Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2873
Revised: April 5, 2010
Accepted: April 12, 2010
Published online: June 21, 2010
AIM: To investigate the effects of the chemokine stromal cell-derived factor-1 (CXCL12) receptor (CXCR4) antagonist AMD3100 on colonic inflammation and epithelial barrier in dextran sulfate sodium (DSS)-induced colitis in mice.
METHODS: Experimental colitis was induced by administration of 5% DSS for 7 d, and assays performed on intestinal segments from the ileocecal valve to the anus. Colonic morphology was examined by hematoxylin and eosin staining. Colonic cytokines were determined by enzyme-linked immunosorbent assay. Myeloperoxidase (MPO) activity (indicator of inflammatory infiltration) was observed spectrophotometrically. Gut permeability was assessed by mucosal-to-serosal clearance of fluorescein isothiocyanate-conjugated dextran 4000 (FD4) in everted gut sacs. The apoptosis of colonic epithelium was assessed by Hoechst-33342 staining. To further elucidate the role of CXCR4 in colonic inflammation, we also investigated the effect of AMD3100 on migration and cytokine production of isolated peripheral blood mononuclear cells (PBMCs).
RESULTS: DSS-induced colitis was characterized by morphologic changes, as well as increased colonic cytokines, inflammatory infiltration, epithelial apoptosis, and intestinal permeability in mice. In AMD3100-treated mice, epithelial destruction, inflammatory infiltration, and submucosal edema were markedly reduced; colonic tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interferon-γ (IFN-γ) levels, as well as MPO activity were significantly decreased. Increased intestinal permeability in DSS-treated mice was significantly reduced by AMD3100. The number of apoptotic cells in colitis mice was markedly increased after DSS administration, and decreased when treated with the CXCR4 antagonist AMD3100. In pre-activated PBMCs, CXCL12 stimulation significantly increased the migration of PBMCs, and was inhibited by AMD3100. Moderately increased TNF-α, IL-6, and IFN-γ from CXCL12-treated PBMCs were also reduced by AMD3100.
CONCLUSION: The CXCR4 antagonist AMD3100 exerts therapeutic effects on experimental colitis by inhibiting colonic inflammation and enhancing epithelial barrier integrity.
- Citation: Xia XM, Wang FY, Xu WA, Wang ZK, Liu J, Lu YK, Jin XX, Lu H, Shen YZ. CXCR4 antagonist AMD3100 attenuates colonic damage in mice with experimental colitis. World J Gastroenterol 2010; 16(23): 2873-2880
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2873.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2873
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD), clinically characterized by bloody diarrhea, fever, weight loss, and anemia[1,2]. Previous studies demonstrated that pathogenesis of UC involves the interaction of environmental, genetic, microbial, and immune factors, which can initiate an abnormal immune response, and consequent intestinal inflammation[3,4]. Uncontrolled local inflammation disrupts the epithelial lining, resulting in mucosal edema and ulceration, and even crypt abscess in the bowel wall[5]. As in other inflammatory processes, polymorphonuclear leukocytes infiltrating into colonic mucosa are believed to be the main source of cytokine and inflammatory mediators[6]. Chemokines, including growth-regulated oncogene-α and interferon-γ-inducible protein-10, which are expressed on various cells of the intestinal tissues, have been reported to regulate the recruitment of inflammatory cells[7,8]. Block of these chemokines or their receptors significantly attenuates intestinal inflammation and mucosal damage in UC animals, suggesting a pivotal role of chemokines in the pathogenesis of UC[2,8].
The chemokine stromal cell-derived factor-1 (CXCL12) is firstly characterized as a pre-B cell growth stimulating factor and its specific receptor is chemokine stromal cell-derived factor-1 receptor (CXCR4), which also functions as an entry receptor for human immunodeficiency virus[9]. The CXCL12/CXCR4 chemokine axis has an important role in the development of cardiovascular, hematopoietic, and central nervous systems[10,11], and is also involved in several inflammatory diseases such as rheumatoid arthritis, acute lung injury, and sepsis[12-15]. Recent studies demonstrated that CXCL12 and CXCR4 are constitutively expressed on intestinal epithelial cells, lamina propria T cells, and peripheral blood T cells of control patients, and the expression is increased in those of UC patients[16,17]. Block of CXCR4 significantly ameliorates murine experimental colitis[4], indicating a possible role of this chemokine axis in intestinal inflammatory response.
In UC patients, increased number of apoptotic epithelial cells is detected throughout the crypt[18]. Local massive apoptosis of epithelial cells disturbs the epithelial barrier, which can facilitate the infiltration of inflammatory cells, and aggravate mucosal damage[19]. Whether the CXCL12/CXCR4 chemokine axis plays a role in epithelial apoptosis and barrier function, however, needs to be further defined.
In the present study, we firstly assessed the effect of a CXCR4 antagonist, AMD3100, on dextran sulfate sodium (DSS)-induced colitis in mice. Morphology, colonic cytokines, myeloperoxidase (MPO) activity (an indicator of inflammatory infiltration), gut permeability, and epithelial apoptosis were all examined in the first series. To further elucidate the role of the CXCL12/CXCR4 interaction in colonic inflammation, we also investigated the effect ofAMD3100 on isolated peripheral blood mononuclear cells (PBMCs). In this series, cell migration and cytokine production were assessed.
Female BALB/c mice (9 wk of age, weighing 20-22 g) were obtained from the Animal Facility of the Jinling Hospital (Nanjing, China). Animals were housed under controlled temperature, humidity and day-night cycles, with free access to standard laboratory feed and water. The Animal Studies Ethics Committee of Jinling Hospital approved all of the experiments.
For the induction of colitis, mice were given 5% DSS (5000 daltons; obtained from Wako Pure Chemical Industry, Japan) in their drinking water for 7 d. Normal control mice received regular drinking water throughout the experiment. The CXCR4 antagonist, AMD3100, was obtained from Sigma (St. Louis, MO, USA). Twenty-five micrograms of AMD3100 dissolved in 200 μL of phosphate-buffered saline (PBS) or 200 μL of PBS alone were administered intraperitoneally once daily during the study period. Eight mice were studied in each experimental group. On day 8, all mice were anesthetized with intraperitoneal administration of ketamine (50 mg/kg) and acepromazine (2 mg/kg), and the intestinal segments from the ileocecal valve to the anus (5-6 cm in length) were collected for subsequent assays.
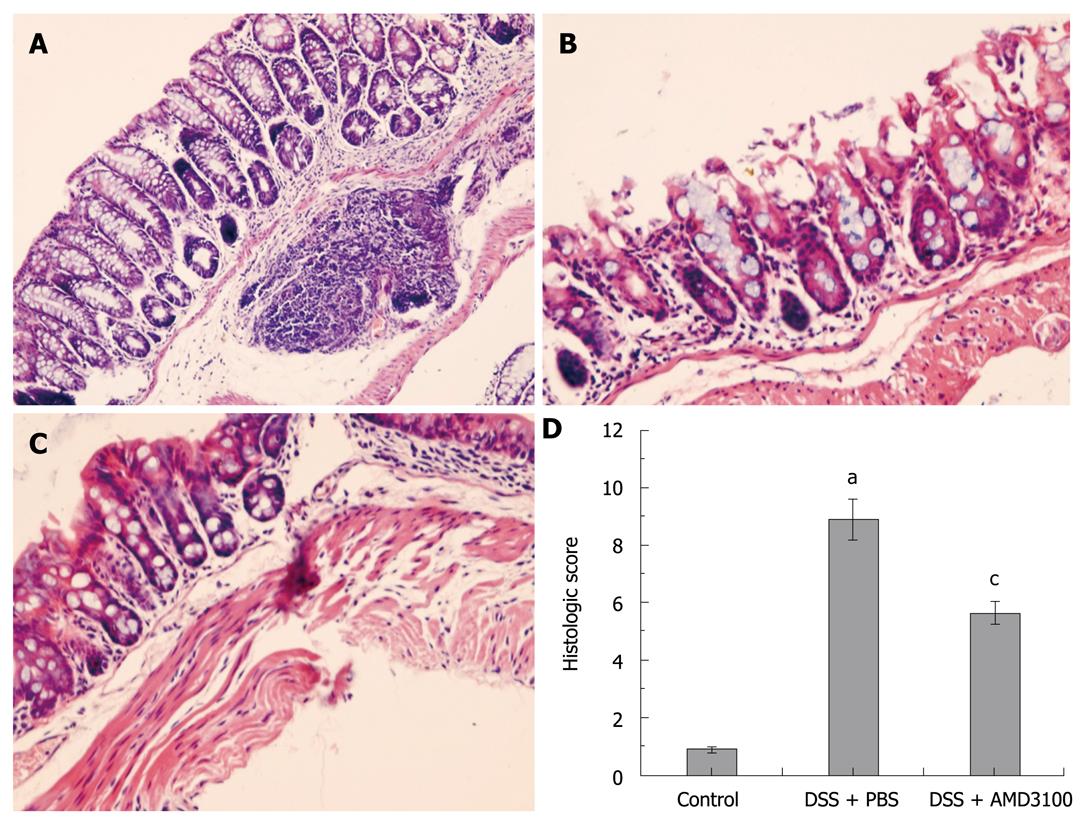
The distal segments of the colon (2 cm from the anal verge) were washed in PBS, fixed in 10% neutral buffered formalin, and embedded in paraffin wax. Five micrometers sections were deparaffinized with xylene, stained with hematoxylin and eosin, and examined by 2 experienced pathologists in blinded fashion. The following morphological criteria were considered: score 0, no damage; score 1 (mild), focal epithelial necrosis; score 2 (moderate), diffuse necrosis of the villi; score 3 (severe), necrosis with neutrophil infiltrate in the submucosa; score 4 (highly severe), widespread necrosis with massive neutrophil infiltrate and hemorrhage[1].
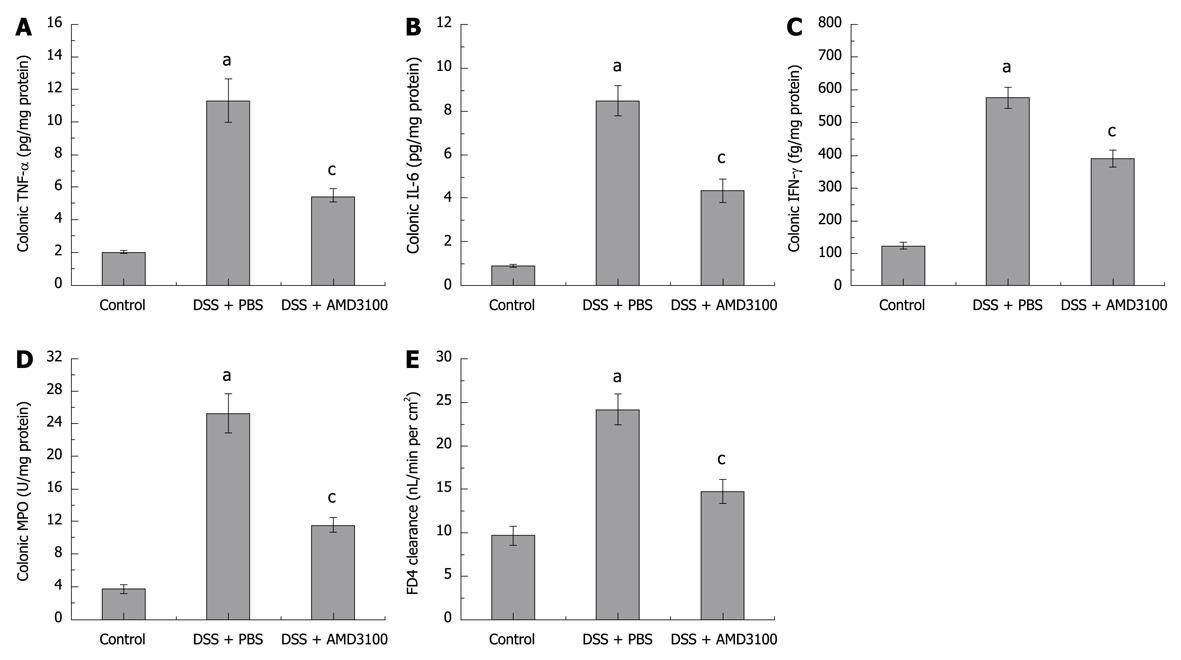
The colonic levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interferon-γ (IFN-γ) were evaluated using commercial colorimetric kits (Jingmei Biotech, Beijing, China) according to the manufacturer’s instructions. The tissue homogenate enzyme-linked immunosorbent assay was determined with respect to the concentration of protein, and expressed as pg/mg tissue protein.
Sequestration of leukocytes within the colon was evaluated by measuring tissue MPO activity[2]. Briefly, tissue samples were homogenized with 0.5% hexadecyltrimethylammonium bromide (Sigma, ST. Louis, MO, USA) in 50 mmol/L phosphate buffer (pH 6.0). The homogenate was sonicated for 10 s, freeze-thawed 3 times, centrifuged at 14 000 rpm for 15 min, and the resulting suspension was used for assay. The assay mixture consisted of 20 μL of supernatant, 10 μL of tetramethylbenzidine (final concentration 1.6 mmol/L), and 70 μL of H2O2 (final concentration 3.0 mmol/L). MPO activity was assessed photometrically at 630 nm. The results were expressed with respect to the concentration of protein, and expressed as the activity per mg protein of the tissue (U/mg protein).
Intestinal permeability was assessed by the mucosal-to-serosal clearance of fluorescein isothiocyanate-conjugated dextran (4000 daltons, FD4; obtained from Sigma, St.Louis, MO, USA) in everted gut sacs, as described in previous studies[20,21]. Intestinal segments from the ileocecal valve to the anus were excised, and prepared in ice-cold modified Krebs-Henseleit bicarbonate buffer (KHBB contained in mmol/L: HEPES 10, NaCl 137, KCl 5.5, NaHCO3 4.2, Na2HPO4 0.3, KHPO4 0.4, MgSO4 0.4, MgCl2 0.5, CaCl2 1.3, glucose 19.5). The intestinal segment first underwent lavage with 3 mL of PBS to remove fecal material, and was then closed at one end with a 4-0 silk ligature. The gut sac was everted using a thin metal rod, then connected to a 1 mL syringe containing 0.4 mL of the KHBB solution, and secured with a 4-0 silk ligature 4 cm from the tip. The everted gut sac was gently distended with 0.4 mL of KHBB, suspended in a 100 mL beaker containing FD4 (20 μg/mL) in KHBB, maintained at 37°C in a water bath, and continuously bubbled with a gas mixture containing 95% oxygen and 5% CO2.
At the beginning of the incubation, a 1 mL sample was withdrawn from the beaker to determine the initial external (mucosal surface) FD4 concentration (FD4muc). After a 30-min incubation period, the gut sac was removed from the beaker, its diameter (D) and length (L) were measured, and the fluid on the serosal side was aspirated into the syringe to determine the FD4 concentration (FD4ser). The serosal and mucosal samples were centrifuged for 10 min at 1000 g. One hundred microliters of the supernatant was diluted with PBS (900 μL), and fluorescence was measured (λex = 492 nm, slit width = 1.5 nm; λem = 515 nm, slit width = 10 nm) in a spectrofluorometer (model F-7000, Hitachi, Japan). The mucosal-to-serosal clearance of FD4 was calculated using the following equations:
Mucosal surface area (A) = πLD.
Mass of FD4 in the gut sac after 30 min incubation (M) = (FD4ser) × 0.4.
Mucosal-to-serosal permeation rate of FD4 (PR, ng/min) = M/30 min.
Mucosal-to-serosal clearance of FD4 (C, nL/min per cm2) = (PR/FD4muc)/A.
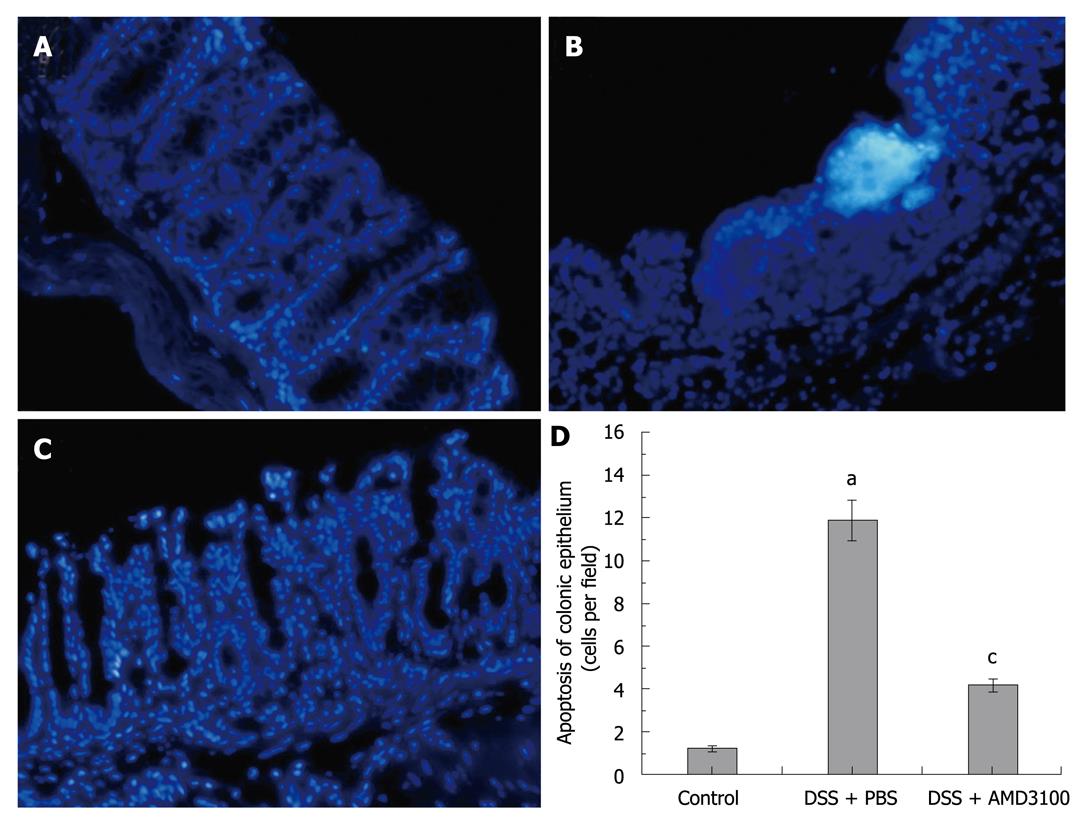
Apoptosis of the colonic epithelium was assessed using a nuclear stain Hoechst-33342 (Sigma, St.Louis, MO, USA). Briefly, tissue sections (5 μm) were dewaxed with xylene and graded alcohols, and finally washed in tap water. Endogenous peroxidase activity was blocked by 3% (v/v) H2O2, and the antigen was recovered by microwave in 0.1 mol/L PBS (pH 7.2). Sections were immersed in Hoechst dye (working concentration 5 μg/mL) for 5 min, and then washed in PBS, 5 min for 3 times. The signals were visualized by a fluorescence microscope (model IX71, Olympus, Japan), and the number of positive cells/high power field was counted in 5 fields for each section as described by Cuzzocrea et al[22].
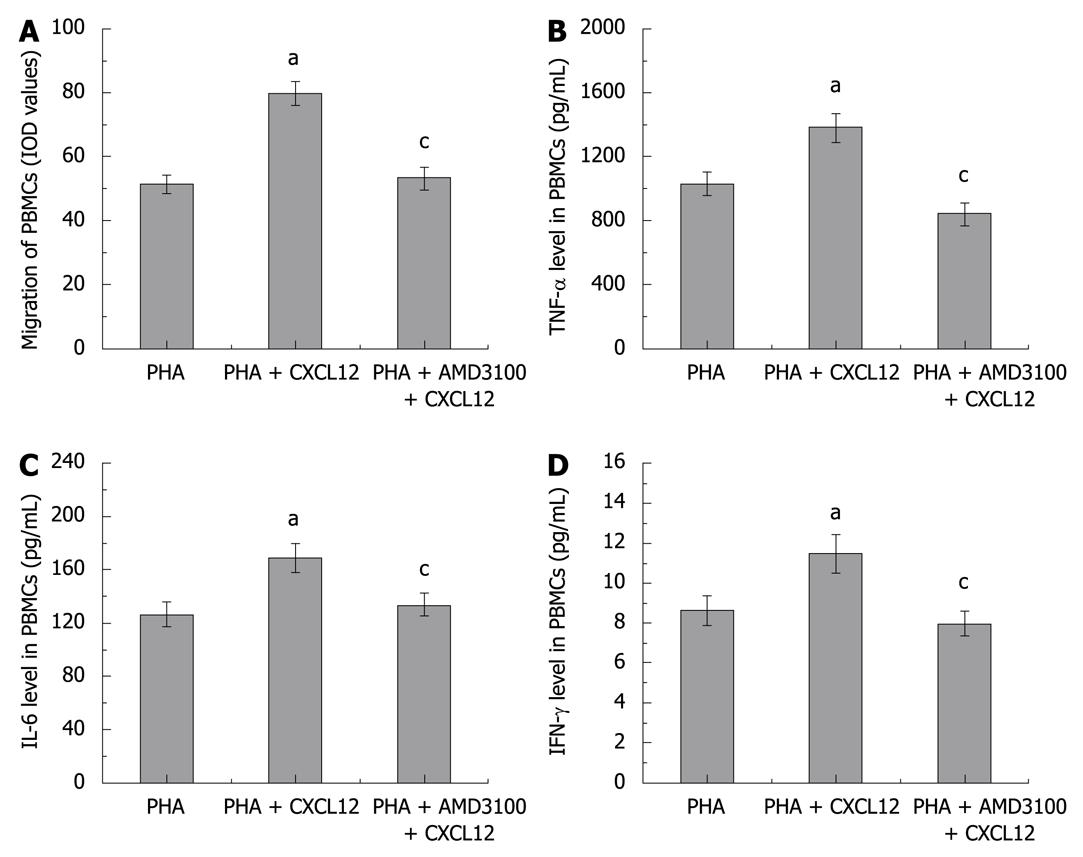
For isolation of PBMCs, 12 normal mice were anesthetized with intraperitoneal administration of ketamine (50 mg/kg) and acepromazine (2 mg/kg). Blood samples were obtained from the inferior cava vein by direct puncture. PBMCs were isolated by Ficoll-Hypaque plus density gradient centrifugation. The isolated PBMCs were resuspended in F-12K Nutrient Mixture (Invitrogen, Carlsbad, USA) with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 100 ng/mL phytohemagglutinin (PHA, activator of PBMCs; obtained from Sigma, St.Louis, MO, USA). Six hours after resuspension, the PBMCs were split into 3 groups (1.5 × 106 cells/mL). Group 1 was cultured in medium with PBS. Group 2 was re-stimulated with 50 ng/mL CXCL12 (Chemicon, MA, USA), and group 3 was pre-treated with 20 μmol/L AMD3100 (30 min before re-stimulation), and then treated with 50 ng/mL CXCL12. The cells were incubated for 24 h, and the cytokine production in supernatant was measured using a colorimetric commercial kit (Jingmei Biotech, Beijing, China) according to the manufacturer’s instructions.
For assessment of cell migration, a 24-well transwell migration chamber with 8 μm pores (Greiner Bio-One, Frickenhausen, Germany) was used[23]. The isolated PBMCs was resuspended in serum-free medium with 0.2% bovine serum albumin to an appropriate concentration (1 × 106 cells/mL), then placed in the top chambers of the transwell (200 μL/well). The lower chambers were filled with 600 μL of control medium (containing 2.5% fetal bovine serum) with or without 50 ng/mL of CXCL12. Some of the cells were preincubated with the CXCR4 antagonist AMD3100 (20 μmol/L) for 30 min before being placed in the top chambers. Twelve hours after incubation, PBMCs that had not migrated to the lower chambers were removed from the upper surface of the transwell membrane with a cotton swab. Migrating cells on the lower membrane surface were fixed with methanol, stained with 10% Giemsa, and photographed under a light microscope. Values of integrated optical density (IOD) from the pictures were employed to assess the migration of PBMCs.
Results are presented as mean and standard error of the mean ± SE. One-way repeated-measures ANOVA (followed by multiple pair-wise comparisons using the Student-Newman-Kleus method) were used for the analysis of differences between the experimental and control groups. All statistical analyses were carried out using the SPSS version 11.5 for Windows (Chicago, IL, USA), with statistical significance set at P < 0.05.
After induction of colitis with DSS, the colonic mucosa showed congestion, erosion, and hemorrhagic ulcerations. Histological findings demonstrated marked epithelial destruction, inflammatory infiltration, crypt distortion, and submucosal edema (Figure 1B). In AMD3100-treated mice, epithelial destruction, inflammatory infiltration, and submucosal edema were markedly reduced as compared with nontreated mice (Figure 1C). No histological alteration was observed in the intestinal segments from control mice (Figure 1A). As a result, the histological score in mice with DSS-induced colitis was significantly higher than that in control mice, and treatment with AMD3100 markedly reduced the histological score in mice with colitis (Figure 1D).
Induction of DSS-induced colitis was assessed by histology and local cytokine production. In the present study, we also evaluated the effect of AMD3100 on colonic inflammation. As shown in Figure 2A-C, colonic TNF-α, IL-6, and IFN-γ levels were significantly elevated at 7 d after induction of colitis, as compared with control mice; treatment with AMD3100 markedly reduced colonic cytokines production in mice with colitis.
MPO activity, as a marker of local leukocyte sequestration, was also examined in this study. Figure 2D shows that colonic MPO activity was significantly increased 7 d after induction of colitis; treatment with AMD3100 decreased the colonic MPO level. These findings indicated that AMD3100 could inhibit the recruitment of inflammatory cells into the colonic segment.
The mucosal-to-serosal clearance of permeability probe FD4 in everted gut sacs was measured to assess the epithelial barrier. The mucosal-to-serosal passage of FD4 was low in control mice, and the calculated clearance was 9.10 ± 1.10 nL/min per cm2. DSS-administered mice demonstrated a marked increase in gut permeability, with the calculated clearance reaching 24.18 ± 1.83 nL/min per cm2. In AMD3100 treated mice, there was a significant reduction in permeability, and the calculated clearance was 14.71 ± 1.43 nL/min per cm2 (Figure 2E). These findings demonstrated that AMD3100 could enhance the colonic epithelial barrier.
According to the manufacturer of Hoechst-33342, the nuclei of apoptotic cells stain bright white, and are accompanied by apoptotic fragments, while the nuclei of live cells are stained as a uniform blue color. As in Figure 3, sporadic apoptotic cells were observed in the intestinal segments from control mice (apoptotic cells were 1.25 ± 0.12 per field). The number of apoptotic cells in the intestinal segments was markedly increased 7 d after induction of colitis, the apoptotic bodies mainly localized in the epithelium (apoptotic cells were 11.98 ± 0.81 per field). However, the presence of apoptotic cells was significantly decreased in the intestinal segments from AMD3100 treated mice (apoptotic cells were 4.23 ± 0.28 per field).
To evaluate whether AMD3100, the CXCR4 antagonist, could block PBMC migration, we performed an in vitro PBMCs chemotaxis assay. To mimic the in vivo inflammatory response, the PBMCs were pre-activated with PHA. The IOD values were employed to assess the number of migrated PBMCs. Figure 4A shows that CXCL12 treatment significantly increased the migration of PBMCs, as compared with the control group, the IOD values being 79.68 ± 3.76 and 51.28 ± 2.97, respectively, and the migration of PBMCs were inhibited by pretreatment with AMD3100 (IOD values 53.27 ± 3.70).
We also measured the cytokine production from isolated and PHA pre-activated PBMCs. As shown in Figure 4B-D, the supernatant levels of TNF-α, IL-6, and IFN-γ from CXCL12-treated PBMCs were moderately higher than that in the control group, and AMD3100 pre-treatment could attenuate the increase in TNF-α, IL-6, and IFN-γ in PBMCs.
The present study demonstrated that administration of the CXCR4 antagonist, AMD3100, decreased the severity of DSS-induced colitis in mice, as assessed by morphology, colonic cytokines, epithelial apoptosis, and gut permeability. More importantly, the CXCR4 antagonist also inhibited the migration and cytokine production of activated PBMCs. These results strongly suggest that the CXCR4 antagonist has a therapeutic effect on experimental colitis.
The CXCL12/CXCR4 axis is an efficacious leukocyte chemoattractant, which can attract lymphocytes and mononuclear cells from the bloodstream to the site of inflammation[24,25]. A previous study demonstrated that CXCL12 could promote natural killer cell migration into tissues by stimulating the production of matrix metalloproteinase-1 and subsequent degradation of the extracellular matrix proteins[26]. By provoking activation of protein kinase C, CXCL12 also induced adhesion of monocytes to human umbilical vein endothelial cells[25]. In patients with bursitis or acute lung injury, increased local CXCL12 and CXCR4 expression was associated with aggravated tissue damage, which could be attenuated by CXCL12 or CXCR4 blockade, suggesting a role of the CXCL12/CXCR4 chemokine axis in local inflammation[12-14,27].
Recently, several studies have been performed to investigate the role of the CXCL12/CXCR4 chemokine axis in IBD, particularly in UC. Katsuta et al[28] have previously demonstrated that the expression of CXCL12 mRNA in colonic mucosa of UC patients was higher than that in patients with Crohn’s disease. Dotan et al[17] also reported that CXCL12 and CXCR4 were constitutively expressed on human intestinal epithelial cells (IECs), and were upregulated in IBD IECs; CXCR4+ cells were increased in the lamina propria of IBD patients, and antibodies against CXCR4 or CXCL12 significantly blocked the migration of lamina propria and peripheral blood T cells, identifying an inflammatory role for the CXCL12/CXCR4 axis in the intestinal mucosa. In patients with refractory UC, increased CXCR4 expression was also observed on peripheral blood T cells[16]. In DSS-induced colitis, both CXCR4 expression on leukocytes and CXCL12 expression in colonic tissue were significantly increased; administration of a CXCR4 antagonist TF14016 ameliorates colonic inflammation in DSS-induced colitis and IL-10 knockout mice, and also reduced TNF-α and IFN-γ production in mesenteric lymph node cells, indicating a possible role of this chemokine axis in the pathophysiology of UC[4].
Consistent with previous reports, our present study also demonstrated marked mucosal damage and inflammatory responses in DSS-induced colitis. MPO activity, as an indicator of inflammatory infiltration, was significantly elevated 7 d after induction of colitis, in parallel with increased colonic TNF-α, IL-6, and IFN-γ. Administration of the CXCR4 antagonist, AMD3100, decreased the histological score, colonic cytokines, and inflammatory infiltration in DSS-induced colitis in mice. These results are in agreement with a previous report that the CXCR4 antagonist, TF14016, could also ameliorate DSS-induced colitis[4].
The intestinal epithelium is a single cell layer that forms the barrier between the body and the luminal gastrointestinal contents[29]. The central role of the intestinal epithelium is to limit access of toxins and microbes to underlying tissues, and intestinal barrier dysfunction has strong potential to result in IBD[30]. Accordingly, our results demonstrated that mice subjected to DSS developed significant intestinal barrier failure, as evidenced by a marked increase in gut permeability to FD4, while AMD3100 administration preserved the intestinal barrier function by reducing gut permeability. Although there is an unanswered question regarding IBD as to whether dysfunction of the intestinal barrier is a primary contributor to inflammation, or is a consequence of the action of inflammatory mediators[31], our present study clearly showed that AMD3100 treatment reduced both the inflammatory response and gut permeability in mice with colitis, identifying a therapeutic effect of the CXCR4 antagonist in experimental colitis.
A previous study also demonstrated that the maintenance of the intestinal epithelium was mainly dependent upon the regulation of proliferation and apoptosis[32]. Local massive apoptosis of epithelial cells would disturb the epithelial barrier, facilitate the infiltration of inflammatory cells, and aggravate mucosal damage[19,33]. In the present study, we found that the number of apoptotic cells in the colonic epithelium was markedly increased in DSS-induced colitis in mice. This was consistent with a previous study which demonstrated that apoptotic epithelial cells in the crypt of UC patients were much more abundant than in the control group[18]. Our present study also reported that AMD3100 treatment significantly decreased the presence of apoptotic cells in intestinal segments of DSS-induced colitis in mice, indicating that the CXCL12/CXCR4 axis also plays a role in epithelial apoptosis during the UC.
The infiltration of leucocytes into the colonic mucosa is believed to play an important role in tissue damage and clinical symptoms in both human and experimental UC[2,3]. Thus, in the present study, we further clarified the effect of the CXCR4 antagonist on migration and cytokine production of PBMCs. To mimic the in vivo inflammatory response, the PBMCs were pre-activated with PHA. We demonstrated that, although CXCL12 increased PBMC migration, the CXCR4 antagonist significantly attenuated the migration of PBMCs. In addition, AMD3100 moderately decreased cytokine production in PBMC culture. Taken together, the present data suggest that the amelioration of DSS-induced colitis by the CXCR4 antagonist can be mainly attributed to its effect on migration of PBMCs.
In conclusion, the present study demonstrated that the CXCR4 antagonist, AMD3100, decreased the severity of DSS-induced colitis in mice, by inhibiting the migration and cytokine production of activated PBMCs, and identified a therapeutic effect of the CXCR4 antagonist in experimental colitis. These data also suggested a pivotal role of the CXCL12/CXCR4 chemokine axis in the pathogenesis of UC.
Ulcerative colitis (UC) is characterized by frequent diarrheal attacks and anal bleeding. Histologic characteristics of UC are the invasion of the crypt epithelium and lamina propria by peripheral blood mononuclear cells (PBMCs), disruption of the epithelial lining, and consequently mucosal ulceration and crypt abscess formation in the bowel wall. Regulation of the migration of inflammatory leukocytes into the intestinal tissues is considered to be a therapeutic option for patients with UC.
Chemokine stromal cell-derived factor-1 receptor (CXCR4) is specific receptor for chemokine chemokine stromal cell-derived factor-1 (CXCL12), and the latter is a potent chemoattractant for PBMCs. The expression of CXCL12 and CXCR4 on intestinal epithelial cells, lamina propria T cells and PBMCs are significantly increased in UC patients, and block of CXCR4 ameliorates the colonic inflammation in experimental colitis. Whether a CXCR4 antagonist enhances epithelial barrier function, however, has not been unequivocally addressed.
A recent report has highlighted the importance of CXCR4 in colonic inflammation of experimental colitis. Whether CXCR4 plays a role in the epithelial barrier is so far uncharacterized. This is the first study to report that, in addition to inflammation inhibition, the CXCR4 antagonist, AMD3100, also decreased epithelial apoptosis and gut permeability in experimental colitis, and consequently enhanced the epithelial barrier function.
By understanding the role of CXCR4 in colonic inflammation and epithelial barrier, this study may represent a future strategy for therapeutic intervention in the treatment of patients with UC.
The authors present a concise argument for conducting the study which is the participation of the lymphocyte CXCR4 receptor in the augmentation of the immune response found in ulcerative colitis. Their contention that blocking this receptor with an experimental CXCR4 antagonist would ameliorate dextran sulfate sodium induced colitis was demonstrated by using the specific CXCR4 blocking agent known as AMD3100.The experimental model is well thought out and the conclusions are justified by the results obtained. The discussion is adequately developed and focused on the experimental results.
| 1. | Mazzon E, Cuzzocrea S. Absence of functional peroxisome proliferator-activated receptor-alpha enhanced ileum permeability during experimental colitis. Shock. 2007;28:192-201. |
| 2. | Buanne P, Di Carlo E, Caputi L, Brandolini L, Mosca M, Cattani F, Pellegrini L, Biordi L, Coletti G, Sorrentino C. Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol. 2007;82:1239-1246. |
| 3. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. |
| 4. | Mikami S, Nakase H, Yamamoto S, Takeda Y, Yoshino T, Kasahara K, Ueno S, Uza N, Oishi S, Fujii N. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008;327:383-392. |
| 5. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. |
| 6. | Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. 1999;155:331-336. |
| 7. | Alzoghaibi MA, Al-Mofleh IA, Al-Jebreen AM. Neutrophil chemokines GCP-2 and GRO-alpha in patients with inflammatory bowel disease. J Dig Dis. 2008;9:144-148. |
| 8. | Suzuki K, Kawauchi Y, Palaniyandi SS, Veeraveedu PT, Fujii M, Yamagiwa S, Yoneyama H, Han GD, Kawachi H, Okada Y. Blockade of interferon-gamma-inducible protein-10 attenuates chronic experimental colitis by blocking cellular trafficking and protecting intestinal epithelial cells. Pathol Int. 2007;57:413-420. |
| 9. | Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O, Matsushima K, Yoshida N, Springer TA, Kishimoto T. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci USA. 1996;93:14726-14729. |
| 10. | Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591-594. |
| 11. | Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595-599. |
| 12. | Haas CS, Martinez RJ, Attia N, Haines GK 3rd, Campbell PL, Koch AE. Chemokine receptor expression in rat adjuvant-induced arthritis. Arthritis Rheum. 2005;52:3718-3730. |
| 13. | Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD, Rai PR, Brown KK, Weiss DJ, Poynter ME. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148-8157. |
| 14. | Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. J Immunol. 2000;165:499-508. |
| 15. | Ding Z, Jia SH, Marshall JC, Downey GP, Waddell TK. Up-regulation of functional CXCR4 expression on human lymphocytes in sepsis. Crit Care Med. 2006;34:3011-3017. |
| 16. | Nakase H, Mikami S, Chiba T. Alteration of CXCR4 expression and Th1/Th2 balance of peripheral CD4-positive T cells can be a biomarker for leukocytapheresis therapy for patients with refractory ulcerative colitis. Inflamm Bowel Dis. 2009;15:963-964. |
| 17. | Dotan I, Werner L, Vigodman S, Weiss S, Brazowski E, Maharshak N, Chen O, Tulchinsky H, Halpern Z, Guzner-Gur H. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16:583-592. |
| 18. | Shichijo K, Ihara M, Razzaque MS, Matsuu-Matsuyama M, Nakayama T, Nakashima M, Sekine I. Expression of apoptotic epithelial cells within lamina propria beneath the basement membrane triggers dextran sulfate sodium-induced colitis. Dig Dis Sci. 2008;53:2443-2451. |
| 19. | Heller F, Fromm A, Gitter AH, Mankertz J, Schulzke JD. Epithelial apoptosis is a prominent feature of the epithelial barrier disturbance in intestinal inflammation: effect of pro-inflammatory interleukin-13 on epithelial cell function. Mucosal Immunol. 2008;1 Suppl 1:S58-S61. |
| 20. | Garcia Soriano F, Liaudet L, Marton A, Haskó G, Batista Lorigados C, Deitch EA, Szabó C. Inosine improves gut permeability and vascular reactivity in endotoxic shock. Crit Care Med. 2001;29:703-708. |
| 21. | Qin X, Caputo FJ, Xu DZ, Deitch EA. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock. 2008;29:372-376. |
| 22. | Cuzzocrea S, Ayroldi E, Di Paola R, Agostini M, Mazzon E, Bruscoli S, Genovese T, Ronchetti S, Caputi AP, Riccardi C. Role of glucocorticoid-induced TNF receptor family gene (GITR) in collagen-induced arthritis. FASEB J. 2005;19:1253-1265. |
| 23. | Moskovits N, Kalinkovich A, Bar J, Lapidot T, Oren M. p53 Attenuates cancer cell migration and invasion through repression of SDF-1/CXCL12 expression in stromal fibroblasts. Cancer Res. 2006;66:10671-10676. |
| 24. | Okabe S, Fukuda S, Kim YJ, Niki M, Pelus LM, Ohyashiki K, Pandolfi PP, Broxmeyer HE. Stromal cell-derived factor-1alpha/CXCL12-induced chemotaxis of T cells involves activation of the RasGAP-associated docking protein p62Dok-1. Blood. 2005;105:474-480. |
| 25. | Takahashi K, Shimokado K, Yoshida M. SDF-1-induced adhesion of monocytes to vascular endothelium is modulated by azelnidipine via protein kinase C inhibition. Eur J Pharmacol. 2006;552:162-169. |
| 26. | Goda S, Inoue H, Umehara H, Miyaji M, Nagano Y, Harakawa N, Imai H, Lee P, Macarthy JB, Ikeo T. Matrix metalloproteinase-1 produced by human CXCL12-stimulated natural killer cells. Am J Pathol. 2006;169:445-458. |
| 27. | Kim YS, Bigliani LU, Fujisawa M, Murakami K, Chang SS, Lee HJ, Lee FY, Blaine TA. Stromal cell-derived factor 1 (SDF-1, CXCL12) is increased in subacromial bursitis and downregulated by steroid and nonsteroidal anti-inflammatory agents. J Orthop Res. 2006;24:1756-1764. |
| 28. | Katsuta T, Lim C, Shimoda K, Shibuta K, Mitra P, Banner BF, Mori M, Barnard GF. Interleukin-8 and SDF1-alpha mRNA expression in colonic biopsies from patients with inflammatory bowel disease. Am J Gastroenterol. 2000;95:3157-3164. |
| 29. | Edelblum KL, Washington MK, Koyama T, Robine S, Baccarini M, Polk DB. Raf protects against colitis by promoting mouse colon epithelial cell survival through NF-kappaB. Gastroenterology. 2008;135:539-551. |
| 30. | McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100-113. |
| 31. | Welcker K, Martin A, Kölle P, Siebeck M, Gross M. Increased intestinal permeability in patients with inflammatory bowel disease. Eur J Med Res. 2004;9:456-460. |
| 32. | Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569-3577. |
| 33. | Yan F, John SK, Wilson G, Jones DS, Washington MK, Polk DB. Kinase suppressor of Ras-1 protects intestinal epithelium from cytokine-mediated apoptosis during inflammation. J Clin Invest. 2004;114:1272-1280. |
Peer reviewers: Dr. Jianyuan Chai, PhD, MS, BS, Assistant Professor, Research (09-151), VA Long Beach Healthcare System, 5901 E. 7th St, Long Beach, CA 90822, United States; Didier Merlin, PhD, Associate Professor, Department of Medicine Division of Digestive Diseases, Emory University, 615 Michael Street, Atlanta, GA 30322, United States; Jay Pravda, MD, Inflammatory Disease Research Center, Gainesville, Florida, 32614-2181, United States
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH