Published online Apr 21, 2010. doi: 10.3748/wjg.v16.i15.1934
Revised: February 20, 2010
Accepted: February 27, 2010
Published online: April 21, 2010
Gastric adenomyoma (AM) is a rare benign tumor characterized by gland-like structures embedded within a smooth muscle stroma. We report a case of a 68-year-old man with gastric AM admitted to our hospital for melana. Endoscopic examination revealed a gastric mass of about 4 cm in diameter, located in the antrum. Histologic examination of the excised specimen showed irregularly arranged glands and interlacing smooth muscle bundles surrounding the glandular elements. Although gastric AM is rare, it should be considered in differential diagnosis of extramucosal gastric tumor.
- Citation: Zhu HN, Yu JP, Luo J, Jiang YH, Li JQ, Sun WY. Gastric adenomyoma presenting as melena: A case report and literature review. World J Gastroenterol 2010; 16(15): 1934-1936
- URL: https://www.wjgnet.com/1007-9327/full/v16/i15/1934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i15.1934
Gastric adenomyoma (AM) is a rare, benign tumor, characteristically composed of glands and cysts, lined by columnar, flattened epithelia and a prominent smooth muscle stroma. The entity was first described by Magnus-Alsleben[1] in 1903. Patients with gastric AM may be asymptomatic, or have nonspecific gastrointestinal symptoms such as epigastric pain, vomiting[2-5]. We describe, herein, an extremely rare case of gastric AM presenting as melana.
A 68-year-old man was referred to our hospital with a 1-week history of melena. He had no remarkable past medical history and alcohol consumption or history of smoking or drugs. Upon hospitalization, physical examination, routine laboratory parameters and tumor markers including carcinoembryonic antigen, cancer antigen 19-9, and alpha fetoprotein were normal, except for markers of hypochromic anemia including hemoglobin 89 g/L (normal range: 120-160 g/L), hematocrit 28.6% (normal range: 40%-50%), mean corpuscular hemoglobin concentration 311 g/L (normal range: 320-360 g/L), serum ferritin 8.31 μg/L (normal range: 21.81-274.66 μg/L).
Abdominal computed tomography (CT) scanning showed a heterogeneous mass in the gastric antrum. Tumor tissue was slightly enhanced after injection of a contrast medium. Neither further invasion beyond the gastric wall, nor visible metastatic lesions in the liver or enlarged lymph nodes were observed.
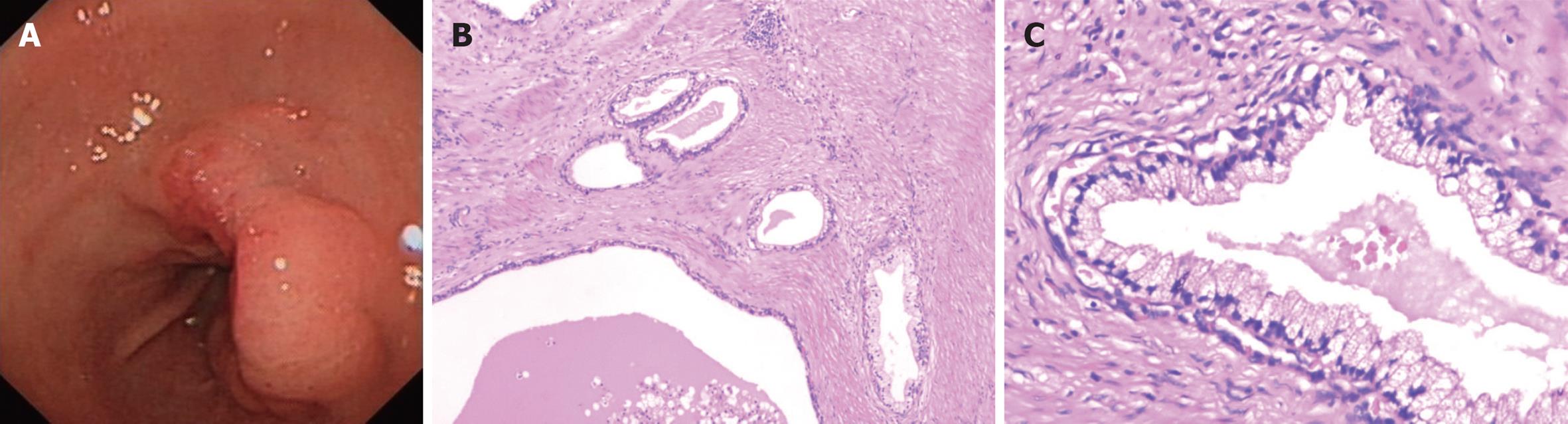
Endoscopic examination revealed an ulcerated mass of about 4 cm in diameter, located on the lesser curvature and posterior wall of the gastric antrum, with the duodenum partly involved (Figure 1A). The esophagus and the remaining parts of stomach were normal. Histopathological examination of mass biopsies showed several dilated glands with mild atypia in an inflamed stroma and necrosis. A decision was made to proceed with surgery in order to relieve the symptoms. As frozen sections revealed the possibility of a gastric AM, a distal subtotal gastrectomy was performed. Postoperative recovery was uneventful.
Macroscopic examination revealed a 50 mm × 43 mm × 23 mm ulcerated mass with sharp margins and a greyish-white cut surface. It was solid and cystic with clear, mucinous fluid in the lumens. The cysts ranged up to 8 mm. Microscopic examination showed that the tumor was located mainly in submucosa and muscularis propria of the stomach, composed of glands and cysts surrounded by bundles of smooth muscle tissue (Figure 1B). Epithelial cells lining the glands and cysts were columnar and flattened mucinous cells (Figure 1C). Cellular stratification was minimal and nuclei were basally located with only slight atypia. Mitotic activity was absent. Complete sectioning of this mass failed to demonstrate exocrine or endocrine pancreatic structures. Accumulations of inflammatory mononuclear cells were noted near the glands.
AM of the gastrointestinal tract is rare, most frequently observed in the duodenum, gall bladder and stomach. Most cases of gastric AM occur in adults with no symptom or have nonspecific gastrointestinal symptoms. Scare clinical manifestations such as localized peritonitis have been reported[6]. To the best of our knowledge, this is the first case of gastric AM presented with melana. We ascribe the hemorrhage to mucosal ulcer.
In spite of the availability of newer diagnostic techniques, including CT and endoscopic ultrasonography[7], it is still difficult to diagnose gastric AM before operation. Endoscopic examination cannot differentiate gastric AM from other extramucosal lesions, such as gastrointestinal stromal tumor, lipoma, neurilemmoma, hemangioma, gastrointestinal autonomic nerve tumor, carcinoid, lymphoma or even gastric carcinoma. In the majority of cases, endoscopic biopsies are superficial and fail to obtain representative tumor tissue. Therefore, frozen section is very helpful to establish the intraoperative diagnosis and to avoid unnecessary extensive operations. In the present case, two biopsies were not diagnostic, frozen section revealed the possibility of gastric AM.
Histologically, gastric AM should be differentiated from high-grade adenocarcinoma and gastritis cystica profunda. Gastric adenocarcinoma associated with gastric AM has been reported[8]. The common features that favor an adenocarcinoma are epithelial atypia, mitotic figures, and fibrous stromal reaction. Gastritis cystica profunda (GCP) is characterized by elongation of gastric foveola with hyperplasia and cystic dilatation of gastric glands, extending into the gastric submucosal layer. The absence of smooth muscle bundles around the cysts helps to differentiate GCP from AM.
Based on the similarities of epithelial component of AM to pancreatic ducts, several authors consider gastric AM as a variant of ectopic pancreas without exocrine or endocrine components[9,10]. Yamagiwa et al[11] divided ectopic pancreas into type I (with all elements of normal pancreatic tissues), type II (with pancreatic tissues but no islets), and type III (with pancreatic ducts only). Gastric AM represents type III. However, Takeyama et al[6] considered AM as a hamartoma based on the disordered proliferation of smooth muscle.
In summary, although gastric AM is rare, it should be always considered in the differential diagnosis of extramucosal gastric lesions. Despite the development of modern diagnostic techniques, its diagnosis remains challenging. If in doubt, frozen section can help to avoid unnecessary radical operation.
| 1. | Magnus-Alsleben E. Adenomyome des Pylorus. Virchows Arch. 1903;173:137–155. |
| 2. | Park HS, Lee SO, Lee JM, Kang MJ, Lee DG, Chung MJ. Adenomyoma of the small intestine: report of two cases and review of the literature. Pathol Int. 2003;53:111-114. |
| 3. | Takahashi Y, Fukushima J, Fukusato T, Mori S. Adenomyoma with goblet and Paneth cells of the ileum. Pathol Res Pract. 2006;202:549-553. |
| 4. | Babál P, Zaviacic M, Danihel L. Evidence that adenomyoma of the duodenum is ectopic pancreas. Histopathology. 1998;33:487-488. |
| 5. | Al-Zahem A, Arbuckle S, Cohen R. Combined ileal heterotopic pancreatic and gastric tissues causing ileocolic intussusception in an infant. Pediatr Surg Int. 2006;22:297-299. |
| 6. | Takeyama J, Sato T, Tanaka H, Nio M. Adenomyoma of the stomach mimicking infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2007;42:E11-E12. |
| 8. | Chapple CR, Muller S, Newman J. Gastric adenocarcinoma associated with adenomyoma of the stomach. Postgrad Med J. 1988;64:801-803. |
| 9. | Lasser A, Koufman WB. Adenomyoma of the stomach. Am J Dig Dis. 1977;22:965-969. |
| 10. | Erberich H, Handt S, Mittermayer C, Tietze L. Simultaneous appearance of an adenomyoma and pancreatic heterotopia of the stomach. Virchows Arch. 2000;436:172-174. |
| 11. | Yamagiwa H, Ishihara A, Sekoguchi T, Matsuzaki O. Heterotopic pancreas in surgically resected stomach. Gastroenterol Jpn. 1977;12:380-386. |
Peer reviewer: Dr. Selin Kapan, Associate Professor of General Surgery, Dr. Sadi Konuk Training and Research Hospital, Department of General Surgery, Kucukcekmece, Istanbul 34150, Turkey
S- Editor Wang JL L- Editor Wang XL E- Editor Lin YP