Published online Mar 28, 2010. doi: 10.3748/wjg.v16.i12.1458
Revised: January 18, 2010
Accepted: January 25, 2010
Published online: March 28, 2010
AIM: To explore the anti-fibrotic effect of Haobie Yangyin Ruanjian Decoction (HYRD) on CCl4-induced hepatic fibrosis in rats and its modulation on the transforming growth factor (TGF) β-Smad signaling pathway.
METHODS: Fifty-six healthy Wistar rats were randomly divided into five groups: normal control group (n = 6), CCl4-induced hepatic fibrosis group (n = 14) and three treatment groups (the treated rats received HYRD via oral administration at daily dosages of 8.2, 2.5 and 0.82 g/kg, respectively) of HYRD (n = 12, respectively). Experimental hepatic fibrosis was induced by subcutaneous injection of carbon tetrachloride solution (CCl4 dissolved in peanut oil, 4:6, V/V) with 0.5 mL/100 g body weight for the first time, and then 0.3 mL/100 g body weight twice a week for 8 wk. In the former 2 wk, rats were raised by feedstuff I (80% corn meal, 20% lard, 0.5% cholesterol). After 2 wk, they were raised by feedstuff II (corn meal and 0.5% cholesterol). Except for the control group, 30% alcohol solution was given orally to each rat every other day from the beginning, 1 mL for each rat. Liver function parameters and hepatic hydroxyproline content were detected by chromatometry. Serum levels of hyaluronic acid (HA), type IV collagen (CIV), type III precollagen (PCIII) and laminin (LN) were assayed with radioimmunoassay. Deposition of collagen was observed with hematoxylin-eosin staining and collagen staining. Gene expression of TGFβ1 and Smad3 were detected with real-time reverse transcriptase-polymerase chain reaction and Western blotting, respectively.
RESULTS: The serum levels of alanine transaminase and aspartate transaminase were increased in the model group compared with the control group (P < 0.01), and they were decreased in the three treatment groups compared with the model group. The serum levels of total protein and albumin were decreased in the model group and increased in the three treatment groups. The hepatic hydroxyproline content and serum levels of PCIII, HA, LN and CIV were markedly increased in the model group compared with the control group, and decreased in the treatment groups. The gene expression of TGFβ1 and Smad3 was enhanced in the model group compared with the control group, and HYRD could down regulate their expression.
CONCLUSION: HYRD can inhibit hepatic fibrosis induced by CCl4 in rats, which is probably associated with its down-regulation on fibrogenic signal transduction of TGFβ-Smad pathway.
-
Citation: Yang FR, Fang BW, Lou JS. Effects of
Haobie Yangyin Ruanjian Decoction on hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol 2010; 16(12): 1458-1464 - URL: https://www.wjgnet.com/1007-9327/full/v16/i12/1458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i12.1458
A variety of pathological factors, including viral hepatitis (especially hepatitis B and C), alcohol and drug abuse, metabolic diseases due to overload of iron or copper, autoimmunity against hepatocytes or bile duct epithelium, and congenital abnormalities can cause hepatic injury. Lifestyle changes (mainly exercise withdrawal and weight gain) have probably raised the prevalence of non-alcoholic fatty liver disease(NAFLD), which is the first cause of chronic liver disease in Western world. All these chronic hepatic diseases can cause hepatic fibrosis (HF)[1]. If hepatic fibrosis treatment was delayed, hepatic cirrhosis would be developed. Hepatic fibrosis, cirrhosis in particular, is associated with significant morbidity and mortality. Hepatic fibrosis is characterized by imbalance between extracellular matrix synthesis and degradation. Extracellular matrix mainly results from hepatic stellate cells (HSCs) which can be transformed into myofibroblast initially. Transforming growth factor (TGF) β-Smad signal pathway plays an important role in this process[2], it can activate HSCs and promote collagen synthesis[3]. Research has shown TGF-β1 is a key cytokine in determining fatty liver and non-alcoholic steatohepatitis (NASH)[4]. Therefore, TGF β-Smad signal pathway has become a main target in hepatic fibrosis treatment[5]. Although new therapeutic approaches have recently been proposed, there is no established therapy for hepatic fibrosis. Haobie Yangyin Ruanjian Decoction (HYRD) is a kind of traditional Chinese Medicine. The aim of the present study was to investigate its protective effects and mechanism in a rat model of CCl4-induced hepatic fibrosis.
The composition of HYRD include Herba Artemisiae Annuae, Carapax Trionycis, Salviae Miltiorrhizae, Rhizoma Polygoni Cuspidati, Radix Curcumae.
Fifty-six healthy Wistar rats, female and male, weighing 237.8 ± 8.5 g were obtained from the Experimental Animal Center of Academy of Medical Science of Chinese People’s Liberation Army (Beijing, China). Animal certificate: SCXK-(Army)2007-004. The rats were randomly divided into normal control group (n = 6), model group (n = 14), and three treatment groups (n = 12, respectively). Except for the normal control group, all the rats were subcutaneously injected with solution of carbon tetrachloride dissolved in peanut oil (CCl4: peanut oil = 4:6, V/V), 0.5 mL/100 g body weight for the first time, and then 0.3 mL/100 g body weight twice a week for 8 wk. In the former 2 wk, rats were raised with feedstuff I (80% corn meal, 20% lard, 0.5% cholesterol). After 2 wk, they were raised with feedstuff II (corn meal and 0.5% cholesterol). Except for the normal control group, 30% alcohol solution was given orally to each rat every other day from the beginning, 1 mL for each rat. For the normal control group, the peanut oil was injected to each rat subcutaneously.
The rats in the three treatment groups, including a high-dose group (8.2 g/kg per day, calculated as Decoction), a medium-dose group (2.5 g/kg per day), and a low-dose group (0.82 g/kg per day), were given HYRD daily via oral administration, 1 mL/100 g body weight. Except for the dead, all the rats were anesthetized with ether. Blood was taken from the abdominal vein, centrifuged at 4°C, 3000 r/min, for 20 min, and serum was kept at -20°C for assay.
Serum levels of alanine transaminase (ALT), aspartate transaminase (AST), albumin (Alb) and total protein (TP) were measured using commercially available kits (Jiancheng Inst. Biotechnology, Nanjing, China) according to the manufacturer’s instructions.
Liver tissue (100 mg) was prepared for hydroxyproline (Hyp) determination according to a modified method by Jamall et al[6]. The Hyp content of the liver as an indirect measure of tissue collagen content was expressed as milligram per gram of dry weight (mg/g).
Serum levels of hyaluronic acid (HA), type IV collagen (CIV), type III precollagen (PCIII) and laminin (LN) were determined by radioimmunoassay (RIA) using commercially available kits (Beifang Inst. Biotechnology, Beijing, China) according to the manufacturer’s instructions.
Liver tissues were taken from the left lobe of the liver of each rat, and fixed in 15% buffered paraformaldehyde, and dehydrated in a graded alcohol series. Specimens were embedded in paraffin blocks, cut into 5 μm-thick sections and placed on glass slides. The sections were stained with hematoxylin-eosin (HE) and ponceau’s[7], respectively. Fibrosis was graded according to the method of Scheuer[8] as follows: stage 0: no fibrosis; stage 1: an increase of collagen without the formation of septa (small satellite expansion of the portal fields), expansion of portal tracts without linkage; stage 2: formation of incomplete septa not interconnecting with each other, from the portal tract to the central vein; stage 3: complete but thin septa interconnecting with each other, which divide the parenchyma into separate fragments; and stage 4: complete cirrhosis, similar to stage 3 with thicker septa. Pathological examination was performed by the same pathologist who was blinded to the treatment assignment for the rats.
Total RNA was extracted from liver tissues of each group with the tissue/cell total RNA isolation kit (Trizol Reagent, Invitrogen, USA) according to the manufacturer’s protocol. The quantity and purity of RNA were detected by determining absorbance at 260/280 nm using a spectrophotometer (BECKMAN COULTER Co., USA). Total RNA was reversibly transcribed into complementary DNA (cDNA) using the cDNA synthesis kit [TaKaRa RNA PCR Kit(AMV)Ver 3.0, Dalian, China] according to the manufacturer’s protocol. The ABI PRISM 7900 HT Real Time PCR System (ABI Co., USA) and real-time PCR kit (2 × SYBGreen RT-PCR Master Mix, UCL, USA) were employed based on the manufacturer’s instructions. The specific primers for the target gene and β-actin were synthesized by Dalian TaKaRa Biotechnology Company. TGF-β1: 5'-TGGCGTTACCTTGGTAACC-3' (forward); 5'-GGTGTTGAGCCCTTTCCAG-3' (reverse). β-actin: 5'-ACCCTTAAGGCCAACCGTGAAAAG-3' (forward); 5'-TCATGAGGTAGTCTGTCAGGT-3' (reverse).
Two-step PCR procedure was used as follows: pre-denaturation for 30 s at 95°C, 1 cycle; 94°C for 15 s and 56°C for 40 s, 40 cycles. The final products were identified by electrophoresis in 1.5% agarose gel and melt curve analysis. Melt curve detection: 95°C 15 s, 60°C 15 s and 95°C 15 s. The final results were described with the relative values (2-ΔΔCt). The calculation and analysis were performed by the Sequence Detection Software version 2.1 in the ABI PRISM 7900 HT Real Time PCR System (ABI Co., USA).
Total protein was extracted from liver tissue and analyzed with bicinchoninic acid (BCA) protein concentration assay kit (Beyotime Inst. Biotechnology, Jiangsu, China). Sample protein was separated by electrophoresis in 12% SDS-PAGE separating gel with Bio-Rad electrophoresis system (Bio Rad Laboratories, Hercules, CA, USA). The primary antibodies (rabbit anti Smad3 antibody, 1:1000 dilution, MILLIPORE Inc., USA) were incubated at 4°C overnight. The corresponding horseradish peroxidase-conjugated secondary antibodies (anti-rabbit IgG, 1:5000 dilution, Zhongshanjinqiao Biotechnology Inc., China) were incubated at room temperature. Immobilon™ Western Chemiluminescent HPR Substrate (MILLIPORE Inc., USA) and Quantity ONE (BIO-RAD) were employed for revealing and quantitative analysis of the blots. β-actin protein was used as the internal control.
All values were expressed as mean ± SD. Comparisons were analyzed by one-way ANOVA using the SPSS 12.0 statistical package. Differences were considered statistically significant if the P < 0.05.
The serum levels of ALT and AST were increased in the model group compared with the control group (P < 0.01). Compared with the model group, the serum levels of ALT and AST were decreased in the three treatment groups. The serum levels of TP and Alb were decreased in the model group compared with the control group. Compared with the model group, the serum levels of TP and Alb were increased in the treatment groups (Table 1).
| Groups | ALT (U/L) | AST (U/L) | TP (g/L) | Alb (g/L) |
| Control | 20.94 ± 8.76b | 26.11 ± 11.81b | 76.9 ± 13.1a | 36.3 ± 5.2b |
| Model | 62.40 ± 25.59 | 99.93 ± 38.76 | 45.8 ± 14.8 | 24.3 ± 5.3 |
| Low-dosage HYRD group | 47.92 ± 19.65 | 70.72 ± 28.53a | 53.2 ± 23.5 | 28.0 ± 7.4 |
| Medium-dosage HYRD group | 44.36 ± 20.50a | 74.06 ± 25.14a | 58.8 ± 24.9 | 30.6 ± 7.5a |
| High-dosage HYRD group | 34.29 ± 11.42b | 49.95 ± 19.47b | 67.1 ± 20.2a | 33.0 ± 5.6b |
Hepatic hydroxyproline content was markedly increased in the model group compared with the control group (P < 0.01). Compared with the model group, the levels of hydroxyproline were significantly decreased in the treatment groups (Table 2).
The serum levels of PCIII, HA, LN and CIV were increased in the model group compared with the control group. Compared with the model group, the serum levels of PCIII, HA, LN and CIV were decreased in the treatment groups (Table 3).
| Groups | PCIII (μg/mL) | HA (ng/mL) | LN (ng/mL) | CIV (ng/mL) |
| Control | 15.16 ± 15.12b | 205.30 ± 48.92a | 82.02 ± 8.86 | 21.71 ± 1.76 |
| Model | 35.73 ± 17.90 | 563.82 ± 335.54 | 89.57 ± 7.59 | 29.20 ± 6.17 |
| Low-dosage HYRD group | 27.87 ± 10.13 | 464.19 ± 283.41 | 79.86 ± 9.52 | 26.38 ± 8.61 |
| Medium-dosage HYRD group | 24.72 ± 10.87a | 256.46 ± 95.98b | 86.34 ± 8.30 | 24.41 ± 4.46 |
| High-dosage HYRD group | 20.34 ± 13.92b | 161.51 ± 48.29b | 83.26 ± 13.20 | 26.22 ± 7.97 |
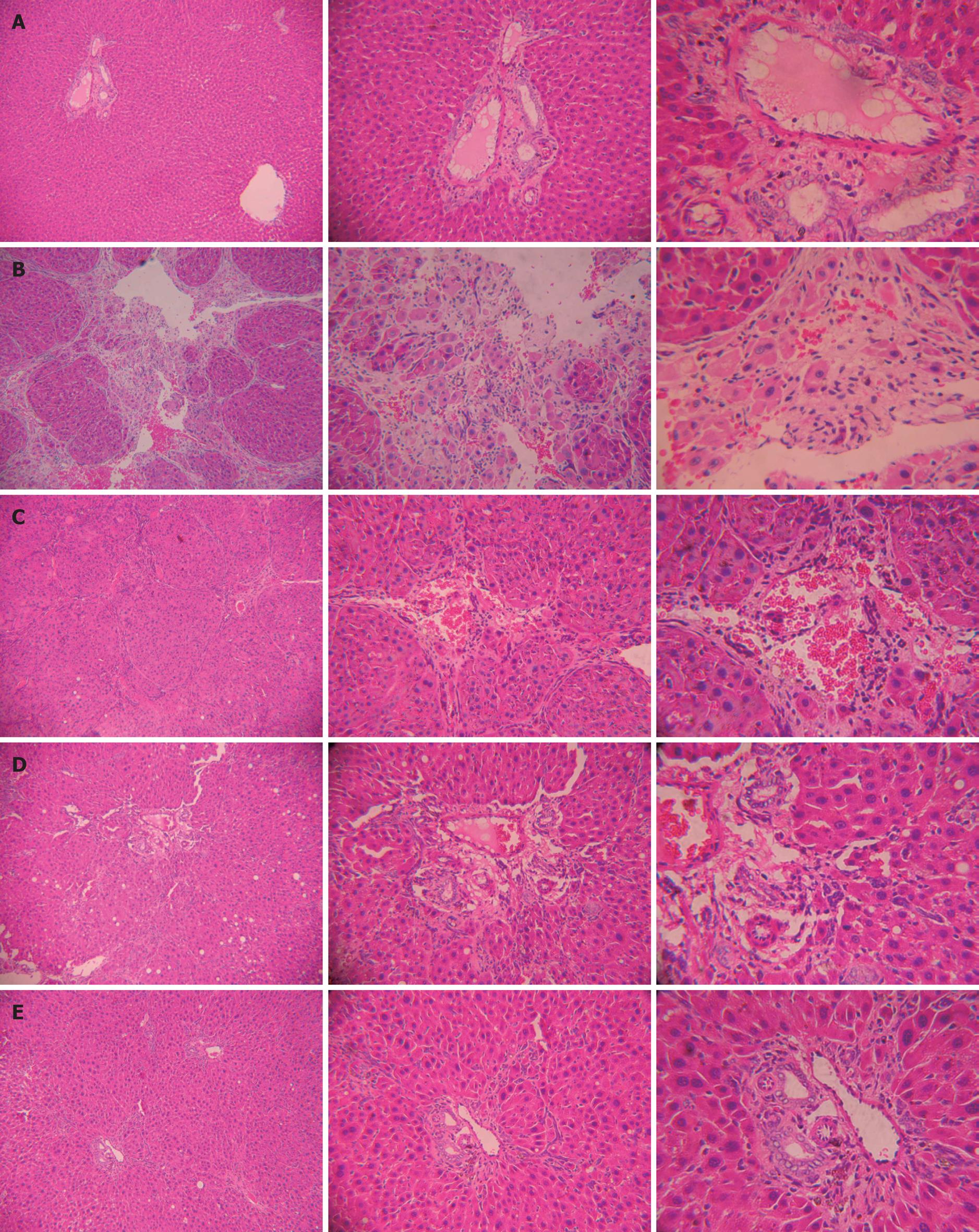
At the end of the study, normal hepatic lobules, without fibroplasia and inflammatory cell infiltration could be observed in normal rats (Figure 1A). Complete septa interconnecting with each other were formed, which divided the parenchyma into separate fragments and a great number of inflammatory cells were infiltrated in intralobules and interlobules, cell degeneration and focal necrosis were found in rats with hepatic fibrosis (Figure 1B), which were improved after HYRD treatment (Figure 1C-E).
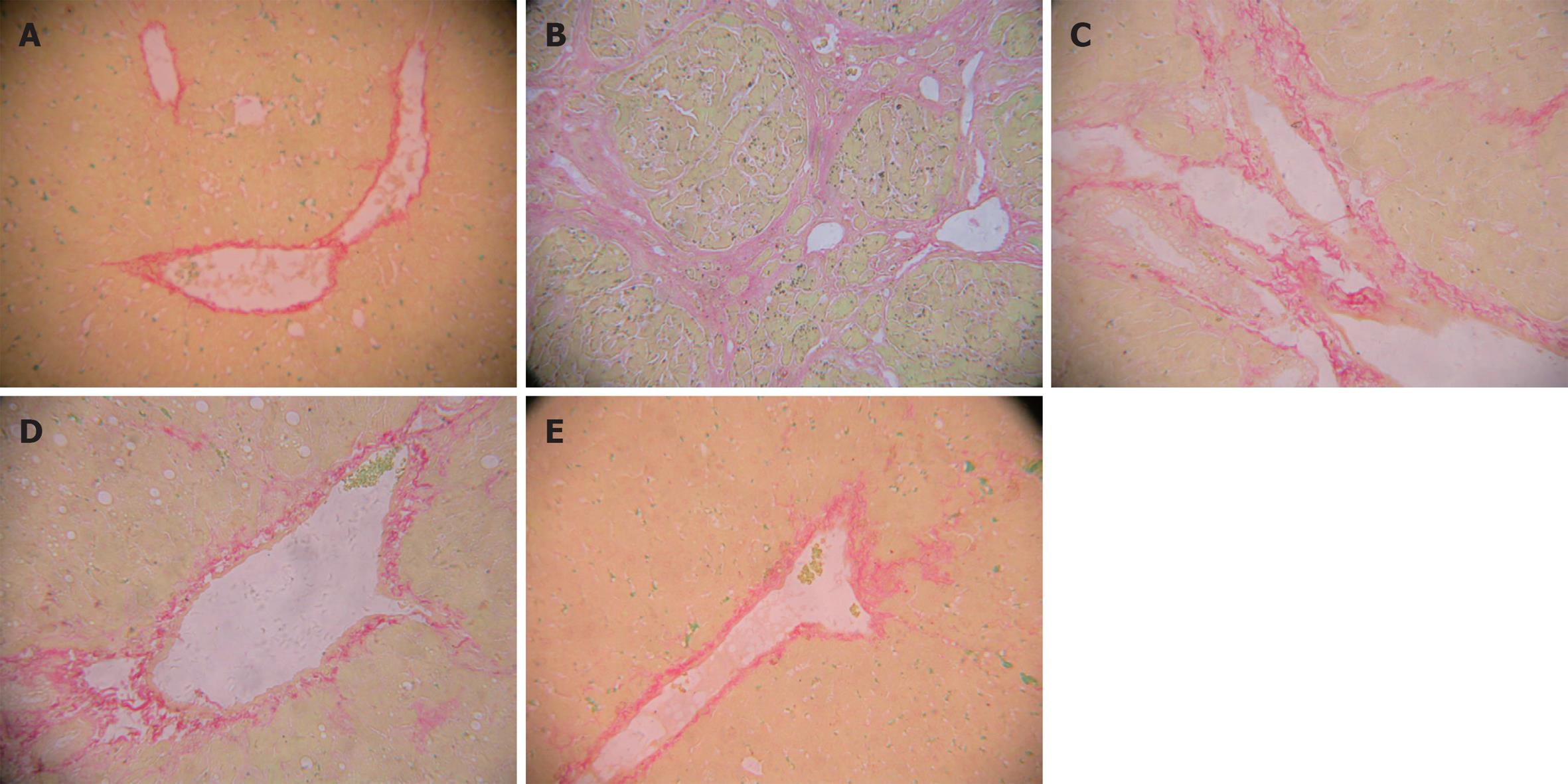
The rat liver was stained with ponceau’s, the collagen fiber was shown red. Collagen deposition was markedly increased in the model group compared with the control group (P < 0.01). Compared with the model group, collagen deposition was significantly decreased in the treatment groups (P < 0.01) (Table 4, Figure 2).
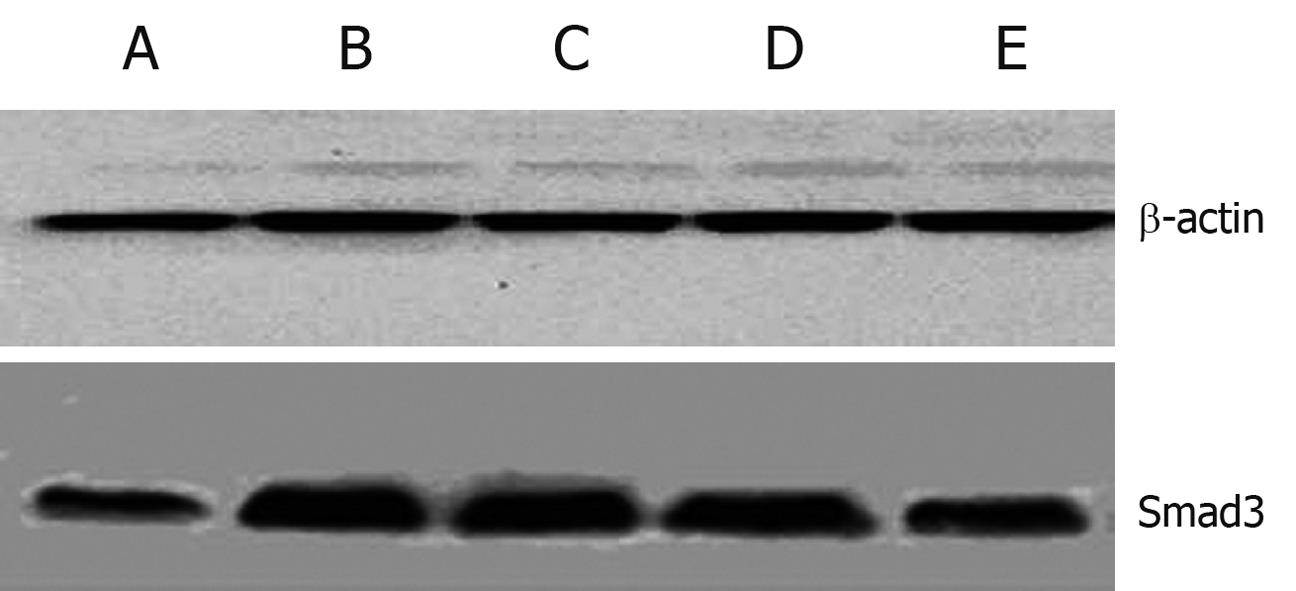
Expression of TGF-β1 and Smad3 in liver was increased significantly in the model group (P < 0.01), and decreased obviously in the treatment groups (Table 5, Figure 3).
Hepatic fibrosis is thought to be a reversible disease, CCl4-induced hepatic fibrosis in rats is a reproducible model for studying the pathogenesis of hepatic fibrosis and cirrhosis[9]. CCl4 can cause oxidative stress reaction via toxic free radicals. Lipid peroxidation of cytomembrane plays an important role in early stage of hepatic injury, which can activate lipocyte (HSCs). The activation of HSCs “induced by some critical cytokines” is considered to be of great importance during the long period of hepatic fibrosis[10]. This activated HSC then becomes the main source of most cytokines and collagen proteins. Among the cytokines mediating factors, TGF-β1 has been demonstrated in most researches to be an essential pro-fibrogenesis factor[11-15]. In addition to that, TGFβ-Smad signaling pathway is the main pathway of TGF-β1[16-18], which transfers the stimulating signal from outside into the affected cells. The Smad proteins consist of a large family of transcription factors. Smads are TGF-β receptor substrates with a demonstrated ability to propagate signals. Briefly, two different transmembrane protein serine/threonine kinases, named TGF-β receptor type I and II, respectively, are brought together by the ligand, which acts as a receptor assembly factor[19]. Before this occurs, receptor I is inactive. During the TGF-β signal transduction, receptor II is activated first. TGF-β and its receptor then form an activated complex. In the ligand-induced complex, active receptor II activates the receptor I kinase. The type I receptors specifically recognize the Smad subgroup known as receptor-activated Smads (R-Smads), which are Smad 2 and Smad 3[20]. Then R-Smads are activated and form a complex consisting of R-Smads and Smad 4, which belong to Co-Smad. The Smads-complex then is accumulated in the nucleus. This procedure leads to the formation of the functional transcriptional complexes. Both the R-Smads and the Co-Smads in this complex may participate in DNA-binding and recruitment of transcriptional cofactors[21,22]. After transferring into the nucleus, the transcriptional complex binds to the certain domain of the target gene and cause the gene expression such as collagen production. The excess collagen production would lead to collagen deposition in liver tissue and hepatic fibrosis or cirrhosis at last. Thus, the down- regulation of TGFβ expression, modulation of TGFβ-Smad signaling and inhibition of the accumulation of activated HSCs by modulating either their activation and/or proliferation or promoting their apoptosis are important therapeutic strategy.
According to the Chinese Medicine theories, hepatic fibrosis is caused by internal damp (Shi), heat (Re), poison (Du), blood stasis (Yu), and both Qi and Yin asthenia. In this study, not only CCl4 but also cholesterol, lard and alcohol were used to establish a hepatic fibrosis model. CCl4 is poison, cholesterol, lard and alcohol produce damp and heat, which cause healthy energy asthenia, blood stasis exacerbation, unrelievable damp and heat, and induce hepatic fibrosis. Thus, the main Chinese Medicine approach to hepatic fibrosis is to eliminate heat, dispel damp, activate blood, promote Qi and cultivate Yin[23,24]. HYRD is composed of Herba Artemisiae Annuae, Carapax Trionycis, Salviae Miltiorrhizae, Rhizoma Polygoni Cuspidati, Radix Curcumae. The Decoction can activate blood and remove stasis, clear heat and eliminate damp, soften hard lumps and dispel nodes.
Herba Artemisiae Annuae can improve immunocyte activity, whose derivative artesunate can inhibit hepatic fibrosis[25], and Carapax Trionycis can inhibit hepatic collagen deposition,promote collagen degradation[26]. Salviae Miltiorrhizae and Rhizoma Polygoni Cuspidati can inhibit lipid peroxidation, reinforce organism immunity, improve hepatic microcirculation, inhibit adhesion of leucocyte and platelet to endotheliocyte, promote hepatocyte regeneration and collagen degradation[27,28]. Radix Curcumae can activate blood and remove stasis, clear heat and eliminate damp[29].
In conclusion, HYRD can inhibit lipid peroxidation, improve hepatic function, lessen collagen deposition, and prevent hepatic fibrosis via modulating TGFβ-Smad signaling pathway.
In China, the incidence of hepatic cirrhosis is still high. If treated properly at fibrosis stage, cirrhosis can be prevented. However, no effective antifibrotic drugs are available at present. According to the Chinese Medicine theories, hepatic fibrosis is caused by internal damp (Shi), heat (Re), poison (Du), blood stasis (Yu), and both Qi and Yin asthenia. Haobie Yangyin Ruanjian Decoction (HYRD) can activate blood and remove stasis, clear heat and eliminate damp, soften hard lumps and dispel nodes.
Recent research showed hepatic fibrosis can be reversed by regulating collagen metabolism, inhibiting hepatic stellate cell (HSC) activation or by promoting HSC apoptosis. Hepatic extracellular matrix mainly results from HSC, which can be activated by fibrogenesis signal pathway.
This study has confirmed that HYRD can improve liver function, alleviate hepatic fibrosis, which is probably associated with its down-regulation on fibrogenic signal transduction of transforming growth factor (TGF) β-Smad pathway.
The HYRD can prevent hepatic fibrosis, which implies that it will be a good medicine for patients with chronic hepatic injury, this article can provide some scientific data for its application and development.
This paper has reinforced my conviction that there is some good in this therapeutical approach. The study is statistically well-managed. Data are clear and convincing.
| 1. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. |
| 2. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. |
| 3. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. |
| 4. | Tarantino G, Conca P, Riccio A, Tarantino M, Di Minno MN, Chianese D, Pasanisi F, Contaldo F, Scopacasa F, Capone D. Enhanced serum concentrations of transforming growth factor-beta1 in simple fatty liver: is it really benign. J Transl Med. 2008;6:72. |
| 5. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. |
| 6. | Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70-75. |
| 7. | Gong ZJ, Zhan RZ. Common special staining methods. Pathological technique. Shanghai: Shanghai Science and Technology Press 1994; 70. |
| 8. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. |
| 9. | Mu YP, Liu P, Long AH. [Study on pathogenesis of CCl4 induced cirrhosis formation in rats based on the recipe used]. Zhongguo Zhongxiyi Jiehe Zazhi. 2006;26:344-347. |
| 10. | Safadi R, Friedman SL. Hepatic fibrosis--role of hepatic stellate cell activation. MedGenMed. 2002;4:27. |
| 11. | Tahashi Y, Matsuzaki K, Date M, Yoshida K, Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y, Inoue K. Differential regulation of TGF-beta signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology. 2002;35:49-61. |
| 12. | Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83-92. |
| 13. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. |
| 14. | Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659-693. |
| 15. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. |
| 16. | Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176-184. |
| 17. | Runyan CE, Poncelet AC, Schnaper HW. TGF-beta receptor-binding proteins: complex interactions. Cell Signal. 2006;18:2077-2088. |
| 18. | Xu L. Regulation of Smad activities. Biochim Biophys Acta. 2006;1759:503-513. |
| 19. | Hill CS. Identification of a Smad phosphatase. ACS Chem Biol. 2006;1:346-348. |
| 20. | Wicks SJ, Grocott T, Haros K, Maillard M, ten Dijke P, Chantry A. Reversible ubiquitination regulates the Smad/TGF-beta signalling pathway. Biochem Soc Trans. 2006;34:761-763. |
| 21. | Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211-215. |
| 22. | Verrecchia F, Mauviel A. Control of connective tissue gene expression by TGF beta: role of Smad proteins in fibrosis. Curr Rheumatol Rep. 2002;4:143-149. |
| 23. | Liu P, Hu YY, Ni LQ. [On establishing comparative reference system for syndrome classification study from the thinking characteristics of syndrome differentiation dependent therapy]. Zhongguo Zhongxiyi Jiehe Zazhi. 2006;26:451-454. |
| 24. | Zhang Q, Liu P, Chen HF, Chen L, Cao SH, Liu Y, Wei JJ, Fang ZH, Wu DZ. Multi-analysis of characteristics of traditional Chinese medical syndrome of hepatocirrhosis. Zhongguo Zhongxiyi Jiehe Ganbing Zaizhi. 2003;13:69-72. |
| 25. | Lai LN, Fang BW. Effects of artesunate on proliferation of HSCs. Zhongyao Yaoli Yu Linchuang. 2006;22:25-27. |
| 26. | Kuang WH, Li JH. Effect of Biejia Jian in preventing rat hepatic fibrosis. Zhongyao Xinyao Yu Linchuang Yaoli. 2004;15:314-317. |
| 27. | She SF, Huang XZ, Tong GD. [Clinical study on treatment of liver fibrosis by different dosages of Salvia injection]. Zhongguo Zhongxiyi Jiehe Zazhi. 2004;24:17-20. |
| 28. | Zhang B, WangLT , ChengJJ , WangZH , ZhaoY . Clinical observation of "HuzhangQingganDecoction" on chronic hepatitis and on hepatic fibrosis formation. Shanghai Zhongyiyao Daxue Xuebao. 2007;21:37-39. |
| 29. | Wang JB, Zhang Y. Clinical application of Curcuma aromatica. Jiangsu Zhongyiyao. 2005;26:59-61. |
Peer reviewer: Giovanni Tarantino, MD, Professor, Department of Clinical and Experimental Medicine, Federico II University Medical School, VIA S. PANSINI, 5, Naples 80131, Italy
S- Editor Wang JL L- Editor Ma JY E- Editor Lin YP