided data on recurrence and mortality at year 1, 2, and 3 failed to demonstrate significant differences but only a trend of superiority in late recurrence, although it showed significant benefit in the 2- and 3-year survival. Besides problems with the limited number of studies and small sample sizes, it may be partially explained by the fact that the emergence of YMDD mutations (e.g. rtM204V/I) reduces the benefit of lamivudine therapy, which is relatively frequent in clinical trials and generally associated with worse clinical outcomes, such as hepatitis flares, hepatic decompensation, fatal liver failure and death[28,37]. In vitro studies have shown that the rtM204V/I mutations decreased susceptibility of the viral polymerase to lamivudine and reduced functional capacity of the viral polymerase[37]. As a result, serum HBV DNA remains at or relapses to a high level, which leaves the liver prone to HCC recurrence, as shown by one report that, during a median follow-up of 35 mo, patients in the non-viraemia (< 100 000 copies/mL) group had a lower 5-year cumulative recurrence rate than those in the viraemia group (54.7% vs 72.9%, P = 0.043), and sustained viraemia independently increased recurrence after surgery in multivariate analysis (P = 0.041)[38]. Therefore, antiviral therapy may be indicated in patients with detectable serum HBV DNA since lower viral load is important to reduce HCC recurrence; meanwhile, periodic assessment of serum HBV DNA using a sensitive assay should be applied to monitor the antiviral resistance. It is hypothesized that potent oral NAs with higher genetic barrier to resistance, such as entecavir, could overcome this disadvantage, but it needs further studies to confirm.
In conclusion, this meta-analysis demonstrates the benefit of antiviral treatment, IFN in particular, in reducing short-term and long-term HCC recurrence and mortality after curative therapies such as hepatic resection or ablation for primary HCC. The effects seem particularly striking for patients with pure HCV infection, and patients who have achieved SVR after IFN therapy may expect fewer recurrences and longer survival. These data are highly encouraging and should call for further evaluation and development of new regimens. For HBV-related HCCs, future randomized control trials with larger sample size, longer follow-up, and regular HBV DNA monitoring will be needed to substantiate the beneficial effects of antiviral therapies, such as IFN and entecavir, on the prognosis of HCC. Finally, based on these results, we hypothesize that if compared with other adjuvant therapies, such as systematic chemotherapy, molecular targeted therapy, some locoregional therapies, antiviral therapy may serve as a cost-effective and favorable alternative to improve the prognosis of patients with HBV/HCV-related HCCs because the underlying carcinogenic background can be reformed, which also warrants in-depth investigations.
Hepatocellular carcinoma (HCC) is common worldwide, particularly in Asia where chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is the most common etiology. The prevention of recurrence remains as one of the most important challenges to improve the prognosis after curative therapies. Up till now, although several clinical trials have evaluated adjuvant antiviral therapies with interferon or lamivudine, no clear recommendations have been proposed.
As a quantitative technique for therapeutic evaluation, meta-analysis may be used when controversy exists, particularly when trials have insufficient statistical power. In the present study, this mathematical tool is utilized to verify the hypothesis that antiviral therapy may serve as an alternative strategy to improve the prognosis of patients with HBV/HCV-related HCCs.
Although antiviral therapies using interferons or lamivudine have shown promising results in chronic hepatitis B or hepatitis C, their effects on patients with HCC after curative therapy remain controversial, mainly due to the small sample size and short follow-up time. By pooling data from available literature, the present study for the first time demonstrates the benefit of antiviral therapies, interferon in particular, in reducing both short-term and long-term HCC recurrence as well as mortality after curative therapies. The effects seem more remarkable for patients with HCV-related HCC. For HBV-related HCCs, this study warrants future randomized control trials with larger sample size and longer follow-up. This study intends to remind surgeons that basic treatment that targets underlying carcinogenic background might be more efficient than the so-called novel techniques which are attractive but still questionable.
The results of study indicate that postoperative antiviral therapy, interferon in particular, may serve as a favorable alternative to reduce recurrence and mortality in patients with HBV/HCV-related HCCs.
This is a meta-analysis of postoperative antiviral treatment on tumor recurrence and survival in patients with chronic HBV or HCV infection-related HCC after curative therapy, which has been an interesting issue but still has no solid evidence. The authors conclude that postoperative antiviral treatment may reduce short-term and long-term HCC recurrence and mortality after curative therapy. Antiviral therapy may serve as a favorable alternative to improve the prognosis of patients with HBV/HCV-related HCCs. The authors have nicely analyzed the recent publications. Overall, these results are well laid out and logical.
| 1. | Kardinal CG, Moertel CG, Wieand HS, Schutt AJ, O’Connell MJ, Wright K, Wiesenfeld M, Tschetter LK, Krook JE. Combined doxorubicin and alpha-interferon therapy of advanced hepatocellular carcinoma. Cancer. 1993;71:2187-2190. |
| 2. | Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, Curley SA, Ellis LM. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol. 2003;21:421-427. |
| 3. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. |
| 4. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. |
| 5. | Papatheodoridis GV, Dimou E, Dimakopoulos K, Manolakopoulos S, Rapti I, Kitis G, Tzourmakliotis D, Manesis E, Hadziyannis SJ. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005;42:121-129. |
| 6. | Bae SH, Yoon SK, Choi JY, Jang JW, Cho SH, Yang JM, Han NI, Ahn BM, Chung KW, Sun HS. Timing of lamivudine administration according to Child class in patients with decompensated cirrhosis. J Gastroenterol Hepatol. 2005;20:1527-1532. |
| 7. | Tseng PL, Lu SN, Tung HD, Wang JH, Changchien CS, Lee CM. Determinants of early mortality and benefits of lamivudine therapy in patients with hepatitis B virus-related decompensated liver cirrhosis. J Viral Hepat. 2005;12:386-392. |
| 8. | Omata M, Yoshida H, Shiratori Y. Prevention of hepatocellular carcinoma and its recurrence in chronic hepatitis C patients by interferon therapy. Clin Gastroenterol Hepatol. 2005;3:S141-S143. |
| 9. | Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, Koike Y, Yoshida H, Omata M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299-306. |
| 10. | Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Wenming C, Zhengfeng Y, Yuxiang Z, Peijun W. Antiviral therapy using lamivudine and thymosin alpha1 for hepatocellular carcinoma coexisting with chronic hepatitis B infection. Hepatogastroenterology. 2006;53:249-252. |
| 11. | Cheng SQ, Wu MC, Chen H, Shen F, Yang JH, Cong WM, Zhao YX, Wang PJ. [Anti-viral therapy using lamivudine and thymosin is helpful to prevent recurrence in hepatocellular carcinoma with coexisting active hepatitis B]. Zhonghua Zhongliu Zazhi. 2005;27:114-116. |
| 12. | Nishiguchi S, Tamori A, Kubo S. Effect of long-term postoperative interferon therapy on intrahepatic recurrence and survival rate after resection of hepatitis C virus-related hepatocellular carcinoma. Intervirology. 2005;48:71-75. |
| 13. | Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Kinoshita H. Randomized clinical trial of long-term outcome after resection of hepatitis C virus-related hepatocellular carcinoma by postoperative interferon therapy. Br J Surg. 2002;89:418-422. |
| 14. | Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Yamazaki O, Shiomi S, Tamori A, Oka H, Igawa S. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med. 2001;134:963-967. |
| 15. | Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437-1447. |
| 16. | Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, Wong J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831-842. |
| 17. | Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458-465. |
| 18. | Takahashi S, Kudo M, Chung H, Inoue T, Nagashima M, Kitai S, Tatsumi C, Minami Y, Ueshima K, Fukunaga T. Outcomes of nontransplant potentially curative therapy for early-stage hepatocellular carcinoma in Child-Pugh stage A cirrhosis is comparable with liver transplantation. Dig Dis. 2007;25:303-309. |
| 19. | Miyaguchi S, Watanabe T, Takahashi H, Nakamura M, Saito H, Ishii H. Interferon therapy for hepatocellular carcinoma patients with low HCV-RNA levels. Hepatogastroenterology. 2002;49:724-729. |
| 20. | Suou T, Mitsuda A, Koda M, Matsuda H, Maruyama S, Tanaka H, Kishimoto Y, Kohno M, Hirooka Y, Kawasaki H. Interferon alpha inhibits intrahepatic recurrence in hepatocellular carcinoma with chronic hepatitis C: a pilot study. Hepatol Res. 2001;20:301-311. |
| 21. | Kubo S, Takemura S, Uenishi T, Yamamoto T, Ohba K, Ogawa M, Hai S, Ichikawa T, Kodai S, Shinkawa H. Second hepatic resection for recurrent hepatocellular carcinoma in patients with chronic hepatitis C. World J Surg. 2008;32:632-638. |
| 22. | Jeong SC, Aikata H, Katamura Y, Azakami T, Kawaoka T, Saneto H, Uka K, Mori N, Takaki S, Kodama H. Effects of a 24-week course of interferon-alpha therapy after curative treatment of hepatitis C virus-associated hepatocellular carcinoma. World J Gastroenterol. 2007;13:5343-5350. |
| 23. | Jeong S, Aikata H, Katamura Y, Azakami T, Kawaoka T, Saneto H, Uka K, Mori N, Takaki S, Kodama H. Low-dose intermittent interferon-alpha therapy for HCV-related liver cirrhosis after curative treatment of hepatocellular carcinoma. World J Gastroenterol. 2007;13:5188-5195. |
| 24. | Akamatsu M, Yoshida H, Shiina S, Teratani T, Obi S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Sustained viral response prolonged survival of patients with C-viral hepatocellular carcinoma. Liver Int. 2006;26:536-542. |
| 25. | Hung CH, Lee CM, Wang JH, Tung HD, Chen CH, Lu SN. Antiviral therapy after non-surgical tumor ablation in patients with hepatocellular carcinoma associated with hepatitis C virus. J Gastroenterol Hepatol. 2005;20:1553-1559. |
| 26. | Sakaguchi Y, Kudo M, Fukunaga T, Minami Y, Chung H, Kawasaki T. Low-dose, long-term, intermittent interferon-alpha-2b therapy after radical treatment by radiofrequency ablation delays clinical recurrence in patients with hepatitis C virus-related hepatocellular carcinoma. Intervirology. 2005;48:64-70. |
| 27. | Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N, Kumada H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228-232. |
| 28. | Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929-1935. |
| 29. | Piao CY, Fujioka S, Iwasaki Y, Fujio K, Kaneyoshi T, Araki Y, Hashimoto K, Senoh T, Terada R, Nishida T. Lamivudine treatment in patients with HBV-related hepatocellular carcinoma--using an untreated, matched control cohort. Acta Med Okayama. 2005;59:217-224. |
| 30. | Someya T, Ikeda K, Saitoh S, Kobayashi M, Hosaka T, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Arase Y. Interferon lowers tumor recurrence rate after surgical resection or ablation of hepatocellular carcinoma: a pilot study of patients with hepatitis B virus-related cirrhosis. J Gastroenterol. 2006;41:1206-1213. |
| 31. | Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543-1554. |
| 32. | Lin SM, Lin CJ, Hsu CW, Tai DI, Sheen IS, Lin DY, Liaw YF. Prospective randomized controlled study of interferon-alpha in preventing hepatocellular carcinoma recurrence after medical ablation therapy for primary tumors. Cancer. 2004;100:376-382. |
| 33. | Kubo S, Nishiguchi S, Hirohashi K, Shuto T, Kuroki T, Minamitani S, Ikebe T, Yamamoto T, Wakasa K, Kinoshita H. Clinicopathological criteria for multicentricity of hepatocellular carcinoma and risk factors for such carcinogenesis. Jpn J Cancer Res. 1998;89:419-426. |
| 34. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. |
| 35. | Omata M, Yoshida H. Resolution of liver cirrhosis and prevention of hepatocellular carcinoma by interferon therapy against chronic hepatitis C. Scand J Gastroenterol Suppl. 2003;47-51. |
| 36. | Dunk AA, Ikeda T, Pignatelli M, Thomas HC. Human lymphoblastoid interferon. In vitro and in vivo studies in hepatocellular carcinoma. J Hepatol. 1986;2:419-429. |
| 37. | Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology. 2009;49:S174-S184. |
Peer reviewers: Sang Hoon Ahn, MD, PhD, Associate Professor, Department of Internal Medicine, Institute of Gastroenterology and Hepatology, Yonsei University College of Medicine, Severance Hospital, 250 Seongsanno, Seoul, South Korea; Chao-Hung Hung, MD, Associate Professor, Division of Hepatogastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, 123 Ta Pei Road, Niao Sung, Kaohsiung 833, Taiwan, China
S- Editor Wang JL L- Editor Ma JY E- Editor Ma WH