Published online May 7, 2010. doi: 10.3748/wjg.v16.i17.2134
Revised: January 5, 2010
Accepted: January 12, 2010
Published online: May 7, 2010
AIM: To evaluate the in vitro immunomodulation capacity of various non-pathogenic yeast strains and to investigate the ability of some of these food grade yeasts to prevent experimental colitis in mice.
METHODS: In vitro immunomodulation was assessed by measuring cytokines [interleukin (IL)-12p70, IL-10, tumor necrosis factor and interferon γ] released by human peripheral blood mononuclear cells after 24 h stimulation with 6 live yeast strains (Saccharomyces ssp.) and with bacterial reference strains. A murine model of acute 2-4-6-trinitrobenzene sulfonic acid (TNBS)-colitis was next used to evaluate the distinct prophylactic protective capacities of three yeast strains compared with the performance of prednisolone treatment.
RESULTS: The six yeast strains all showed similar non-discriminating anti-inflammatory potential when tested on immunocompetent cells in vitro. However, although they exhibited similar colonization patterns in vivo, some yeast strains showed significant anti-inflammatory activities in the TNBS-induced colitis model, whereas others had weaker or no preventive effect at all, as evidenced by colitis markers (body-weight loss, macroscopic and histological scores, myeloperoxidase activities and blood inflammatory markers).
CONCLUSION: A careful selection of strains is required among the biodiversity of yeasts for specific clinical studies, including applications in inflammatory bowel disease and other therapeutic uses.
- Citation: Foligné B, Dewulf J, Vandekerckove P, Pignède G, Pot B. Probiotic yeasts: Anti-inflammatory potential of various non-pathogenic strains in experimental colitis in mice. World J Gastroenterol 2010; 16(17): 2134-2145
- URL: https://www.wjgnet.com/1007-9327/full/v16/i17/2134.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i17.2134
Yeasts, as an inevitable part of the microflora of various fermented foods and beverages, are found in a wide range of foods from plant or animal origin, where they have a significant impact on food safety and organoleptic properties. Both baker’s and brewer’s yeasts [Saccharomyces cerevisiae (S. cerevisiae)] have been available for the last decades as dietary supplements because of their high contents of vitamin B, proteins, peptides, amino-acids and trace minerals. Regardless of their non-human origin, such non-pathogenic yeasts fulfill the major criteria for probiotic definition[1]. Interest in probiotic yeasts has increased, especially in relation to animal feed but also for human applications. Hence, yeasts are rarely (if ever) associated with outbreaks or cases of food-borne illness and, through their history, many yeast species are recognized as safe, as confirmed by their Qualified Presumption of Safety status, assigned by the European Food Safety Authority (http://www.efsa.europa.eu). However, some rare cases of Saccharomyces boulardii (S. boulardii) fungemia have been reported, although they are essentially restricted to immunocompromised patients or associated with patients being contaminated through a central venous catheter[2]. Also, identification of the contaminating yeast strains in some cases could be questioned, as it was often based on cross-reacting anti-yeast mannan circulating antibodies, shared with Candida species.
Although S. boulardii was originally selected using rather empiric methods, this yeast species has many proven benefits in various gastrointestinal diseases[3-5] and is well recognized as the non-bacterial prototype of a probiotic. Multiple mechanisms have been suggested that explain the broad health-promoting effects of consuming food grade yeasts, ranging from local general trophic effects to action on both innate and/or adaptive immunity[6,7]. The yeast-induced probiotic signals might be either prophylactic or therapeutic in a defined pathologic context or in a specific application.
Most reported effects of yeasts as probiotic organisms in clinical trials stand for alleviation of (1) antibiotic-associated diarrhea; (2) infectious diarrhea [including recurrent Clostridium difficile (C. difficile) related diseases, CDAD]; (3) irritable bowel syndrome; and (4) inflammatory bowel diseases (IBD). Focusing on IBD, S. boulardii efficacy was both observed in Crohn’s disease[8] and by favoring remission of ulcerative colitis[9]. Most of the therapeutic approaches in IBD were also using anti-inflammatory drugs such as mesalamine concomitantly and the exclusive use of yeast as possible ecological treatment has not been tested, most probably for ethical reasons. The anti-inflammatory potential of yeasts has been addressed by many in vitro studies elaborating on multiple mechanisms[4,7]. Surprisingly, data using yeasts in animal models of colitis are very scarce and the precise mechanism(s) of action by which such intervention may exert its beneficial effects in vivo is consequently poorly documented. Dalmasso et al[10] have reported a beneficial action of secreted yeast molecules on T cell migration in the chronic model of CD4+CD45RBhi T-lymphocytes transfer into SCID mice. The same authors have also reported the preventive effect of S. boulardii in an acute murine 2-4-6-trinitrobenzene sulfonic acid (TNBS) model, characterized by a strong inhibitory activation of NFκB and the reduction of mRNA for pro-inflammatory cytokines together with decreased histological scores of inflammation[11,12]. Consistently, a similar TNBS-induced model of colitis, although developed for a more therapeutic approach in rats, also showed appreciable reduction of histological scores and pro-inflammatory colonic markers by S. boulardii (including cytokines but also metabolites of pro-oxidative pathways such as iNOS). The proposed mechanism depended on PPAR activation by the probiotic yeast[13], a regulator previously demonstrated to reduce intestinal inflammation[14]. Finally, yeast-driven therapy in a DSS-induced colitis model was also successfully demonstrated in mice[15], even when this chemically induced colitis was amplified by Candida albicans colonization. Because the model is known to affect epithelial crypt cells and destroy barrier integrity without intervention of the adaptive immune system[16], it was suggested that the contribution of yeast-mediated protection was restricted to the switching off of the innate mechanisms of colitis and/or to the enhancement of the mucosal barrier integrity.
Very few yeast strains have been studied as possible biotherapeutics agents and, at the moment, S. boulardii is the only yeast commercialized for human use, and consequently the single preparation formally recognized as probiotic. However, other Saccharomyces spp. or members of other yeast genera with similar or possibly better therapeutic properties will certainly be isolated in the future[17,18]. Hence, distinct non pathogenic yeast strains may have their own impact on gut homeostasis, sharing some of the common mechanisms of S. boulardii, but also other more specific consequences or even novel prophylactic or therapeutic effects.
Considering the widespread use of lactic acid bacteria and bifidobacteria as probiotics, it is now well accepted that all strains are not equally beneficial, that each may have individual mechanisms of action. Furthermore, host characteristics (e.g. flora composition or immune status) may determine which probiotic species or strains may be optimal[19]. Peran et al[19] conclude that it would be interesting to compare different probiotics in the same experimental model, in order to establish the best profile in a given setting. Likewise, this reasoning can be extent towards “friendly yeasts” as far as evaluation is conducted for a specific use without generalizations about probiotic effects[20,21].
In this paper, we compared yeast strains in their ability to induce cytokines on human peripheral blood mononuclear cells (PBMC). Our results also demonstrated that some yeast strains exhibit significant anti-inflammatory activities in vivo; whereas other may have weaker inflammation lowering effects or no anti-inflammatory effect at all. Thus, as for probiotic bacteria[20], strain-specific differences were seen for yeasts, suggesting that careful selection of strains for therapeutic use is required for preclinical and further clinical studies, including for applications in IBD.
All Saccharomyces strains used in this study and listed (Table 1), including baker’s yeast S. boulardii and wine-related strains, were originally isolated from a proprietary germoplasm bank of S. cerevisiae (Societe Industrielle Lesaffre 147 Rue Gabriel Peri, BP 6027, 59700 Marcq-en-Baroeul Cedex, France). They were all provided by Lesaffre as a similar dry form with a cell concentration near to 1 × 1010 colony-forming units (CFU)/g. The different dried yeast products were processed and enumerated as follows. For both in vitro and in vivo assays, yeast was rehydrated with phosphate buffered saline (PBS) (pH 7.2) 1:10 (w/v) at room temperature, and adjusted to an appropriate concentration in CFU/mL. For this purpose, cellular counts together with viability, expressed in %, were routinely performed using a hematimeter (type Thoma) combined with a Trypan blue exclusion method. More than 95% viability was preserved following rehydration. Enumeration was also confirmed a posteriori by plating on YPD-agar and allowing growth during 2 d at 30°C and 37°C.
| Strain | Identification | Origin |
| BB536 | Bifidobacterium longum | Morinaga Milk Industry Ltd. |
| Ls33 | Lactobacillus salivarius | Commercial strain/Danisco |
| NCFM | Lactobacillus acidophilus | Commercial strain/Danisco |
| MG1363 | Lactococcus lactis | Cheese starter[22] |
| TG1 | Escherichia coli | Commensal strain[23] |
| LV01/CNCM I-3799 | Saccharomyces boulardii | Lesaffre collection |
| LV02/CNCM I-3856 | Saccharomyces cerevisiae | Lesaffre collection |
| LV03 | Saccharomyces pastorianus | Lesaffre collection |
| LV04 | Saccharomyces bayanus | Lesaffre collection |
| LV06 | Saccharomyces cerevisiae | Lesaffre collection |
| LV09 | Saccharomyces cerevisiae | Lesaffre collection |
Some bacterial strains were used as reference strains for immune cell stimulation as previously described[24], also listed (Table 1). Lactobacillus strains were grown under limited aeration at 37°C in MRS medium (Difco) and a Bifidobacterium strain was grown anaerobically in MRS supplemented with 0.05% L-cysteine-hydrochloride (Sigma). Lactococcus lactis MG1363 was grown at 30°C in M17 medium supplemented with 0.5% glucose. Escherichia coli (E. coli) was grown at 37°C in LB medium (Difco). Bacterial cells were grown until stationary phase, washed and resuspended at 1 × 109 CFU/mL in PBS containing 20% glycerol and stored at -80°C until required for later assays. Some routine analyses were also performed using a portable photometer (Densimat bioMerieux)[25]. Cells were adjusted to McFarland 3 and stored at -80°C.
Groups contained 10 mice. When needed, fecal samples from 5 mice were collected, pooled, weighed and mechanically homogenized in sterile neutral and isotonic buffer at 50 mg/mL of feces. Dilutions were plated onto the “yeast-selective” tetracycline (150 μg/mL) YPD-agar (Yeast extract, Peptone, Dextrose, as described elsewhere[26]. No tetracycline-resistant microorganisms (bacteria or fungi) were detected in non-inoculated mice, indicating no or negligible basal colonization of mice gastrointestinal tract (GIT) by non-pathogenic yeast nor Candida species.
PBMCs were isolated from peripheral blood of healthy donors as previously described[24]. Briefly, after a Ficoll gradient centrifugation (Pharmacia, Uppsala, Sweden), mononuclear cells were collected, washed in RPMI 1640 medium (Live technologies, Paisley, Scotland) and adjusted to 2 × 106 cells/mL supplemented with gentamicin (150 g/mL), L-glutamine (2 mmol/L), and 10% foetal calf serum (Gibco-BRL) supplementation.
PBMC (2 × 106 cells/mL) were seeded in 24-well tissue culture plates (Corning, NY). Twenty microliters of the thawed bacterial or yeast suspensions at 109 CFU/mL in PBS containing 20% glycerol (bacteria:cell or yeast:cell ratio of 10:1) or standardized at homogeneous cell density, were added. PBS containing 20% glycerol was used as a negative (non-stimulated) control. On the basis of preliminary time-course studies, 24 h stimulation corresponded to the best time point for cytokine responses of bacteria stimulated-PBMCs. After 24 h stimulation at 37°C in an atmosphere of air with 5% CO2, culture supernatants were collected, clarified by centrifugation and stored at -20°C until cytokine analysis. Concerning yeast stimulation, freshly rehydrated powders were used at distinct yeast:cell ratios (from 0.1:1 to 5:1), with or without the presence of fungizone (amphotericin B, 10 μg/mL). Neither medium acidification nor bacterial or fungal proliferation was observed. Cytokines were measured by enzyme-linked immunosorbent assay (ELISA) using BD Pharmingen antibody pairs (BD Biosciences, San Jose, Ca, USA) for interleukin (IL)-10, interferon γ (IFNγ) and IL-12p70, and R&D systems (Minneapolis, Mn, USA) for human tumor necrosis factor (TNF), according to the manufacturer’s recommendations.
BALB/c mice (female, 7 to 8 wk old) were obtained from Charles River (St Germain sur l’Arbresle, France). A standardized murine TNBS colitis model was used in which moderate levels of inflammation were induced[27]. Briefly, a 50 μL solution of 100 mg/kg TNBS (Sigma) in 50 % ethanol was slowly administered in the colon via a 3.5 F catheter. Freshly rehydrated yeast suspensions (100 μL), containing 1 × 109 CFU/mL in NaHCO3 buffer were administered intragastrically to mice each day, starting 4 d before until the day of TNBS administration while control mice received the corresponding buffer. Mice were weighed, bled from the retro-orbital venous plexus and killed 72 h after TNBS administration. Cleared sera were frozen and stored until cytokine assays at -20°C. Colons were removed, washed and opened longitudinally. Inflammation grading was performed by two blinded observers, using the Wallace scoring method[28]. Results are expressed as % protection, corresponding to the reduction of the mean macroscopic inflammation score of treated mice (n = 10) in comparison to the mean score of TNBS-treated control mice (NaHCO3 buffer-treated mice, n = 10)[27]. Histological analysis was performed on May Grünwald-Giemsa stained 5 μm tissue sections from colon samples fixed in 10% formalin and embedded in paraffin and tissue lesions were scored according to the Ameho criteria[29]. Additionally, the degree of polymorphonuclear neutrophil infiltration of the distal colon was assessed by quantifying myeloperoxidase (MPO) - a neutrophil granule enzyme - as reported earlier[30]. A commercial preparation of prednisone (Cortancyl, Sanofi Aventis, France) was used as a positive control of protection and was orally administered for 2 subsequent days at 10 mg/kg starting at the day before TNBS administration. When needed, heparinized whole blood was collected by retro-orbital puncture and, after separation, mice sera samples were stored at -20°C for subsequent analysis.
Murine IL-6 and serum amyloid A (SAA) protein levels were measured by ELISA using commercial antibodies from Pharmingen antibody pairs (BD Biosciences, San Jose, Ca, USA), and Biosource International (Camarillo, Ca, USA), respectively, with a lower limit of sensitivity of 15 pg/mL for IL-6 and 30 ng/mL for SAA.
All analyses were performed as comparison of experimental groups with respective controls by the non-parametric one-way analysis of variance Mann-Whitney U-testing, or by Student-T testing where appropriate. Differences were judged to be statistically significant when the P value was < 0.05. Data are presented as mean ± SE.
All experiments were performed in an accredited establishment (number 59-35009; Institut Pasteur de Lille, France) and using approved guidelines according to French Ethical Committee and European Union Normatives (number 86/609/CEE).
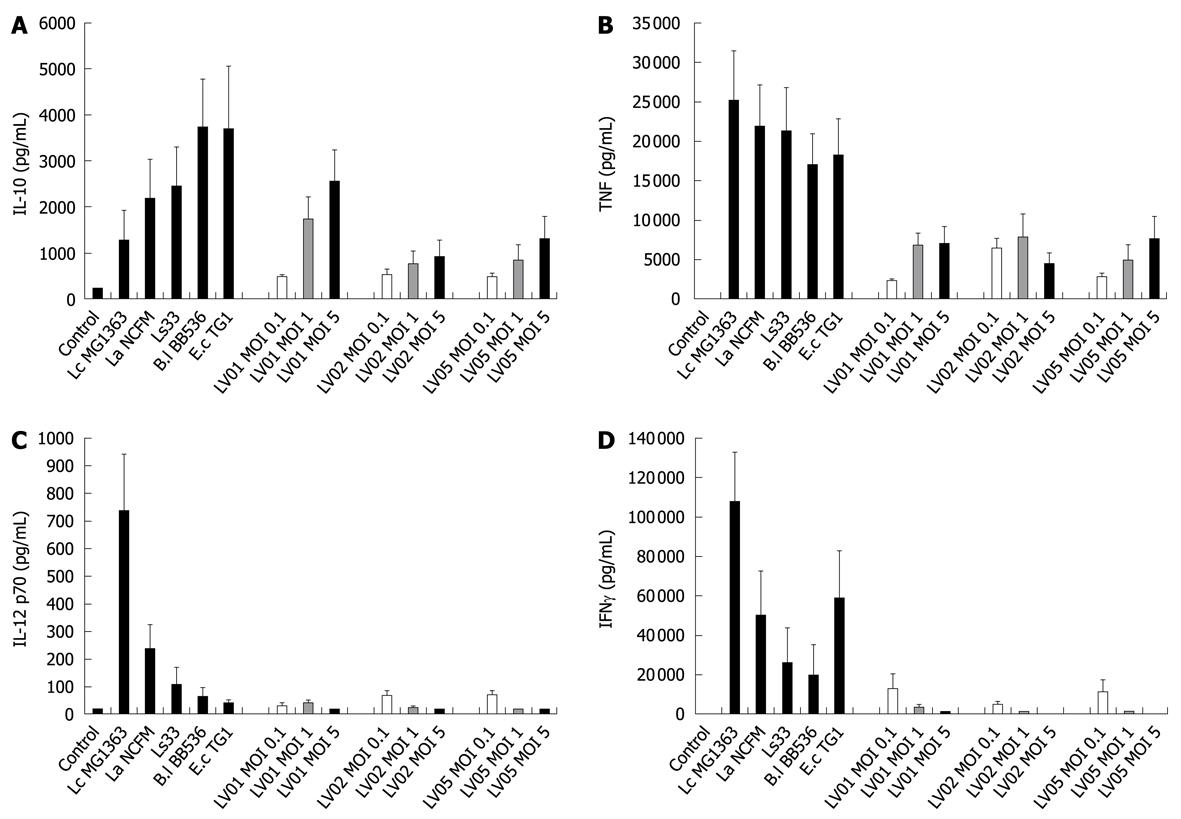
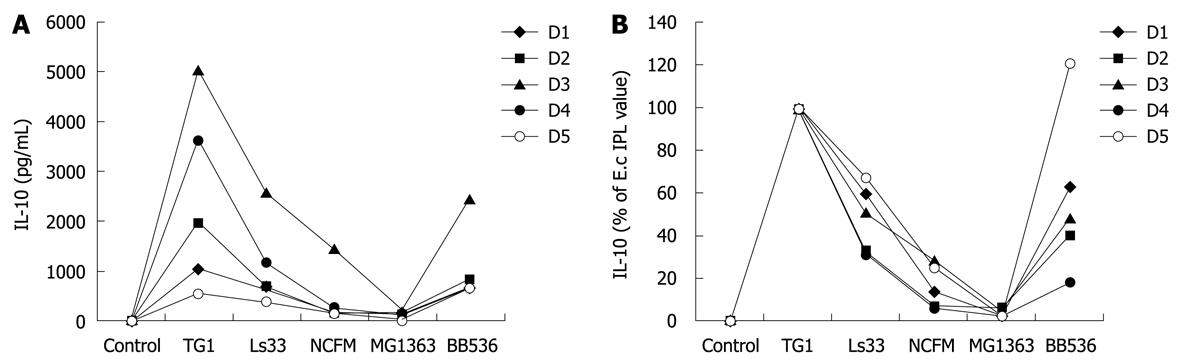
To determine the optimal dose of yeast that can induce cytokine production in human PBMCs, immuno-competent cells were first stimulated with three different multiplicity levels of infection (0.1:1, 1:1 and 5:1) of three live yeast strains and compared with immuno-stimulatory effects of reference bacteria (ranging from weak to strong inducers). Substantial amounts of IL-10, and to a lesser extent for TNF were dose-dependently induced by yeast cells (Figure 1A and B, respectively) while no or very low levels of the pro-inflammatory indicators IL-12 p70 and IFNγ could be detected following yeast stimulation (Figure 1C and D). Following these results, the yeast:host cell ratio of 1:1 was retained for further comparative analyses between strains in order to mimic physiological interactions between cells but without saturating the stimulatory capacity. In addition, the variable responsiveness due to individual blood donors was highly minimized by expressing data as % of a reference strain instead of in pg/mL, as shown (Figure 2, panel A and B). PBMCs from 5 donors were stimulated with each of the six yeast strains covering distinct subspecies and origins (Table 1).
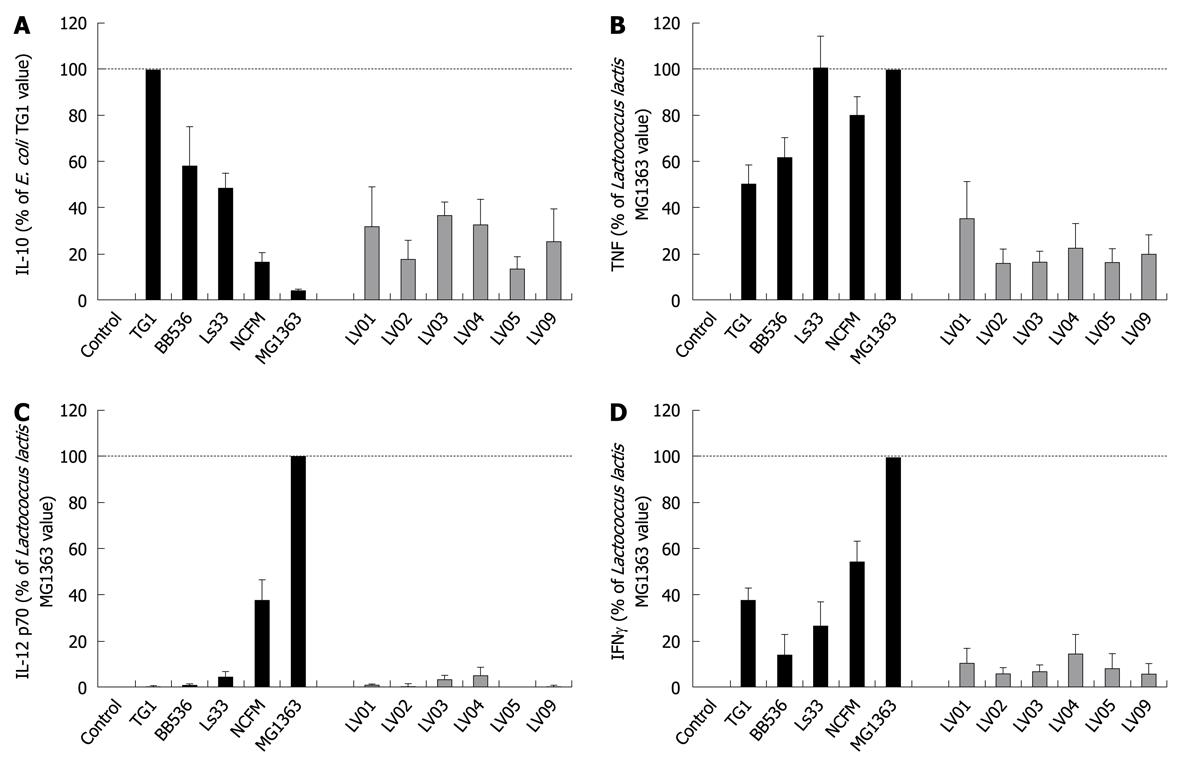
All individual strains of yeast induced relatively high levels of the anti-inflammatory IL-10, reaching the values generally obtained with lactobacilli (mean/max values 535/2300 pg/mL). However, in contrast to bacteria, no significant strain-specificity was observed among different strains of yeasts, which all induced similar levels of cytokines (Figure 3A). Analysis of the cell culture supernatants revealed moderate induction of TNF after yeast stimulation, as compared with bacterial stimulation, again without a clear strain dependency (Figure 3B). Yeasts induced extremely low levels of the pro-inflammatory signals IL-12 and IFNγ as compared to some bacteria such as Lactococcus lactis MG1363 or Lactobacillus acidophilus NCFM (Figure 3C and D). Concerning the intrinsic immuno-stimulatory capacity of yeast cells in vitro, we can conclude that no specific stimulation pattern could be discriminated among the six strains, while all strains favored anti-inflammatory profiles with a high IL-10/IL-12 ratio, low induction of TNF and negligible levels of IFNγ.
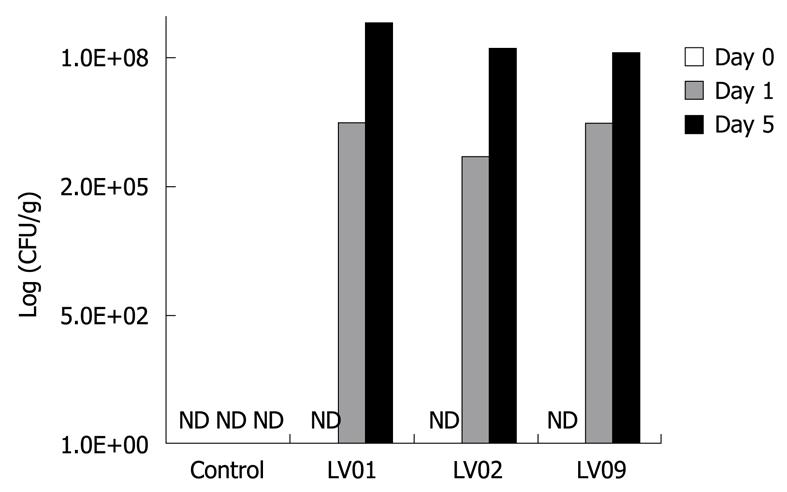
Next to the effect of yeasts on cytokine induction on PBMCs in vitro, we compared the ability of the 3 strains LV01, LV02 and LV09 to survive the digestive tract of mice. Following 0, 1 and 5 consecutive days of intra-gastric feeding of 108 CFU of each of the yeasts, faecal enumeration showed equivalent survival counts for all the strains with a progressive increase with the duration of the treatment (Figure 4). The systematic increase by 2 log units between day 1 and day 5 suggests that proliferation occurs in the gut, rather than simple transit and shedding. Although we cannot speculate on the long term persistence and colonization of the yeast species, a 5-d-treatment clearly leads to a noticeable presence of living yeasts in the colon lumen (up to 108 CFU/g for the 3 strains). A significant faecal recovery of yeast colonies from healthy mice was still detectable 10 d after the last feeding (more than 105 CFU/g, data not shown). It is noteworthy to mention that neither diarrhoea nor slight changes in faecal character, nor weight disturbance or other deleterious signs were observed in mice treated with those food-grade yeasts.
In order to evaluate whether the administration of distinct yeast strains equally impacted on TNBS-induced colitis, we compared the performance of the three strains LV01, LV02 and LV09 with the anti-inflammatory corticoid drug (prednisone) which we and others previously used in this experimental setting[31,32]. Since some anti-inflammatory effects of lactobacilli were observed both after oral and systemic administration[33,34], we assessed whether intraperitoneal administration of the yeast LV01 could also rescue mice from colitis.
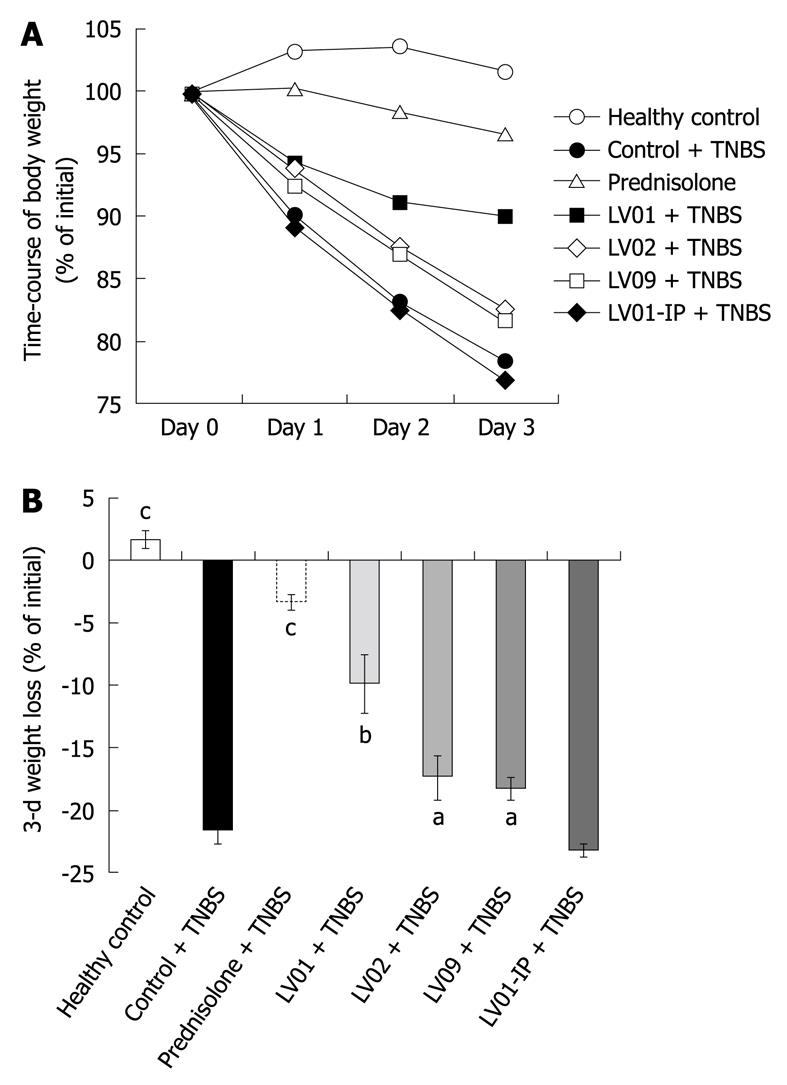
Rectal administration of TNBS causes progressive weight loss in vehicle-treated mice, reaching up to 22.5% of the initial weight 72 h after induction of colitis (Figure 5A and B). As expected, prednisone prevented body weight loss associated with experimental colitis (-3.3%, P < 0.001). The oral treatments by the 3 yeast strains also significantly attenuated the deleterious weight changes for the LV01 (-9.9%, P < 0.01), and to a lesser extent for LV02 and LV09 (18%, P < 0.05). In contrast, the systemic administration of the strain LV01 (LV01-IP) had no additional effect on weight.
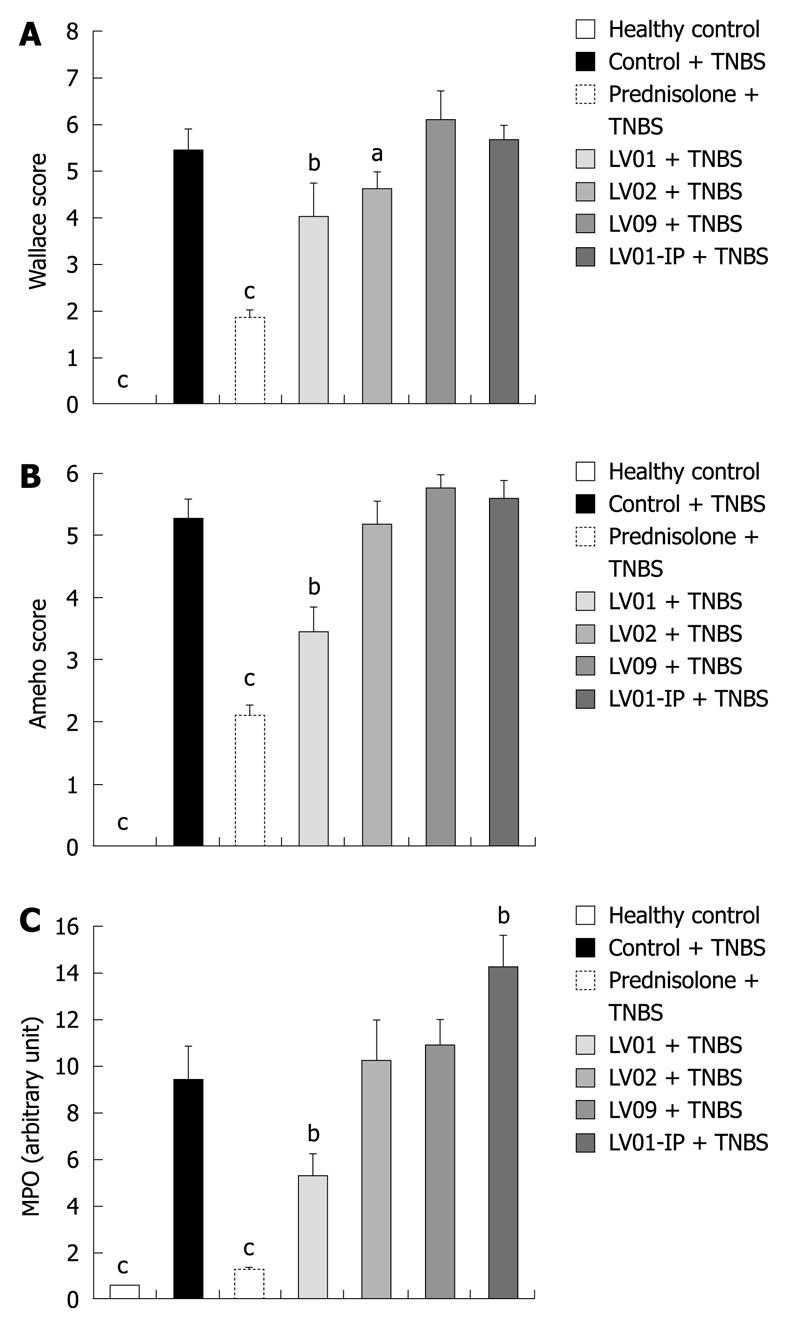
After the onset of colitis, anti-inflammatory effects were measured macroscopically for the prednisone-treated as well as for the LV01-treated mice (66% and 27% reduction of macroscopic damage, respectively, P < 0.01, Figure 6A). More moderately LV02 reduced the colonic insult (18% of control, P < 0.05) while intra-peritoneal injection of LV01 failed to influence the macroscopic scores.
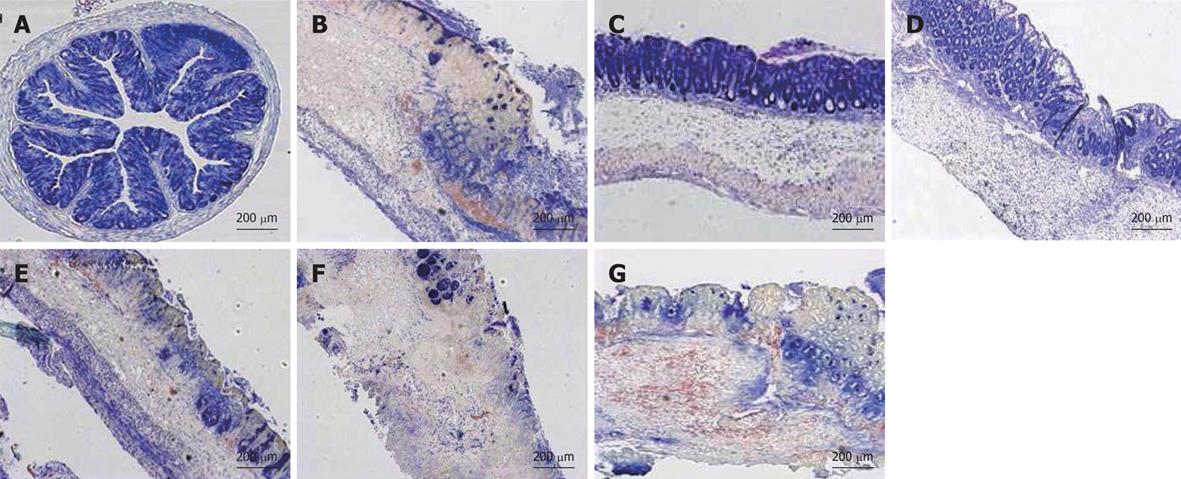
Considering the histological assessment of colitis in various conditions (Figure 6B), both the prophylactic treatment by prednisone and LV01 significantly reduced the microscopic scores of inflammation (by 60.3% and 35%, respectively, P < 0.05), while neither LV02 nor LV09 treament could be associated with a significant reduction of the histopathological features observed during active colitis (mucosal erosions, oedema, ulcerations areas and even necrosis). These observations, including thickening of the submucosa and inflammatory infiltrates, were correlated with the colonic myeloperoxidase activities measured (MPO, Figure 6C) and with similar reductions for the steroid drug and LV01 (P < 0.01). In contrast, no improvement, and even some aggravation, was depicted for strain LV01 provided by the systemic route, as supported by massive neutrophil infiltrates. Representative aspects of the corresponding colon sections are given (Figure 7). Mouse colons with vehicle-treated TNBS-induced colitis exhibited massive goblet cell depletion and showed disorganized mucosal architecture, including muscle layer necrosis (Figure 7B). By contrast, corticoid-treated animals showed a well-conserved appearance, more similar to normal structures despite a slight thickening of the submucosa. Only minor thickening of submucosa and superficial lesions, mainly oedema, were seen in mice treated with the yeast LV01, in complete absence of necrosis. Erosions were more important in LV02-pretreatment, although necrosis was restricted to the upper luminal compartment and the crypt’s architecture was still identifiable. Treatment with strain LV09 given orally and strain LV01 after intraperitoneal administration were characterized by prominent inflammatory infiltrates and transmural inflammation with necrosis reaching the deeper muscle layers.
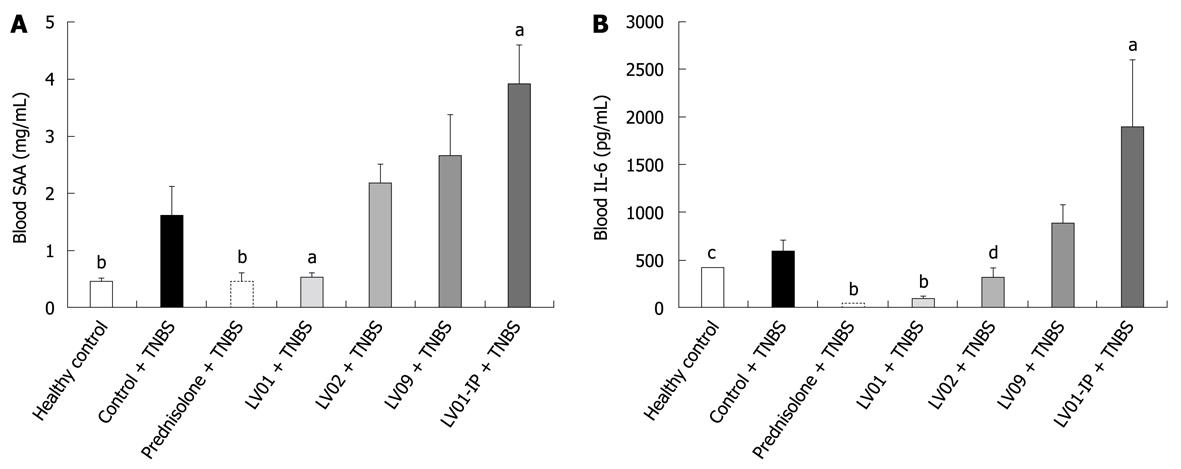
The levels of the blood markers of inflammation SAA and IL-6, while significantly increased after TNBS administration, were strikingly reduced by prednisone and LV01 treatments, approaching baseline values (P < 0.01). LV02 strain also minimized the pro-inflammatory cytokine IL-6 in the serum (P < 0.05), with a similar trend observed for SAA (P = 0.055, Figure 8).
In conclusion, TNBS caused increases in mucosal and systemic inflammatory symptoms that can be prevented by an orally administered anti-inflammatory drug (prednisone) as well as the food-grade yeast LV01. This was substantiated on the basis of reduction of body-weight loss, macroscopic damage, histological scores, colonic inflammatory infiltrates and serum markers. The yeast strain LV02 showed an intermediate protection capacity with sometimes partial and borderline effects in the degree of attenuation, depending on the marker considered. LV09 only lowered the weight loss with no improvement of other colitis-related symptoms. These results support the relevance of yeast for colitis protection, but distinct performance of various strains highly suggests strain-dependent mechanisms.
Evidence that yeasts can be used as a probiotic with therapeutic potential in IBD has emerged from clinical trials and experimental models of colitis in animals. However, these studies remained scarce and essentially used the strain S. boulardii, formerly S. cerevisiae var boulardii. In this study we aimed at questioning the differential anti-inflammatory potential of other yeast strains. Whether food-borne strains of S. cerevisiae or other yeast species possess probiotic properties is of fundamental importance in developing, understanding and handling of new potential biotherapeutic agents, both concerning the pharmaceutical and the (functional) food industry. Attempts for such a screening of yeast biodiversity were already carried out in vitro[17,35,36], mainly by studying selection criteria such survival at low acid pH and tolerance to bile salts or adhesion on epithelial cells. The predictability for the in vivo situation, however, is limited, as the importance of colonization in strain efficacy remains to be determined in vivo[37]. An example was recently given with a Lactobacillus strain that fulfilled adhesion and survival criteria in vitro but was unable to persist in mice, although exerting immunomodulatory effects[38]. To our knowledge, the comparison of distinct yeast strains based on their ability to induce cytokine secretions by immunocompetent cells was never reported.
Results obtained in this study showed that, in contrast to bacteria[22,39,40], in vitro immunomodulation did not discriminate among various strains. This can be attributed to the more conserved cell-wall structure of the yeasts. However, yeast cells are not inert from an immunological point of view and substantial induction of IL-10, on PBMCs, associated with weak TNF and no release of IFNγ and IL-12, favor, in general, a more anti-inflammatory profile than bacteria. This is in agreement with very recent studies using germ-free mice[41].
The three strains used in the murine TNBS-induced colitis exhibited similar colonization patterns in vivo, thus allowing accurate comparisons. Standardization of experimental settings for all evaluations and, as far as possible into the same study, is highly recommended[42]. The present work confirms that a yeast strain (S. boulardii) is able to significantly prevent symptoms in an acute experimental colitis in mice. The 30% protection observed, lower than the protection obtained by a traditional anti-inflammatory drug, is consistent with previous reports using the same yeast strain in a rat model of TNBS[13]. By contrast, in another study in rats, TNBS-induced inflammation was only weakly lowered with S. boulardii with the 18% trend, which did not passed the statistics test, while conventional IBD drugs and other probiotic preparations did show significance[43,44]. Such differences between studies may depend on experimental design including distinct outcomes when considering preventive vs therapeutic applications, as reported e.g. for bacteria in the TNBS colitis model[45], in the experimental DSS model[46] or even in clinical studies[47]. Hence, these observations emphasize the necessity to compare distinct treatments and strains in a single study. We enlightened here for the first time that, like probiotic bacteria, probiotic yeasts are not equivalent in their capacity to alleviate colitis, despite a similar colonization profile.
The “main contribution” of yeast-mediated anti-inflammatory effects is suggested to more address the intestinal barrier properties and epithelial cells rather than an immunocompetent cell-based immunity, because (1) protection triggered by yeasts in vivo is strain-dependent, whereas together with the immune screening in vitro, was poorly discriminating; and (2) the failure of S. boulardii to induce protection by the systemic route, in contrast to the oral route. Indeed, in contrast to some beneficial bacteria which are able to exert effects distantly from the inflammation site[33,34,48], emphasizing a possible role of migratory cells such as dendritic cells (DC)[31], yeast-mediated protection seems to take place predominantly in the intestinal mucosa. An intestinal barrier dysfunction in IBD is obvious, although it is not clear yet whether this is a cause or a consequence of the disease[49]. Prophylactic reinforcement and therapeutic restoration of barrier function by changing the luminal environment may successfully stimulate the mucosal barrier. Probiotics are elements of choice to normalize the barrier function, stabilize tight junction components[50] and limit translocation[51]. Trophic effects[52] as well as enhancement of epithelial integrity mediated by yeasts have been extensively described[4,7]. Reduction of bacterial translocation by oral treatment with yeasts in various models of sepsis also confirmed this functional aspect of probiotic-yeasts[53-55].
However, other mechanisms including direct yeast-DC interactions were also evoked to explain how S. boulardii exhibits its anti-inflammatory properties[56]. Stimulation may occur in Peyer’s patches or in the lamina propria. This mechanism did not seem to be very strain specific and, paradoxically, even Candida albicans was recently reported to induce tolerogenic DC and to positively balance inflammation after adoptive transfer in a mice colitis model[57]. Similarly, soluble β-glucans derived from C. albicans were able to suppress the production of pro-inflammatory cytokines by PBMCs[58] while mannan from S. cerevisiae impaired the ability of macrophages to kill phagocytosed pathogens[59]. On the other hand, colonization of mice by C. albicans exacerbated DSS-colitis[60] and delayed healing of TNBS colitis[61]. Finally, intraperitoneal injection of zymosan, a polysaccharide cell wall component from S. cerevisiae has been widely used as a (self-resolving) model of acute inflammation[62]. By contrast, yeast extracts administered to mice per os resulted in a significant reduction of the C. difficile-toxin A-mediated COX-2 increase and in the toxin-mediated disruption of epithelial cells[63]. Whether yeast or yeast-derived molecules will have a pro- or anti-inflammatory role may thus greatly depend on the route of administration and consequently on the target cells and tissues.
Nevertheless, we can speculate that oral yeast-induced immunomodulation predominantly addresses intestinal permeability and, based on published data, the immune function of intestinal cells. This is consistent with clinical findings indicating that S. boulardii has a positive effect on the maturation of enterocytes, but only a minor influence on lymphocytes[64]. Our results suggest that further screening for probiotic yeasts may favor the use of epithelial cell or mucosa-integrated models, rather than immune-cell based approaches. This will help us to select yeast or fungal strains with important potential in inflammatory conditions or in a context where inflammation is concomitant with enteropathogens such C. difficile and E. coli[65]. Understanding how various strains perform in vivo will allow us to trace specific mechanisms and select or design optimal strains for specific infectious or inflammatory diseases, or even consider new probiotic applications[66]. As some of the yeast-based probiotic mechanisms seem to be distinct from the bacterial ones, possible synergy might be expected from mixing both types of probiotics[67]. This was previously shown in a preclinical study on traveler’s diarrhea, where the combination of yeast and bacteria was found to be more effective than each probiotic alone[68]. Using probiotic Saccharomyces spp. alone or in combination with lactic acid bacteria will thus enhance and extend the actual biotherapeutic/probiotic approach in IBD together with conventional anti-inflammatory treatments. Lastly, the strain-specific differences seen in the anti-inflammatory capacity of yeasts may also be extended for clinical use against Salmonella ssp., C. difficile and Candida species, requiring an optimal and controlled formulation of the probiotic product[69].
As for probiotic bacteria, probiotic yeast such Saccharomyces boulardii (S. boulardii) can be effective in gastrointestinal pathologies such as inflammatory bowel disease (IBD) and bacterial- or enterotoxin-mediated diarrhea and inflammation. However, very few yeast strains have been studied as possible biotherapeutics agents. The underlying mechanisms of action of probiotics are not fully understood but there is some evidence that they exert their beneficial effect by multiple and specific modes of action depending on the type of the probiotic (bacteria, yeast), the species and strain.
The yeast-mediated capacity to lower inflammation is suggested to mainly involve epithelial cells and barrier function, as demonstrated by (1) the weakness of specific immunomodulatory response in vitro; and (2) the lack of protection by S. boulardii when administered by the systemic but not the oral route. In contrast with the performance of some probiotic bacteria which the authors previously showed to be directly correlated with their in vitro anti-inflammatory potential, the immunoregulatory mechanism of probiotic yeasts fails to directly act on the cytokine level.
As for probiotic bacteria, strain specificity also matters for yeast as for probiotics, but mechanisms, and consequently screening methods, partly differ between yeast and bacteria. A careful and appropriate selection of strains is then required to screen the biodiversity of yeasts for specific clinical studies including applications in IBD as well as for other dedicated therapeutic uses. This will impact on future product composition to enhance or synergize gastrointestinal related disorders by using probiotics.
Probiotics are defined as living microorganisms that may have beneficial effects on health, including mainly food-grade bacteria and non-pathogenic yeasts.
The paper by Benoit Foligné and co-workers investigated the anti-inflammatory effects of various yeasts strains in experimental colitis in mice. The overall design of the study is fair and the results are stimulating for future research.
| 1. | Food and Agriculture Organization of the United Nations, World Health Organization. Guidelines for evaluation of probiotic in food. 2002; Available from: http://www.who.int/foodsafety/fs_management_probiotic_guidelines.pdf. |
| 2. | Herbrecht R, Nivoix Y. Saccharomyces cerevisiae fungemia: an adverse effect of Saccharomyces boulardii probiotic administration. Clin Infect Dis. 2005;40:1635-1637. |
| 3. | Vandenplas Y, Brunser O, Szajewska H. Saccharomyces boulardii in childhood. Eur J Pediatr. 2009;168:253-265. |
| 4. | Zanello G, Meurens F, Berri M, Salmon H. Saccharomyces boulardii effects on gastrointestinal diseases. Curr Issues Mol Biol. 2009;11:47-58. |
| 5. | Buts JP. Twenty-five years of research on Saccharomyces boulardii trophic effects: updates and perspectives. Dig Dis Sci. 2009;54:15-18. |
| 6. | Czerucka D, Rampal P. Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes Infect. 2002;4:733-739. |
| 7. | Czerucka D, Piche T, Rampal P. Review article: yeast as probiotics -- Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26:767-778. |
| 8. | Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci. 2000;45:1462-1464. |
| 9. | Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur J Gastroenterol Hepatol. 2003;15:697-698. |
| 10. | Dalmasso G, Cottrez F, Imbert V, Lagadec P, Peyron JF, Rampal P, Czerucka D, Groux H, Foussat A, Brun V. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology. 2006;131:1812-1825. |
| 11. | Dalmasso G, Alexander G, Carlsen H, Imbert , Lagadec P, Peyron JF, Rampal P, Blomhoff R, Czerucka D. Saccharomyces boulardii prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2005;128 Suppl 2:A168. |
| 12. | Dalmasso G, Alexander G, Carlsen H, Imbert , Lagadec P, Peyron JF, Rampal P, Blomhoff R, Czerucka D. The probiotic yeast Saccharomyces boulardii prevents colonic inflammation in TNBS-induced colitis via inhibition of NfkB: use of an in vivo imaging mouse model. Gut. 2007;56 Suppl 3:A4. |
| 13. | Lee SK, Kim YW, Chi SG, Joo YS, Kim HJ. The effect of Saccharomyces boulardii on human colon cells and inflammation in rats with trinitrobenzene sulfonic acid-induced colitis. Dig Dis Sci. 2009;54:255-263. |
| 14. | Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, Auwerx J, Pettersson S, Desreumaux P. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265-1276. |
| 15. | Jawhara S, Poulain D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med Mycol. 2007;45:691-700. |
| 16. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. |
| 17. | Martins FS, Nardi RM, Arantes RM, Rosa CA, Neves MJ, Nicoli JR. Screening of yeasts as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J Gen Appl Microbiol. 2005;51:83-92. |
| 18. | Martins FS, Rodrigues AC, Tiago FC, Penna FJ, Rosa CA, Arantes RM, Nardi RM, Neves MJ, Nicoli JR. Saccharomyces cerevisiae strain 905 reduces the translocation of Salmonella enterica serotype Typhimurium and stimulates the immune system in gnotobiotic and conventional mice. J Med Microbiol. 2007;56:352-359. |
| 19. | Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, Zarzuelo A, Galvez J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103:836-844. |
| 20. | Medina M, Izquierdo E, Ennahar S, Sanz Y. Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol. 2007;150:531-538. |
| 21. | Seksik P, Dray X, Sokol H, Marteau P. Is there any place for alimentary probiotics, prebiotics or synbiotics, for patients with inflammatory bowel disease? Mol Nutr Food Res. 2008;52:906-912. |
| 22. | Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1-9. |
| 23. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press 1989; . |
| 24. | Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236-243. |
| 25. | Araujo R, Rodrigues AG, Pina-Vaz C. A fast, practical and reproducible procedure for the standardization of the cell density of an Aspergillus suspension. J Med Microbiol. 2004;53:783-786. |
| 26. | Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. New York: Cold Spring Harbor Laboratory Press 1986; . |
| 27. | Foligné B, Nutten S, Steidler L, Dennin V, Goudercourt D, Mercenier A, Pot B. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig Dis Sci. 2006;51:390-400. |
| 28. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. |
| 29. | Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487-493. |
| 30. | Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206-209. |
| 31. | Foligne B, Zoumpopoulou G, Dewulf J, Ben Younes A, Chareyre F, Sirard JC, Pot B, Grangette C. A key role of dendritic cells in probiotic functionality. PLoS One. 2007;2:e313. |
| 32. | Fiorucci S, Wallace JL, Mencarelli A, Distrutti E, Rizzo G, Farneti S, Morelli A, Tseng JL, Suramanyam B, Guilford WJ. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736-15741. |
| 33. | Sheil B, McCarthy J, O'Mahony L, Bennett MW, Ryan P, Fitzgibbon JJ, Kiely B, Collins JK, Shanahan F. Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut. 2004;53:694-700. |
| 34. | Foligné B, Grangette C, Pot B. Probiotics in IBD: mucosal and systemic routes of administration may promote similar effects. Gut. 2005;54:727-728. |
| 35. | van der Aa Kühle A, Skovgaard K, Jespersen L. In vitro screening of probiotic properties of Saccharomyces cerevisiae var. boulardii and food-borne Saccharomyces cerevisiae strains. Int J Food Microbiol. 2005;101:29-39. |
| 36. | Pennacchia C, Blaiotta G, Pepe O, Villani F. Isolation of Saccharomyces cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. J Appl Microbiol. 2008;105:1919-1928. |
| 37. | Edwards-Ingram L, Gitsham P, Burton N, Warhurst G, Clarke I, Hoyle D, Oliver SG, Stateva L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl Environ Microbiol. 2007;73:2458-2467. |
| 38. | Bujalance C, Moreno E, Jimenez-Valera M, Ruiz-Bravo A. A probiotic strain of Lactobacillus plantarum stimulates lymphocyte responses in immunologically intact and immunocompromised mice. Int J Food Microbiol. 2007;113:28-34. |
| 39. | Cross ML, Ganner A, Teilab D, Fray LM. Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunol Med Microbiol. 2004;42:173-180. |
| 40. | Kekkonen RA, Kajasto E, Miettinen M, Veckman V, Korpela R, Julkunen I. Probiotic Leuconostoc mesenteroides ssp. cremoris and Streptococcus thermophilus induce IL-12 and IFN-gamma production. World J Gastroenterol. 2008;14:1192-1203. |
| 41. | Martins FS, Silva AA, Vieira AT, Barbosa FH, Arantes RM, Teixeira MM, Nicoli JR. Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch Microbiol. 2009;191:623-630. |
| 42. | Feighery LM, Smith P, O'Mahony L, Fallon PG, Brayden DJ. Effects of Lactobacillus salivarius 433118 on intestinal inflammation, immunity status and in vitro colon function in two mouse models of inflammatory bowel disease. Dig Dis Sci. 2008;53:2495-2506. |
| 43. | Peys E, Varghese J, Suresh P, Vandenkerckhove J, Van Hemel J, Chaniyilparampu RN, Sas B. Effects of Bacillus subtilis 'PB6' (ATCC-PTA 6737) on Clostridium difficile associated diarrhea (CDAD) and inflammatory bowel disease (IBD) in animal models. Am J Infect Dis. 2007;3:254-265. |
| 44. | Sas B, Van Hemel J, Vandenkerkerkove J, Peys E, Tan HM, Cy SE, Ramchan C. The use of Bacillus PB6 for the prophylaxis or treatment of gastrointestinal and immuno-related diseases. US Patent WO 2007/064741. |
| 45. | Mañé J, Lorén V, Pedrosa E, Ojanguren I, Xaus J, Cabré E, Domènech E, Gassull MA. Lactobacillus fermentum CECT 5716 prevents and reverts intestinal damage on TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15:1155-1163. |
| 46. | Herías MV, Koninkx JF, Vos JG, Huis in't Veld JH, van Dijk JE. Probiotic effects of Lactobacillus casei on DSS-induced ulcerative colitis in mice. Int J Food Microbiol. 2005;103:143-155. |
| 47. | Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, Schipper ME, Gooszen HG, Akkermans LM, Kroese AB. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery. 2009;145:157-167. |
| 48. | Laudanno O, Vasconcelos L, Catalana J, Cesolari J. Anti-inflammatory effect of bioflora probiotic administered orally or subcutaneously with live or dead bacteria. Dig Dis Sci. 2006;51:2180-2183. |
| 49. | McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100-113. |
| 50. | Resta-Lenert SC, Barrett KE. Modulation of intestinal barrier properties by probiotics: role in reversing colitis. Ann N Y Acad Sci. 2009;1165:175-182. |
| 51. | Ewaschuk J, Endersby R, Thiel D, Diaz H, Backer J, Ma M, Churchill T, Madsen K. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46:841-850. |
| 52. | Buts JP, De Keyser N. Effects of Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci. 2006;51:1485-1492. |
| 53. | Geyik MF, Aldemir M, Hosoglu S, Ayaz C, Satilmis S, Buyukbayram H, Kokoglu OF. The effects of Saccharomyces boulardii on bacterial translocation in rats with obstructive jaundice. Ann R Coll Surg Engl. 2006;88:176-180. |
| 54. | Sahin T, Aydin S, Yüksel O, Bostanci H, Akyürek N, Memiş L, Başaran N. Effects of the probiotic agent Saccharomyces Boulardii on the DNA damage in acute necrotizing pancreatitis induced rats. Hum Exp Toxicol. 2007;26:653-661. |
| 55. | Karen M, Yuksel O, Akyürek N, Ofluoğlu E, Cağlar K, Sahin TT, Paşaoğlu H, Memiş L, Akyürek N, Bostanci H. Probiotic agent Saccharomyces boulardii reduces the incidence of lung injury in acute necrotizing pancreatitis induced rats. J Surg Res. 2010;160:139-144. |
| 56. | Thomas S, Przesdzing I, Metzke D, Schmitz J, Radbruch A, Baumgart DC. Saccharomyces boulardii inhibits lipopolysaccharide-induced activation of human dendritic cells and T cell proliferation. Clin Exp Immunol. 2009;156:78-87. |
| 57. | Bonifazi P, Zelante T, D'Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2:362-374. |
| 58. | Nakagawa Y, Ohno N, Murai T. Suppression by Candida albicans beta-glucan of cytokine release from activated human monocytes and from T cells in the presence of monocytes. J Infect Dis. 2003;187:710-713. |
| 59. | Mpofu CM, Campbell BJ, Subramanian S, Marshall-Clarke S, Hart CA, Cross A, Roberts CL, McGoldrick A, Edwards SW, Rhodes JM. Microbial mannan inhibits bacterial killing by macrophages: a possible pathogenic mechanism for Crohn's disease. Gastroenterology. 2007;133:1487-1498. |
| 60. | Jawhara S, Thuru X, Standaert-Vitse A, Jouault T, Mordon S, Sendid B, Desreumaux P, Poulain D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis. 2008;197:972-980. |
| 61. | Zwolinska-Wcislo M, Brzozowski T, Budak A, Kwiecien S, Sliwowski Z, Drozdowicz D, Trojanowska D, Rudnicka-Sosin L, Mach T, Konturek SJ. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J Physiol Pharmacol. 2009;60:107-118. |
| 62. | Cash JL, White GE, Greaves DR. Chapter 17. Zymosan-induced peritonitis as a simple experimental system for the study of inflammation. Methods Enzymol. 2009;461:379-396. |
| 63. | Duncan PI, Fotopoulos G, Pasche E, Porta N, Masserey Elmelegy I, Sanchez-Garcia JL, Bergonzelli GE, Corthésy-Theulaz I. Yeast, beef and pork extracts counteract Clostridium difficile toxin A enterotoxicity. FEMS Microbiol Lett. 2009;295:218-225. |
| 64. | Jahn HU, Ullrich R, Schneider T, Liehr RM, Schieferdecker HL, Holst H, Zeitz M. Immunological and trophical effects of Saccharomyces boulardii on the small intestine in healthy human volunteers. Digestion. 1996;57:95-104. |
| 65. | Wu X, Vallance BA, Boyer L, Bergstrom KS, Walker J, Madsen K, O'Kusky JR, Buchan AM, Jacobson K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol. 2008;294:G295-G306. |
| 66. | Chen X, Fruehauf J, Goldsmith JD, Xu H, Katchar KK, Koon HW, Zhao D, Kokkotou EG, Pothoulakis C, Kelly CP. Saccharomyces boulardii inhibits EGF receptor signaling and intestinal tumor growth in Apc(min) mice. Gastroenterology. 2009;137:914-923. |
| 67. | Dixit K, Gandhi DN. Biotherapeutic properties of probiotic yeast Saccharomyces species in fermented dairy foods. Accessed: April 16 2010; Available from: http://www.dairyscience.info/probiotics/105-biotherapeutic-probiotic-yeast.html. |
| 68. | Bisson JF, Hidalgo S, Rozan P, Messaoudi M. Preventive effects of different probiotic formulations on travelers' diarrhea model in wistar rats : preventive effects of probiotics on TD. Dig Dis Sci. 2010;55:911-919. |
| 69. | Martins FS, Veloso LC, Arantes RM, Nicoli JR. Effects of yeast probiotic formulation on viability, revival and protection against infection with Salmonella enterica ssp. enterica serovar Typhimurium in mice. Lett Appl Microbiol. 2009;49:738-744. |
Peer reviewer: Dr. Francesco Costa, Dipartimento di Medicina Interna - U.O. di Gastroenterologia Università di Pisa - Via Roma, 67 - 56122 - Pisa, Italy
S- Editor Wang YR L- Editor O’Neill M E- Editor Zheng XM