Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2913
Revised: April 8, 2010
Accepted: April 15, 2010
Published online: June 21, 2010
AIM: To assess risk factors for bleeding after gastric endoscopic submucosal dissection (ESD) and to develop preventive measures.
METHODS: This retrospective study was performed in a tertiary referral center. A total of 328 patients underwent ESD for 398 gastric neoplasms between July 2007 and June 2009. The main outcome was association between post-ESD bleeding and the following: age; sex; comorbidities; daily use of medicine potentially related to gastric injury/bleeding; location, size, and histological depth of lesions; ulceration; experience of operator coagulating the ulcer floor, and duration of operation. We also determined the relationship between the location of post-ESD bleeding and risk factors for hemorrhage.
RESULTS: Univariate analysis revealed significant risk factors: tumor location [odds ratio (OR), 2.86; 95% CI: 1.21-6.79, P = 0.024], coagulator experience (OR, 4.29; 95% CI: 1.43-12.86, P = 0.009), and medicine potentially related to gastric injury/bleeding (OR, 2.80; 95% CI: 1.14-6.90, P = 0.039). Multivariate logistic regression analysis confirmed significant, independent risk factors: tumor in lower third of stomach (OR, 2.47; 95% CI: 1.02-5.96, P = 0.044), beginner coagulator (OR, 3.93; 95% CI: 1.29-11.9, P = 0.016), and medicine (OR, 2.76; 95% CI: 1.09-6.98, P = 0.032). We classified cases of post-ESD bleeding into two groups (bleeding at the ulcer margin vs bleeding at the center) and found that bleeding at the margin occurred more frequently with beginner coagulators compared with experts (OR, 16.00; 95% CI: 1.22-210.59, P = 0.040).
CONCLUSION: Beginner coagulators, tumor in the antrum, and medicines were significant risk factors for post-ESD bleeding. Bleeding at the ulcer margin frequently occurred with beginner operators.
- Citation: Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol 2010; 16(23): 2913-2917
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2913.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2913
Endoscopic submucosal dissection (ESD) is an excellent treatment for superficial gastric cancer because a large tumor or lesion with ulcer scar can be successfully resected en bloc[1]. Because ESD allows accurate histopathological diagnosis and reduces the risk of local recurrence[2], it is now a standard treatment for early gastric cancer with certain histopathological properties. However, ESD is technically more difficult and can result in more complications compared with conventional endoscopic mucosal resection (EMR)[1,3]. Bleeding after gastric ESD is reported to occur in up to 7% of cases[4] and can occur later than other complications like perforation, sometimes even after discharge from the hospital. Further, bleeding after gastric ESD may lead to serious conditions including massive bleeding and life-threatening hemorrhagic shock[5]; therefore, preventing post-gastric ESD bleeding is essential. Prophylactic coagulation of visible vessels in the resection area has been reported to reduce the bleeding rate[5], but preventive coagulation therapy cannot fully solve the problem of delayed bleeding. Thus, an assessment of risk factors for post-ESD bleeding is needed.
In the present study, we aimed to determine risk factors for post-gastric ESD bleeding and establish techniques to minimize these complications.
A total of 329 patients underwent ESD for 399 gastric neoplasms, including early gastric cancers and gastric adenomas, between July 2007 and June 2009 in our institute. One lesion was excluded from the analysis because ESD for that lesion was stopped halfway during the procedure; it was considered unresectable by ESD because of massive submucosal invasion. Thus, 398 lesions in 328 patients were evaluated.
All patients provided written informed consent before treatment. Patients fasted on the morning of the operation, and lansoprazole infusion (30 mg twice a day) was initiated. ESD was performed under conscious sedation with flunitrazepam and buprenorphine using a video endoscope (GIF-Q260J; Olympus Optical Co., Ltd., Tokyo, Japan) and high-frequency power supply unit (VIO 300D or ICC 200; ERBE, Tübingen, Germany).
The ESD procedure was as follows. First, marks were made 5 mm outside the tumor edge with a Flex Knife (Olympus Optical Co., Ltd.). Epinephrine (1:100 000 solution in saline) was injected into the submucosal layer around the lesion, and the mucosa 5 mm outside the marks was cut using IT-Knife 2 (Olympus Optical Co., Ltd.). After incision of the mucosa, submucosal dissection of the lesion was performed using IT-Knife 2. After resection of the lesion, all visible vessels on the ulcer floor were coagulated with hot biopsy forceps (Hoya Co., Ltd., Pentax Life Care Div., Japan) and VIO 300D (swift coagulation, effect 3, 45 W) or ICC 200 (forced coagulation, 65 W).
Patients who did not develop complications started a soft diet on day 1 after ESD and were discharged 5 d after the procedure. They received oral lansoprazole for 8 wk, beginning on day 1 after ESD. We defined bleeding after ESD as hemorrhage resulting in hematemesis or melena that required endoscopic treatment.
When hemorrhage occurred, we performed urgent endoscopy to determine the vessel responsible for the bleeding. When clots were lodged in the responsible vessel, they were removed and the responsible vessel was exposed. The vessel was grasped or touched with the hot biopsy forceps using the forced coagulation mode (ICC 200, 65 W).
We classified the 398 lesions into two groups: with bleeding and without bleeding. We reviewed patient medical records and collected the following data for each patient: age, sex, comorbidities, daily use of medicine related to gastric injury/bleeding, tumor depth, histological ulceration, tumor diameter, tumor location, and the operator performing coagulation of the ulcer floor (coagulator). The risk factors were analyzed based on individual tumors, because some patients had more than one lesion resected.
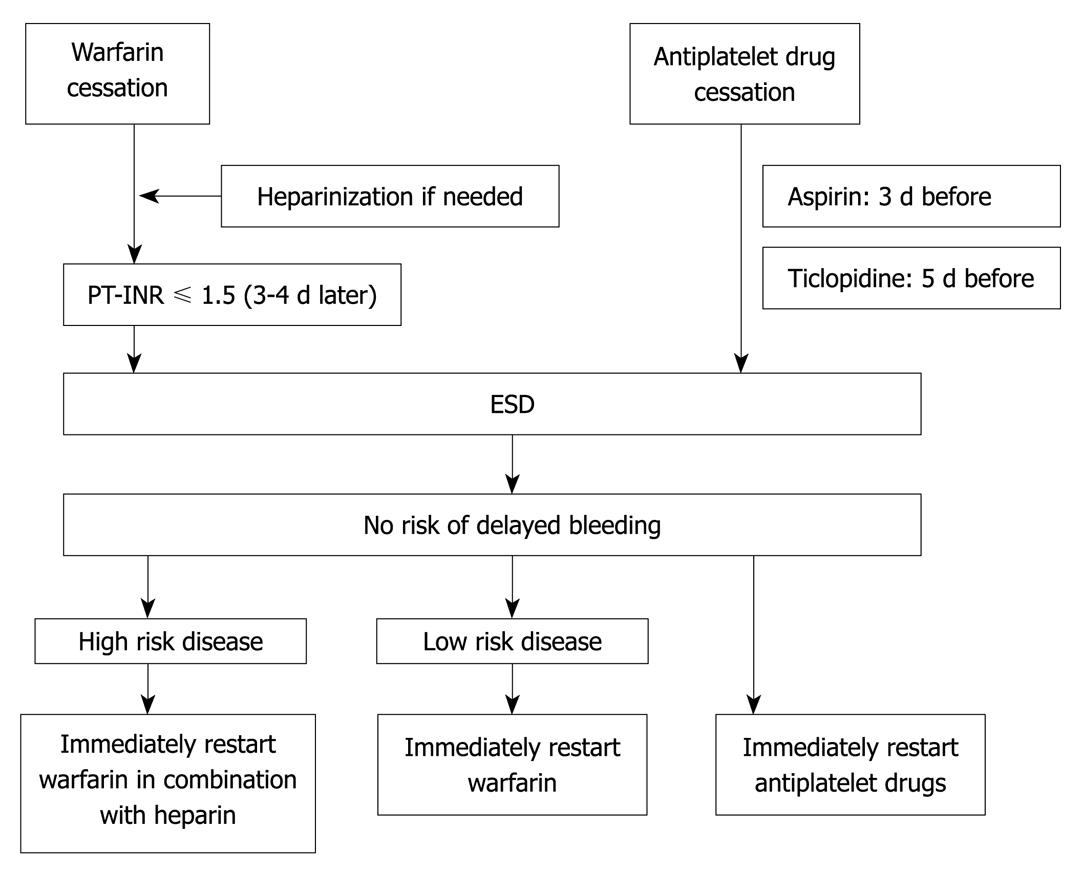
Comorbidities included hypertension (systolic blood pressure > 140 mmHg or antihypertensive treatment) and diabetes mellitus (oral hypoglycemic agent or insulin treatment). Medicines potentially related to gastric injury/bleeding included antiplatelet agents, anticoagulants, steroids, or nonsteroidal anti-inflammatory drugs (NSAIDs). Patients taking steroids or NSAIDs were instructed to discontinue their use 1 wk before ESD to 1 wk after ESD. Patients taking antiplatelet or anticoagulants were instructed to discontinue use according to “measures against patients undergoing antithrombotic regimen in endoscopic treatment” in the 3rd edition of the guidelines for gastroenterological endoscopy[6]. The cessation and restart of antithrombotic drugs in ESD is shown in Figure 1. Ulceration and depth were determined histopathologically after ESD; histological depth was classified as intramucosal (m) or involving the submucosal layer (sm). Based on the Japanese classification of gastric carcinoma[7], tumor location was classified into two groups: upper or middle third of the stomach (UM) or lower third (L). Coagulators were the physicians who coagulated the artificial ulcer floor after resection of the lesion. In our hospital, novice ESD operators begin their training with coagulation of the ulcer floor; therefore, the operator who resected the tumor was not always the one who coagulated the ulcer floor. Ulcer floor coagulators were categorized as beginners (performed < 50 cases of gastric ESD) or experts (performed > 200 cases of gastric ESD). In the present study, there were no coagulators who performed between 50 and 200 ESD procedures.
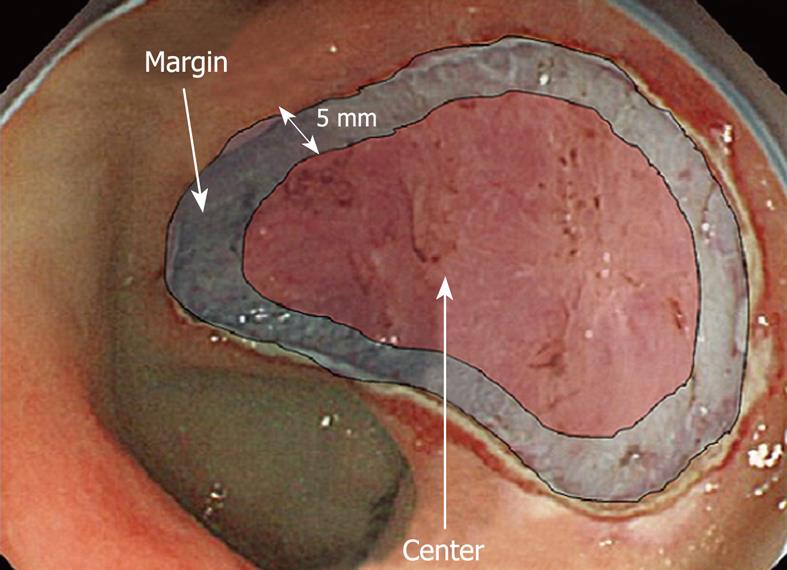
We also determined the relationship between the location of post-ESD bleeding and risk factors for hemorrhage. During the observation period, post-ESD bleeding occurred in 23 lesions (23 patients). The hemorrhage location was categorized as ulcer margin (< 5 mm from the ulcer edge) or center (ulcer floor area other than the margin) (Figure 2).
Categorical data were compared using χ2 test (with Yates correction) and Fisher’s exact test. Differences in the means of continuous data were compared by Student’s t test or Mann-Whitney U test. To identify important risk factors for bleeding after ESD, predictors with P-values < 0.2 in the univariate analysis were included in a backward, stepwise multiple logistic regression model. P-value < 0.05 was considered significant, and all tests were two-sided. Data are expressed as mean ± SD or as median (range). All statistical analyses were performed with PASW Statistics 18 for Windows (SPSS Japan, Tokyo).
During the treatment of the 398 lesions, 23 cases of post-ESD bleeding occurred (5.8%). Baseline characteristics of the study population are displayed in Table 1. A total of six operators (two experts and four beginners) were involved in this study. Among the 23 cases of post-ESD bleeding, 10 (43.5%) occurred within 24 h after ESD. All cases were controlled by endoscopic treatment and did not require surgical intervention; one case required blood transfusion.
| n (%) | |
| Sex (M/F) (% M) | 302/96 (75.6) |
| Age (yr) | 68.0 ± 10.2 |
| Hypertension (-/+) (% positive) | 208/190 (47.7) |
| Diabetes mellitus (-/+) (% positive) | 349/49 (12.3) |
| Drugs potentially related to gastric injury/bleeding (-/+) (% positive) | 330/68 (17.1) |
| Location (UM/L) (% L) | 252/146 (36.7) |
| Depth (m/sm) (% sm) | 343/55 (13.8) |
| Ulcer (-/+) (% positive) | 367/31 (7.8) |
| Diameter of lesion (cm) | 43.1 ± 17.2 |
| Duration of operation (min) | 69.0 ± 56.7 |
| Coagulator (expert/beginner) (% beginner) | 182/216 (54.3) |
The results of univariate analysis are displayed in Table 2. Significant risk factors for bleeding after gastric ESD included: tumor location [L vs UM; odds ratio (OR), 2.86; 95% CI: 1.21-6.79, P = 0.024], coagulators (beginner vs expert; OR, 4.29; 95% CI: 1.43-12.86, P = 0.009), and daily use of medicine potentially related to gastric injury/bleeding (user vs non-user; OR, 2.80; 95% CI: 1.14-6.90, P = 0.039). As an additional datum, the incidence of hemorrhage in relation to tumor location was estimated (U vs M vs L; 4.6% vs 3.2% vs 9.6%, P = 0.042).
| Bleeding (-) (n = 375) | Bleeding (+) (n = 23) | |
| Sex (M/F) (% M) | 283/92 (75.4) | 19/4 (82.6) |
| Age (yr) | 67.8 ± 10.2 | 70.1 ± 10.0 |
| Hypertension (-/+) (% positive) | 198/177 (47.2) | 10/13 (56.5) |
| Diabetes Mellitus (-/+) (% positive) | 329/46 (12.2) | 20/3 (13.0) |
| Drugs potentially related to gastric injury/bleeding (-/+) (% positive) | 315/60 (16.0) | 15/8 (34.8)a |
| Location (UM/L) (% L) | 243/132 (33.2) | 9/14 (60.9)a |
| Depth (m/sm) (% sm) | 324/51 (13.6) | 19/4 (17.4) |
| Ulcer (-/+) (% positive) | 347/28 (7.4) | 20/3 (13.0) |
| Diameter of lesion (cm) | 42.7 ± 17.2 | 48.3 ± 17.3 |
| Duration of operation (min) | 68.8 ± 56.7 | 72.2 ± 56.4 |
| Coagulator (beginner/expert) (% beginner) | 197/178 (52.5) | 19/4 (82.6)a |
Multivariate logistic regression analysis confirmed that the following were independent factors for bleeding after gastric ESD: tumors located in the lower third of the stomach (OR, 2.47; 95% CI: 1.02-5.96, P = 0.044), beginner coagulators (OR, 3.93; 95% CI: 1.29-11.91, P = 0.016), and daily use of medicine potentially related to gastric injury/bleeding (OR, 2.76; 95% CI: 1.09-6.98, P = 0.032) (Table 3).
We classified cases of post-ESD bleeding into two groups (bleeding at the ulcer margin vs bleeding at the center), and determined the relationships between bleeding site and the three risk factors described above. We found that bleeding at the margin occurred more frequently with beginner coagulators compared with experts (OR, 16.00; 95% CI: 1.22-210.59, P = 0.040) (Table 4).
| Marginal bleeding (n = 17) | Central bleeding (n = 6) | |
| Drugs potentially related to gastric injury/bleeding | 6 (35.3) | 3 (50.0) |
| Tumor in the lower third of stomach | 9 (52.9) | 5 (83.3) |
| Beginner coagulator | 16 (94.1) | 3 (50.0)a |
The interval between ESD and post-ESD bleeding was also determined. The median interval was 3 d (range, 0-14 d), and bleeding occurred significantly earlier in L lesions compared with UM lesions (UM vs L; 9 d (range, 0-14 d) vs 1 (0-9), P = 0.019). The relationship between patient medication and the interval between ESD and bleeding was also estimated. In the “medication” group, the median interval to bleeding was 3 d (range, 0-14 d), while in the “no medication” group, the median interval to bleeding was 1 d (range, 0-12 d). Based on the data medication did not appear to affect the interval between ESD and post-ESD bleeding (P = 0.201).
Previous studies[5,8-11] have evaluated measures against post-ESD bleeding. Takizawa et al[5] found that coagulating exposed vessels on the ulcer floor after ESD, which is known as post-ESD coagulation preventive therapy (PEC), reduced the risk for delayed bleeding. However, the incidence of bleeding after ESD was still 3.1% after PEC; therefore, additional measures are needed to prevent delayed bleeding.
In the present study, multivariate logistic regression analysis showed that tumors located in the lower third of the stomach, beginner operators coagulating the ulcer floor, and daily use of medicine potentially related to gastric injury/bleeding were significantly associated with bleeding after ESD.
Takizawa et al[5] also reported that tumor location was an independent risk factor for delayed bleeding. In that study, delayed bleeding occurred more frequently in the upper third compared with the middle and lower thirds of the stomach. In the present study, we compared UM lesions with L lesions, because we thought that antral active peristalsis and bile reflux might lead to a high incidence of post-ESD bleeding in the lower stomach. On the other hand, submucosal artery diameters were smaller in L lesions compared with UM lesions in a dog model[12]. For that reason, bleeding during gastric EMR may occur more frequently in UM lesions. Similarly, during ESD procedures, intraoperative bleeding frequently occurs in UM lesions. Intraoperative bleeding, however, may lead to more careful endoscopic hemostasis, which ultimately protects against delayed bleeding. In another study, an increased risk of delayed bleeding has been reported when immediate bleeding occurred during endoscopic mucosal resection[13]. However, sites of delayed bleeding often differed from the sites of immediate bleeding, suggesting that well-coagulated arteries seldom bleed.
Previous studies have reported that daily use of medicine potentially related to gastric injury/bleeding is not a risk factor for post-ESD bleeding[5,13]. However, multivariate logistic regression analysis in our study showed that use of these medicines was an independent risk factor for post-ESD bleeding. On the other hand, daily use of these drugs did not appear to affect the interval between ESD and bleeding. The median interval was 3 d, which was before medication was restarted; therefore, direct effects of these drugs on post-ESD bleeding appear to be limited. The mechanism underlying the association between daily use of medicine potentially related to gastric injury/bleeding and post-ESD bleeding is not known.
Our study also showed that post-ESD bleeding occurred significantly more often when beginners performed coagulation of the ulcer floor after ESD. The incidence of hemorrhage was 2.2% when performed by experts, but 8.8% with beginners (Table 2). Beginners caused bleeding in the ulcer margin significantly more often. Coagulation of exposed vessels on the ulcer floor after ESD reduces the risk of hemorrhage, but potentially hemorrhagic vessels are often hidden in a fat layer that remains on the ulcer floor, especially at the edge of the ulcer. Thus, careful coagulation of vessels at the ulcer margin is important in preventing delayed bleeding.
In L lesions, hemorrhage occurred significantly earlier compared with UM lesions. The median interval between ESD and postoperative hemorrhage in L lesions was 1 d (range, 0-9 d), suggesting that second-look endoscopy 1 d after ESD may be helpful in preventing post-ESD bleeding in L lesions.
Limitations of the present study include its single-center retrospective study design.
In conclusion, risk factors for bleeding after gastric ESD included beginner operators coagulating the ulcer floor after ESD, tumor location in the lower third of the stomach, and medicine potentially related to gastric injury/bleeding. Careful coagulation of the vessels in the ulcer floor after ESD, especially around the ulcer margin, may reduce the incidence of post-ESD hemorrhage. In addition, a second-look endoscopy 1 d after ESD may be helpful in preventing post-ESD bleeding in tumors in the lower third of the stomach.
Endoscopic submucosal dissection (ESD) is an excellent treatment for early gastric cancer, but bleeding after ESD occurs in up to 7% of cases and remains a severe complication.
Prophylactic coagulation of visible vessels in the resection area has been reported to reduce the bleeding rate, but preventive coagulation therapy cannot fully solve the problem of delayed bleeding. Thus, an assessment of risk factors for post-ESD bleeding is needed. The authors reviewed their 398 ESD cases and evaluated risk factors for post-ESD bleeding.
Beginner coagulators, tumor in the lower third of the stomach, and medicines potentially related to gastric injury/bleeding were significant risk factors for post-ESD bleeding. Bleeding at the ulcer margin occurred more frequently with beginner coagulators than with experts. Therefore, novice ESD operators should pay special attention to coagulating vessels in the ulcer edge after ESD. As to tumors in the lower third of the stomach, post-ESD bleeding happened earlier than in other areas. A second-look endoscopy 1 d after ESD may be helpful.
By overcoming post-ESD bleeding, ESD for gastric neoplasms will become a safer and more popular treatment.
In this paper, experts/beginners were defined as endoscopists that have performed more than 200/less than 50 cases of gastric ESD.
This is well written and important paper. Although it is not a prospective study, the topic is important for all gastroenterologists and gastrointestinal (GI) surgeons dealing with surgical endoscopy of the upper GI tract. The authors’ work gives important information about one of the typical complications i.e. bleeding after ESD.
Peer reviewer: Jan Kulig, Professor, MD, Head, 1st Department of General and GI Surgery, Jagiellonian University Medical College, 40 Kopernika St., 31-501 Kraków, Poland
S- Editor Tian L L- Editor O'Neill M E- Editor Lin YP
| 1. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. |
| 2. | Fujishiro M. Endoscopic submucosal dissection for gastric cancer. Curr Treat Options Gastroenterol. 2008;11:119-124. |
| 3. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. |
| 4. | Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1-11. |
| 5. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. |
| 6. | Ogoshi K, Tada M, Kaneko E. Measures against patients undergoing antithrombotic regimen in endoscopic treatment. The Guideline for Gastroenterological Endoscopy. 3rd edition. Bunkyo, Tokyo: Igaku-shoin Ltd 2006; 16-24. |
| 7. | Association JGC. Japanese classification of gastric carcinoma. 2nd English edition. Gastric Cancer. 1998;1:11. |
| 8. | Jeong HK, Park CH, Jun CH, Lee GH, Kim HI, Kim HS, Choi SK, Rew JS. A prospective randomized trial of either famotidine or pantoprazole for the prevention of bleeding after endoscopic submucosal dissection. J Korean Med Sci. 2007;22:1055-1059. |
| 9. | Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610-1616. |
| 10. | Jang JS, Choi SR, Graham DY, Kwon HC, Kim MC, Jeong JS, Won JJ, Han SY, Noh MH, Lee JH. Risk factors for immediate and delayed bleeding associated with endoscopic submucosal dissection of gastric neoplastic lesions. Scand J Gastroenterol. 2009;1-7. |
| 11. | Ono S, Kato M, Ono Y, Nakagawa M, Nakagawa S, Shimizu Y, Asaka M. Effects of preoperative administration of omeprazole on bleeding after endoscopic submucosal dissection: a prospective randomized controlled trial. Endoscopy. 2009;41:299-303. |
| 12. | Narimiya N, Sato H, Joki M, Odagiri M, Iwasaki M. An experimental study of submucosal vascular structure of the stomach after endoscopic mucosal resection [In Japanese with English abstract]. Gastroenterol Endosc. 1994;36:958-962. |
| 13. | Okano A, Hajiro K, Takakuwa H, Nishio A, Matsushita M. Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc. 2003;57:687-690. |