Published online Jun 14, 2010. doi: 10.3748/wjg.v16.i22.2735
Revised: March 30, 2010
Accepted: April 6, 2010
Published online: June 14, 2010
Acute pancreatitis is a common disease characterized by sudden upper abdominal pain and vomiting. Alcoholism and choledocholithiasis are the most common factors for this disease. The choice of treatment for acute pancreatitis might be affected by local complications, such as local hemorrhage in or around the pancreas, and peripancreatic infection or pseudoaneurysm. Diagnostic imaging modalities for acute pancreatitis have a significant role in confirming the diagnosis of the disease, helping detect the extent of pancreatic necrosis, and for diagnosing local complications. Magnetic resonance imaging (MRI) might be indicated in acute pancreatitis for detecting and characterizing local complications of acute pancreatitis that involve necrotic, hemorrhagic, infectious, vascular, and pseudocyst disorders. The general MRI sequences for pancreatitis require the combined use of T1-weighted, T2-weighted sequences, and magnetic resonance cholangiopancreatography. For imaging of pancreatic necrosis, the combination of T1-weighted and T2-weighted findings with dynamic contrast-enhanced imaging gives a comprehensive evaluation of the extent of necrosis and full range of inflammatory extension. For imaging of infectious complications, dynamic contrast-enhanced examinations might help differentiate pancreatic cellulitis or abscesses, from pancreatic fluid collection or simple pseudocysts. For vascular abnormalities, the combination of cross-sectional pancreatic parenchyma imaging with MRA represents a single diagnostic modality for the full evaluation of peripancreatic artery and vein involvement, such as arterial pseudoaneurysms and venous thromboses. The purpose of this pictorial review is to examine the MRI appearances of various local complications of acute pancreatitis and to discuss the practical setup of MRI in local complications of acute pancreatitis.
- Citation: Xiao B, Zhang XM, Tang W, Zeng NL, Zhai ZH. Magnetic resonance imaging for local complications of acute pancreatitis: A pictorial review. World J Gastroenterol 2010; 16(22): 2735-2742
- URL: https://www.wjgnet.com/1007-9327/full/v16/i22/2735.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i22.2735
Acute pancreatitis is a common and protean disease characterized by sudden upper abdominal pain and vomiting, and is triggered by the leakage of activated pancreatic digestive enzymes. Alcoholism and choledocholithiasis are the most common physiological factors for acute pancreatitis[1,2]. Traditional management of acute pancreatitis tends to promote the use of conservative management. However, the choice of treatment can be influenced by local complications, such as local hemorrhage in or around the pancreas, and peripancreatic infection or pseudoaneurysm[2,3].
Increased levels of serum and/or urinary pancreatic amylase and lipase have been detected in most individuals with acute pancreatitis after the onset of symptoms. Therefore, acute pancreatitis is often diagnosed by clinical manifestations and laboratory examinations. However, diagnostic imaging modalities for acute pancreatitis have a significant role in confirming the diagnosis of the disease, helping detect the extent of pancreatic necrosis, and for diagnosing local complications[2,3].
Among a variety of imaging tools, computed tomography (CT) is considered the standard of reference for patients with acute pancreatitis; however, it has an increased radiation burden that can result from follow-up examinations[4] and displays different results for the potential aggravation of acute pancreatitis resulting from the use of iodinated contrast media[5-7]. The specific reasons for using magnetic resonance imaging (MRI) are as follows: (1) MRI is a diagnostic imaging method with no radiation hazard, which might be suitable for patients with multiple follow-up reviews; (2) MRI is a reliable method of staging the severity of acute pancreatitis, which has predictive value for the prognosis of the disease. It also has fewer contraindications than CT[8]; (3) MR cholangiopancreatography (MRCP) has the unique capability of providing noninvasive images of the pancreatic ducts and can demonstrate possible communication of a pancreatic pseudocyst with pancreatic ducts[9]; and (4) MRI in combination with MR angiography (MRA), a single diagnostic modality, is useful for assessing the signal consistency of fluid exudation or pseudocysts to identify local hemorrhage in or around the pancreas or pseudoaneurysm, which might help plan the surgery.
However, the possible drawbacks of MRI with respect to CT are cost and time consumption. Moreover, several MRI sequences require patients to hold their breath, which might be difficult for patients with severe acute pancreatitis.
MRI of pancreatitis and its complications has been reported[10]. However, to our knowledge, there is no report thoroughly assessing MRI for local complications of acute pancreatitis, including necrotic, hemorrhagic, infectious, vascular, and pseudocyst disorders.
In this pictorial essay, after briefly discussing the MRI technique, we review the MRI appearances of various local complications of acute pancreatitis, ranging from relatively common necrotic and hemorrhagic conditions to rare conditions, such as peripancreatic abscess and pseudoaneurysm. Knowledge of the classic MRI findings of these complications allows prompt recognition of the related pathologic condition, and might influence the choice of treatment.
For the appropriate evaluation of local complications of acute pancreatitis, it is necessary to assess all parts of the pancreas, including the peripancreatic parenchyma and the vasculature[1]. The general MRI sequences for pancreatitis require the combined use of T1-weighted sequences [e.g. fast spin-echo (FSE) imaging with multiple breath-hold acquisitions or single-breath-hold gradient echo (GRE) imaging], T2-weighted sequences [e.g. fast recovery fast spin-echo (FRFSE) or single-shot fast spin-echo (SSFSE) imaging], and MRCP (e.g. a thick-slab, SSFSE MRCP). The advantages or limitations of these sequences are as follows: (1) T1-weighted scan with fat suppression improves the delineation of pancreatic borders and the pancreas itself, and it has important value in MRI acquisition for evaluation of hemorrhagic complications of acute pancreatitis[3]. However, T1-weighted sequences with breath holding require patient cooperation, otherwise, respiratory artifacts will occur, which might affect the visualization of the whole pancreas and its adjacent structure[1-3]; (2) T2-weighted sequences have significant advantages in demonstrating fluid-filled lesions in or around the pancreas and the pancreatic duct, and SSFSE T2-weighted sequence can be used to guide acquisition of an MRCP series[2,3]. Using respiratory triggered or navigator-echo-based techniques allows the investigating of less cooperative patients; and (3) MRCP is obtained before gadolinium administration, allows noninvasive evaluation of pancreatic ducts and the whole extrahepatic biliary tract, and provides few respiratory artifacts or susceptibility effects[1-3]. However, the main weakness of MRCP for ductal visibility is the potential overlap of other fluid-containing organs (e.g. the stomach and duodenum)[3].
The methodology of contrast-enhanced MRI for patients with acute pancreatitis in our institute is detailed as follows. A 19-gauge needle was placed in the right antecubital vein. A dose of 0.2 mL/kg Gd-DTPA (469.01 mg/mL Bayer Schering Pharma AG; Berlin, Germany) was then administered. A bolus of 20 mL of physiological saline was given immediately following gadolinium administration. The contrast agent and physiological saline were injected at an infusion rate of 3 mL/s with a power injector (Spectris MR Injector System; Medrad, USA). After gadolinium chelate injection, the optimal dynamic contrast-enhanced method [e.g. T1-weighted acquisition performed with liver acquisition with volume acceleration (LAVA)] was used to assess the extent of pancreatic necrosis for the initial staging of acute pancreatitis, which has been shown to correlate with the course of the disease[1-3]. MRI LAVA scanning starts at 14-16 s after the intravenous administration of gadolinium. It costs a total of 65 s for three-phase dynamic contrast-enhanced scans (15 s for each phase and 10 s of the interval of phases for breathing). A total of (80-112) × 3 contrast-enhanced frames were obtained. Moreover, MRA, the post-processing technique after MRI LAVA, can be performed to supplement the information for visualization of pancreatic vascular network and vascular complications of acute pancreatitis. The detailed protocols used with our 1.5-T MR imager for these sequences are listed in Table 1.
| Sequence | TR (ms) | TE (ms) | Slice thickness (mm) | Slice space (mm) | Matrix | Field of view (mm) | Number of excitation | Flip angle |
| FSE T1W | 300 | 10 | 5 | 0.5-1 | 256 × 192 | 380 × 340 | 2 | 90° |
| GRE T1W | 195 | 1.5 | 5-7 | 0.5-1 | 256 × 192 | 360 × 340 | 1 | 70° |
| FRFSE T2W | 8000-13 500 | 90-120 | 5 | 0.5-1 | 256 × 224 | 400 × 340 | 1 | 90° |
| SSFSE T2W | Not applicable | 80-197 | 5 | 0.5 | 256 × 192 | 390 × 330 | 1 | 90° |
| SSFSE MRCP | Not applicable | 830-1100 | 40-50 | 0 | 256 × 192 | 320 × 240 | 2 | 90° |
| LAVA T1W | 3.8-6.1 | 1.8-2.1 | 51 | 0 | 256 × 224 | 380 × 340 | 1 | 15°-20° |
Pancreatic necrosis refers to a pathological collection of devitalized tissue in the pancreas, which is a relatively common local complication of acute pancreatitis[10].
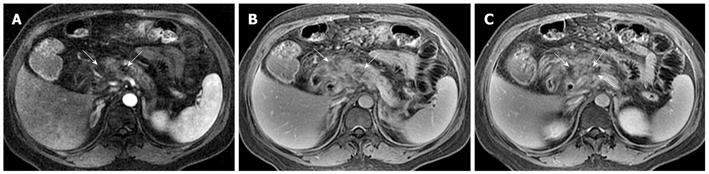
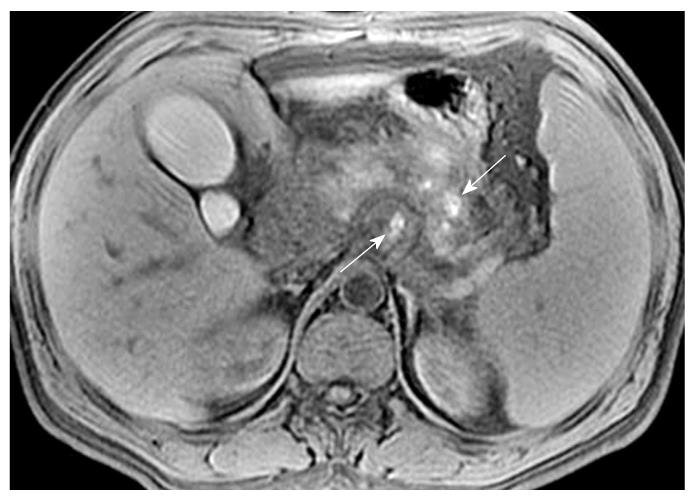
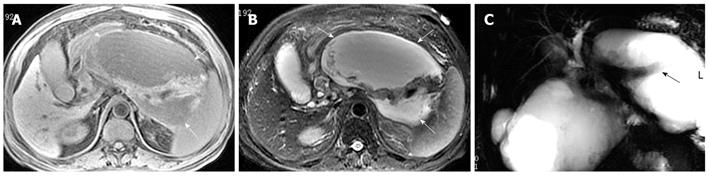
Necrosis is diagnosed when focal or diffuse, conspicuous hypointense areas on T1WI corresponding to hyperintense areas on T2WI and well-marginated areas of non-enhancing pancreatic parenchyma in comparison to the signal intensity of the normal pancreatic parenchyma are present[1-3]. Dynamic contrast-enhanced MRI examination can identify surviving pancreatic tissue[10]. The focal pancreatic necrosis is characterized by multiple spotted, patchy non-enhancing areas (like “pepper”) on contrast-enhanced MR images (Figure 1). The large diffuse non-enhanced area of the pancreas (the so-called “black pancreas”) in acute pancreatitis on enhanced MR images represents diffuse pancreatic necrosis (Figure 2).
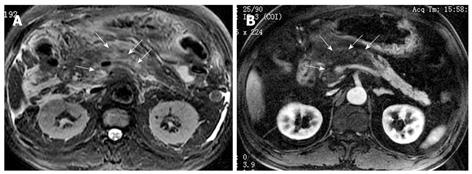
Necrosis of peripancreatic fatty tissue, which is a form of inflammatory extension, involves the spread of peripancreatic intra-abdominal fatty tissue and adipose tissue in the retroperitoneal space[2]. The covering of the pancreas is surrounded by the formation of the capsule, which is only a series of loose connective tissue. Therefore, the pancreatic capsule forms almost no block on the role of inflammatory extension. MRI might show the intra-abdominal inflammatory involvement located in omental or mesenteric fatty contents regions (Figure 3). The affected retroperitoneal space manifests the inflammation extension from the retroperitoneal space to anterior pararenal space of the left kidney, which is complicated by retroperitoneal steatonecrosis (Figure 4)[2,3]. It is important and helpful in evaluating the prognosis of acute pancreatitis to differentiate between necrosis of peripancreatic tissue and central pancreas necrosis. Neoptolemos et al[11] reported that the evaluation of the integrity of the main pancreatic duct might be helpful in making a distinction between mild pancreatitis and severe pancreatitis, and for determining the necessity for surgery for local pancreatic complications in severe pancreatitis.
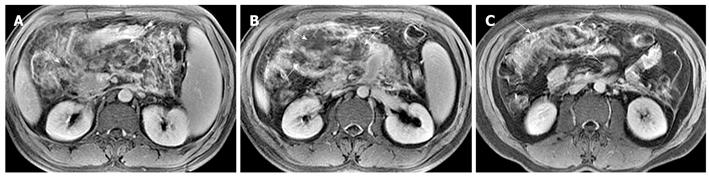
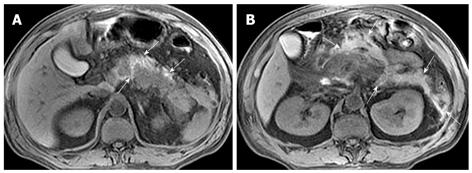
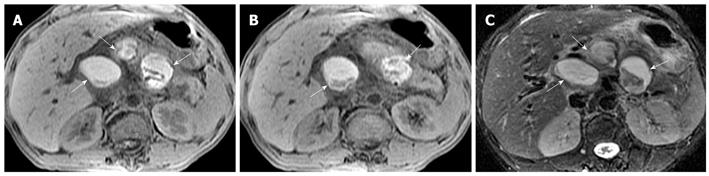
Hemorrhage of the pancreas is seen in 2%-5% of patients with acute pancreatitis and might occur in the setting of severe necrotizing pancreatitis[10]. With conversion from hemoglobin to methemoglobin in the hemorrhage regions, MRI findings of hemorrhagic lesions show spotted or patchy high signal intensity (like “salt”) in T1-weighted images with fat suppression (Figure 5).
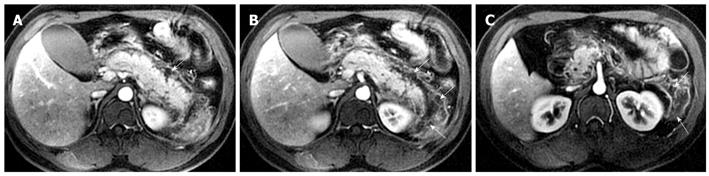
Peripancreatic hemorrhage might be secondary to the necrosis of peripancreatic tissue, which is visualized as patchy or large diffuse areas with hyperintensity on T1-weighted images with fat suppression[2] (Figure 6). Martin et al[12] reported that elevated peripancreatic signal on fat-suppressed T1-weighted spoiled gradient echo images is associated with poor outcome in acute pancreatitis. This might represent a simplified imaging method in regards to its relationship to clinical prognosis. Other local complications of acute pancreatitis, such as pancreatic pseudocysts and peripancreatic vascular invasion, can also show hemorrhage (mentioned below).
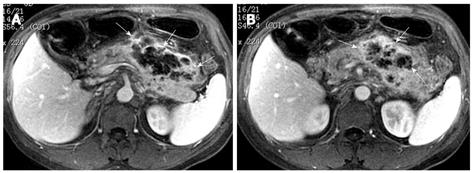
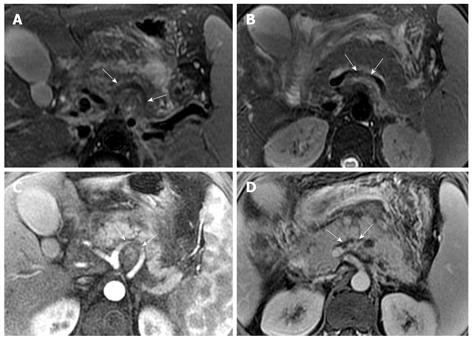
Pancreatic cellulitis is an infectious complication in patients with acute pancreatitis, whose incidence varies from 8.3% to 10.6%, and can be difficult to differentiate clinically from pancreatic abscess[13,14]. It is a clinical or radiological entity in the pancreas and peripancreatic region as a result of pathological pancreatic swelling, inflammatory cell infiltration, and tissue necrosis[15]. Contrast-enhanced MRI examination can show the range of the involvement of pancreatic cellulitis. MRI findings of pancreatic cellulitis include the formation of an ill-defined mass with ring-like and separated enhancement (like “Hornet’s nest”), and non-enhanced regions within the central part of the mass (Figure 7). Pancreatic cellulitis can be absorbed completely and might also progress to a pancreatic abscess.
Pancreatic abscess is a typical infectious manifestation of acute necrotizing pancreatitis, whose incidence varies from 1% to 9%, and can result in high mortality in acute pancreatitis patients[16]. If pancreatic cellulitis was not well controlled, abscesses might occur 3-5 wk after the onset of acute pancreatitis and might be similar to pseudocysts[10]. Pancreatic abscesses with acute pancreatitis induce a systemic infection poisoning reaction resulting in symptoms of fever and local peritonitis. The performance of a pancreatic abscess on MRI might present as an encapsulated liquid-form mass with a significantly enhancing wall (Figure 8).
Pancreatic pseudocysts are local complications of acute or chronic pancreatitis, and they can be classified as acute pseudocysts in patients suffering from acute post-necrotic pancreatitis, and chronic pseudocysts that develop in patients already suffering from chronic pancreatitis[17]. The pathogenesis of pancreatic pseudocysts might be associated with an actively secreting pancreas in acute or chronic pancreatitis; an encapsulated cyst communicating with pancreatic ducts and/or partial pancreatic ductal obstruction. Patients with pancreatic pseudocysts are treated conservatively or percutaneously with aspiration under ultrasound guidance or surgically with external catheter drainage[17].
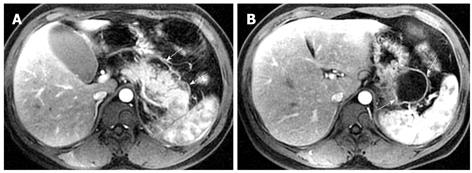
Pancreatic pseudocysts might be located within the pancreatic tissue or surrounding the pancreas. They often occur approximately four weeks after a first episode of acute pancreatitis[18-20]. MRI findings of a simple pseudocyst include a round or oval fluid collection surrounded by a thin or thick wall, with liquid signal performance of hypointensity on T1-weighted images and hyperintensity on T2-weighted images (Figure 9). Pancreatic pseudocysts can also present as complex pseudocysts associated with mucus, protein, and hemorrhage[2]. This often manifests as a heterogeneous lesion dominated by hyperintensity on T1-weighted images with fat suppression (Figure 10). Therefore, compared with CT, MRI might be more favorable when the a suspected pseudocyst with bleeding occurs.
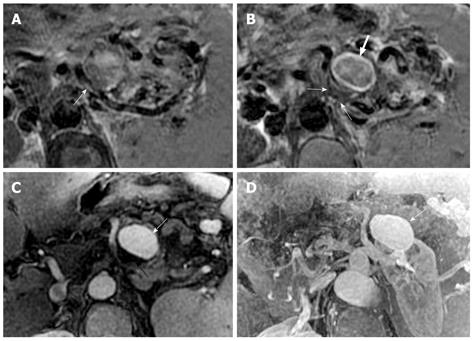
Vascular complications of acute pancreatitis represent a spectrum of vascular abnormalities, such as peripancreatic artery involvement including pseudoaneurysm, and vein invasion including phlebothrombosis[1-4]. Dörffel et al[21] assessed vascular complications in acute pancreatitis by color duplex ultrasonography: the incidence of arterial pseudoaneurysms was 7% in acute pancreatitis, and the incidence of venous thromboses was 30% in acute non-necrotizing pancreatitis and 57% in necrotizing pancreatitis. MRI findings of artery invasion consist of the loss of normal vascular flowing void effect, the blurred edge of the local involving arterial wall and the poor enhancement at the involving segment on contrast-enhanced arterial phase images (Figure 11). The splenic artery, gastroduodenal artery, and pancreaticoduodenal artery are more frequently involved[10,22].
Pseudoaneurysm is an uncommon, usually delayed, complication of acute pancreatitis and might constitute a life-threatening emergency if rupture occurs[10]. MRI can directly show the pseudoaneurysm cavity that communicates with the adjoining arteries and the mural thrombosis in the pseudoaneurysm cavity. On enhanced MR images, the enhancement of pseudoaneurysm cavity corresponds with the arteries, whereas the non-enhanced region is illustrated as the mural thrombus (resembling a Chinese “Yin-Yang” diagram)[23] (Figure 12).
The splenic vein is the most common vein invaded by pancreatic inflammatory extensions due to its proximity to the pancreas[10,22]. The involving vein segment might be complicated by local venous thrombosis. MRI shows the loss of normal vascular flowing void effect at this involving vein segment. After administration of a contrast agent, an intravenous filling defect can be seen on enhanced venous phase images (Figure 11).
Cross-sectional T2-weighted imaging and contrast-enhanced fat-suppressed T1-weighted imaging combined with MRCP can be used to assess the severity of acute pancreatitis before therapeutic planning[1-3]. However, dynamic contrast-enhanced MRI in acute pancreatitis is not routinely performed, because, for the majority of patients, pancreatic change is easy to establish with various T1-weighted and T2-weighted sequences in transverse and coronal sections. Therefore, in our current pancreatic imaging practice, the decision process as to whether patients receive dynamic studies is as follows: (1) For imaging of pancreatic necrosis, the combination of T1-weighted and T2-weighted findings with dynamic contrast-enhanced appearances represents a comprehensive evaluation of the extent of necrosis and full range of inflammatory extension. We recommend that enhanced-MRI is essential; (2) For imaging of infectious complications, dynamic contrast-enhanced examinations might help differentiate pancreatic cellulitis or abscesses from pancreatic fluid collection or simple pseudocysts; and (3) For imaging of vascular abnormalities, the combination of cross-sectional pancreatic parenchyma imaging with MRA offers the possibility of a single diagnostic modality for the full evaluation of peripancreatic artery and vein involvement, such as arterial pseudoaneurysms and venous thromboses.
In summary, MRI might be indicated in acute pancreatitis for detecting and characterizing local complications of acute pancreatitis. Enhanced-MRI is essential for a comprehensive evaluation of the extent of pancreatic necrosis.
| 1. | Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297-306. |
| 2. | Lenhart DK, Balthazar EJ. MDCT of acute mild (nonnecrotizing) pancreatitis: abdominal complications and fate of fluid collections. AJR Am J Roentgenol. 2008;190:643-649. |
| 3. | Matos C, Cappeliez O, Winant C, Coppens E, Devière J, Metens T. MR imaging of the pancreas: a pictorial tour. Radiographics. 2002;22:e2. |
| 4. | Blackstone MO. Contrast medium worsens pancreatitis? Gastroenterology. 1994;107:321-322. |
| 5. | McMenamin DA, Gates LK Jr. A retrospective analysis of the effect of contrast-enhanced CT on the outcome of acute pancreatitis. Am J Gastroenterol. 1996;91:1384-1387. |
| 6. | Uhl W, Roggo A, Kirschstein T, Anghelacopoulos SE, Gloor B, Müller CA, Malfertheiner P, Büchler MW. Influence of contrast-enhanced computed tomography on course and outcome in patients with acute pancreatitis. Pancreas. 2002;24:191-197. |
| 7. | Hwang TL, Chang KY, Ho YP. Contrast-enhanced dynamic computed tomography does not aggravate the clinical severity of patients with severe acute pancreatitis: reevaluation of the effect of intravenous contrast medium on the severity of acute pancreatitis. Arch Surg. 2000;135:287-290. |
| 8. | Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Devière J. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715-723. |
| 9. | Gillams AR, Kurzawinski T, Lees WR. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol. 2006;186:499-506. |
| 10. | Miller FH, Keppke AL, Dalal K, Ly JN, Kamler VA, Sica GT. MRI of pancreatitis and its complications: part 1, acute pancreatitis. AJR Am J Roentgenol. 2004;183:1637-1644. |
| 11. | Neoptolemos JP, London NJ, Carr-Locke DL. Assessment of main pancreatic duct integrity by endoscopic retrograde pancreatography in patients with acute pancreatitis. Br J Surg. 1993;80:94-99. |
| 12. | Martin DR, Karabulut N, Yang M, McFadden DW. High signal peripancreatic fat on fat-suppressed spoiled gradient echo imaging in acute pancreatitis: preliminary evaluation of the prognostic significance. J Magn Reson Imaging. 2003;18:49-58. |
| 13. | Fan ST, Choi TK, Chan FL, Lai EC, Wong J. Pancreatic phlegmon: what is it? Am J Surg. 1989;157:544-547. |
| 14. | Maroun Marun C, Uscanga L, Lara F, Passareli L, Quiroz-Ferrari F, Robles-Díaz G, Campuzano-Fernández M. [Pancreatic phlegmon: a potentially fatal form of acute pancreatitis]. Rev Invest Clin. 1992;44:507-512. |
| 15. | Kune GA, King R. The late complications of acute pancreatitis: pancreatic swelling, cyst and abscess. Med J Aust. 1973;1:1241-1246. |
| 16. | Hill MC, Dach JL, Barkin J, Isikoff MB, Morse B. The role of percutaneous aspiration in the diagnosis of pancreatic abscess. AJR Am J Roentgenol. 1983;141:1035-1038. |
| 17. | Andrén-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part I: classification, pathophysiology, anatomic considerations and treatment. JOP. 2004;5:8-24. |
| 18. | Merkle EM, Görich J. Imaging of acute pancreatitis. Eur Radiol. 2002;12:1979-1992. |
| 19. | Robinson PJ, Sheridan MB. Pancreatitis: computed tomography and magnetic resonance imaging. Eur Radiol. 2000;10:401-408. |
| 20. | Kim YH, Saini S, Sahani D, Hahn PF, Mueller PR, Auh YH. Imaging diagnosis of cystic pancreatic lesions: pseudocyst versus nonpseudocyst. Radiographics. 2005;25:671-685. |
| 21. | Dörffel T, Wruck T, Rückert RI, Romaniuk P, Dörffel Q, Wermke W. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas. 2000;21:126-133. |
| 22. | Mortelé KJ, Mergo PJ, Taylor HM, Wiesner W, Cantisani V, Ernst MD, Kalantari BN, Ros PR. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol. 2004;52:67-72. |
Peer reviewers: Kiichi Tamada, MD, Department of Gastroenterology, Jichi Medical Shool, 3311-1 Yakushiji, Minamikawachi, Kawachigun, Tochigi 329-0498, Japan; Massimo Falconi, MD, Chirurgia B, Department of Anesthesiological and Surgical Sciences Policlinico GB Rossi, Piazzale LA Scuro, 37134 Verona, Italy
S- Editor Tian L L- Editor Stewart GJ E- Editor Ma WH