Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1215
Revised: January 5, 2010
Accepted: January 12, 2010
Published online: March 14, 2010
AIM: To clarify the mechanism by which bone marrow cells promote angiogenesis around transplanted islets.
METHODS: Streptozotocin induced diabetic BALB/c mice were transplanted syngeneically under the kidney capsule with the following: (1) 200 islets (islet group: n = 12), (2) 1-5 × 106 bone marrow cells (bone marrow group: n = 11), (3) 200 islets and 1-5 × 106 bone marrow cells (islet + bone marrow group: n = 13), or (4) no cells (sham group: n = 5). All mice were evaluated for blood glucose, serum insulin, serum nerve growth factor (NGF) and glucose tolerance (GTT) up to postoperative day (POD) 14. Histological assessment for insulin, von Willebrand factor (vWF) and NGF was performed at POD 3, 7 and 14.
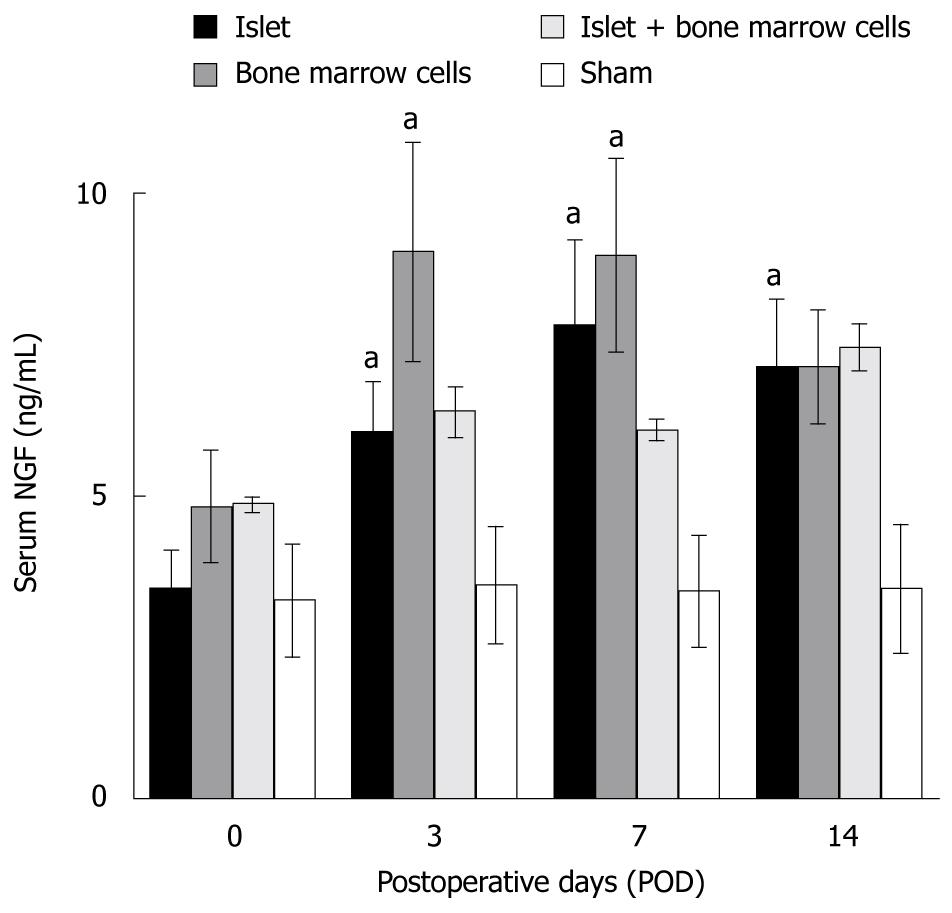
RESULTS: Blood glucose level was lowest and serum insulin was highest in the islet + bone marrow group. Serum NGF increased in islet, bone marrow, and islet + bone marrow groups after transplantation, and there was a significant difference (P = 0.0496, ANOVA) between the bone marrow and sham groups. The number of vessels within the graft area was significantly increased in both the bone marrow and islet + bone marrow groups at POD 14 as compared to the islet alone group (21.2 ± 3.6 in bone marrow, P = 0.01, vs islet group, 22.6 ± 1.9 in islet + bone marrow, P = 0.0003, vs islet group, 5.3 ± 1.6 in islet-alone transplants). NGF was more strongly expressed in bone marrow cells compared with islets.
CONCLUSION: Bone marrow cells produce NGF and promote angiogenesis. Islet co-transplantation with bone marrow is associated with improvement of islet graft function.
- Citation: Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E. Bone marrow cells produce nerve growth factor and promote angiogenesis around transplanted islets. World J Gastroenterol 2010; 16(10): 1215-1220
- URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1215
Islet transplantation is a promising treatment for type 1 diabetes mellitus (T1DM). However, clinical islet transplantation using current protocols has not yet yielded long-term insulin-independence[1]. One of the hurdles to overcome is the lack of a vascular network to support the newly transplanted islets. Initial avascularity in the period during islet isolation, transplantation[2,3], and the establishment of a vascular network around islets renders islets vulnerable to severe hypoxia for up to 14 d after transplantation[4]. Therefore, the promotion of angiogenesis is an important endeavor to prevent islet graft failure.
Recently, bone marrow transplantation as a cell therapy for resolution of clinical diseases has been studied. Endothelial precursor cells (EPCs), a heterogeneous population originating in the hematopoietic compartment of bone marrow, have an important role in the angiogenesis of adult tissues[5]. Transplanted EPCs induce hypoxia-inducible factor-1α (HIF-1α) under hypoxic conditions which leads to upregulation of vascular endothelial growth factor (VEGF) and promotes vascularization[5-7]. Our previous study revealed that transplanted bone marrow produces VEGF and promotes vascularization around the co-transplanted islets[8].
Nerve growth factor (NGF), which plays an important role in promoting growth, differentiation and function of nerve cells[9,10] has been shown to have an important role in angiogenesis by stimulating VEGF[10,11]. Moreover, NGF is secreted by islets and may have a beneficial effect on islet function[12]. In this study, we focused on NGF levels and its effects to clarify the mechanism of angiogenesis brought by bone marrow cell transplantation.
BALB/c male mice (22-27 g, Charles River Laboratories. Inc., Boston, MA, USA) were used as both donors and recipients. The mice were housed under pathogen-free conditions with a 12-h light cycle and free access to food and water. All animal care and treatment procedures were approved by the Institutional Animal Care Use Committee.
Streptozotocin (STZ, 200 mg/kg per mouse, Sigma-Aldrich, St. Lois, MO, USA) was injected intraperitoneally and blood glucose levels were measured by Accu-Chek Aviva glucose monitors (Roche, Indianapolis, IN, USA). We used the recipient mice once the blood glucose level was greater than 19.3 mmol/L (350 mg/dL).
Murine islets were isolated by collagenase (collagenase V, Sigma-Aldrich) digestion, and separated by Ficoll (Sigma-Aldrich) discontinuous gradients and purified as previously described[13]. Collected islets were 133-200 μm in size[14]. Based on previous results[15], 200 islets were considered a marginal islet mass for restoring normoglycemia in streptozotocin-induced diabetes.
The protocol of bone marrow cell isolation was modified from the Soleimani method[16]. Under general anesthesia with 2% isoflurane, hind limb extirpations were performed at the hip, knee and ankle joint. Muscle and connective tissue were dissected away from the femur and tibia, the knee joint and both ends of the bones were cut. A thirty gauge needle with a 1 mL syringe was inserted into the bone, and bone marrow was flushed by injection with Hanks balanced salt solution (HBSS) for collection. After a single wash, bone marrow was dispersed by incubation with 0.05% trypsin/0.53 mmol/L EDTA solution (Mediatech Inc., Manassas, VA, USA).
Cell transplantation was performed under the left kidney capsule. Recipients were divided into four graft groups: (1) islet-alone (200 syngeneic islets per recipient, n = 12), (2) bone marrow (1-5 × 106 syngeneic bone marrow cells per recipient, n = 11), (3) islet + bone marrow (200 syngeneic islets and 1-5 × 106 syngeneic bone marrow cells, n = 13) and (4) sham (skin and renal capsule incisions with no transplantation, n = 5).
Blood glucose and serum insulin were measured at postoperative days (POD) 0, 3, 7 and 14. Glucose tolerance was assessed at POD 7 and 14. Achievement of normoglycemia was defined as a non-fasting blood glucose level of ≤ 11 mmol/L (200 mg/dL). Intraperitoneal glucose tolerance tests (GTT) were performed by overnight fasting for 10 h and then injecting mice with a 2.0 g/kg body weight of glucose solution followed by tail vein blood samples at 0, 15, 30, 60, 90 and 120 min after injection. Blood glucose levels were measured by Accu-Chek Aviva glucose monitors and serum insulin was measured with a rat/mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Linco, MO).
Blood samples for serum NGF were obtained at POD 0, 3, 7 and 14 as for serum insulin. Serum NGF was measured with a NGF ELISA kit (NGF Emax® ImmunoAssay System, Promega, Madison, WI, USA).
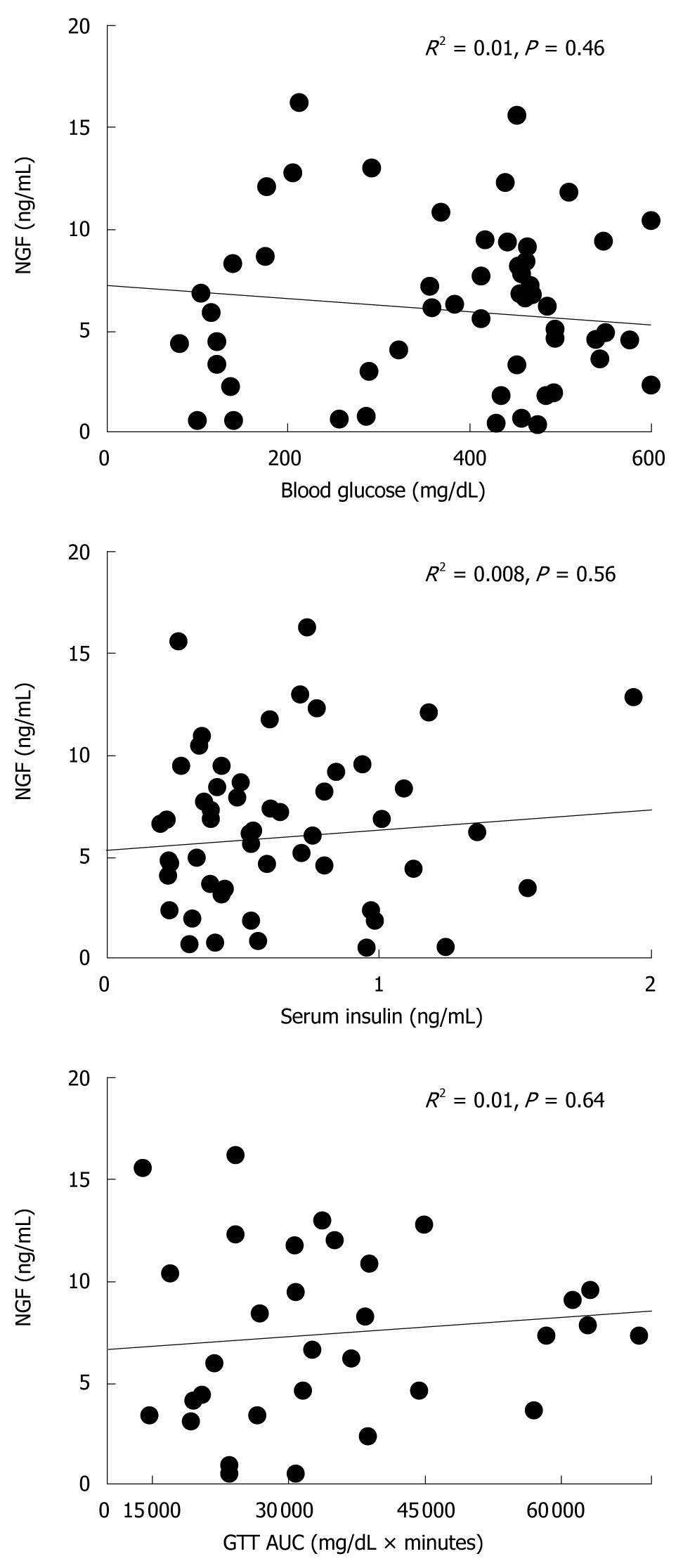
To evaluate whether NGF level affects islet function, simple regression analysis was performed between serum NGF and each of the following parameters: blood glucose, serum insulin and area under the curve (AUC)[17]. All the data acquired from POD 0 to 14 were applied to the analysis.
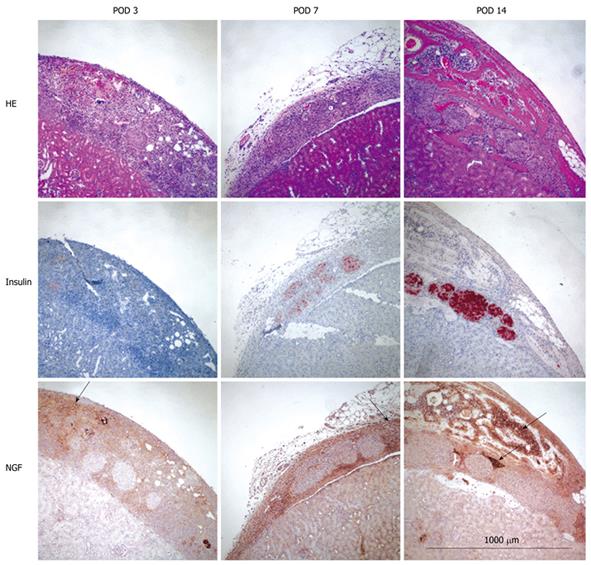
Kidney specimens were obtained from three or more mice at POD 3, 7 and 14 and photographs of the fresh organs were taken to assess the density of new vessels around islets and/or bone marrow. Tissue was then fixed with 10% formalin, embedded in paraffin, and cut into 5 μm sections. Specimens were stained with hematoxylin and eosin (HE) to identify cellular changes. Apoptosis was detected by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) method using an in situ apoptosis detection kit (Promega). Sections were treated with proteinase K and incubated with TdT enzyme for 60 min at 37°C. After washing in Phosphate buffer solution (PBS) the sections were further incubated with streptavidin horseradish peroxidase (HRP) solution and visualized with 3,3'-diaminobenzidine (DAB). Immunohistochemical staining was done for insulin (islets), von Willebrand Factor (vWF, for newly formed blood vessels) and NGF. For vWF, specimens were treated with Proteinase K (Dako, Carpinteria, CA). Primary antibodies were guinea pig anti-insulin antibody (Dako, Carpunteria, CA, USA) diluted 1:100, rabbit anti-vWF (Abcam, Cambridge, MA) diluted with 1:2000 and rabbit anti-NGF (Santa Cruz Biotechnology Inc.) diluted 1:100. After incubating with biotinylated secondary IgG antibody (Vector Laboratories, Burlingame, CA, USA and Santa Cruz Inc.), a peroxidase substrate solution containing DAB (Brown, Dako) or aminoethylcarbazol (AEC, Red for insulin, Dako) was used for visualization and counterstained with hematoxylin.
vWF-positive vessel numbers were calculated from vWF-positive lumens at the transplant site.
Data are expressed as mean ± SE of the mean. All the statistical analyses were performed with JMP 5.0.1J for Macintosh (SAS Institute Inc., Cary, NC, USA). Dunnet t-test or analysis of variance (ANOVA) was performed. Significance was designated at P < 0.05. Correlation coefficients (R2) in regression analysis were defined as follows: Very strong correlation as 0.8 < R2 < 1.0; strong as 0.5 < R2 < 0.8, moderate as 0.25 < R2 < 0.5; weak R2 < 0.25. Correlation was designated positive when the R2 was over 0.25 (moderate).
These data were previously published[8]. In summary, islet co-transplantation with bone marrow yielded lower blood glucose, higher serum insulin, and improved glucose tolerance.
Serum NGF levels were higher in islet, bone marrow and islet + bone marrow groups than in the sham group, and were higher at all times points relative to pre-transplant levels (P < 0.05, Figure 1). The increase was most prominent in the bone marrow versus the sham group (P = 0.0496, ANOVA).
These data were previously published[8]. In summary, bone marrow transplantation with or without islets was associated with enhanced angiogenesis which was more prominent in bone marrow alone than in the combined islet-bone marrow group [21.2 ± 3.6 in bone marrow (P = 0.01, vs islet group), 22.6 ± 1.9 in islet + bone marrow (P = 0.0003, vs islet group), 5.3 ± 1.6 in islet-alone transplants].
NGF was much more strongly expressed in bone marrow cells as compared to islets at every time point (Figure 2). No apoptotic (TUNEL positive) islets were detected in any of the experimental groups (data not shown).
There were no significant correlations between serum NGF and blood glucose, serum insulin or glucose tolerance (Figure 3).
NGF is a neurotrophin that plays a crucial role in promoting growth, differentiation and function of sympathetic nerve cells[9,10,18]. NGF levels decrease with diabetes and are correlated with neuropathy. Thus a therapeutic trial to increase NGF has been performed to improve diabetic neuropathy[19]. Recently, some studies have reported the angiogenic effects of NGF[10,20-25]. Earlier studies showed that NGF promotes the neovascularization of endothelial cells using HUVEC or matrigel assays[20,24].
In diabetes, NGF has been shown to reverse local tissue hypoxia and endothelial cell impairment[23,25]. Treatment with NGF prevents apoptosis of endothelial cells related with neovascular formation and progress[22-25]. Angiogenesis induced by NGF is presumed to help in wound closure[25] and recovery of ischemia in diabetics[23], as well as organ remodeling[26]. NGF may also contribute to the progress, migration and metastasis of tumors[21,27].
We have previously shown that treatment with NGF improved islet function in vitro and in vivo and promoted vessel formation around transplanted islets[12]. NGF may have a role in islet transplantation by promoting angiogenesis and preventing hypoxia at the early post-transplant period. However, this remains to be tested and reproduced in appropriate trials.
In a previous study, we showed an association between bone marrow cell transplantation and angiogenesis around islets, together with enhanced VEGF expression and improved islet function[8]. A potential role for NGF in these improvements was the focus of this study in view of reports that NGF is derived from bone marrow stem cells[28] and stimulates VEGF, promoting angiogenesis[10,11]. NGF’s stronger expression in bone marrow relative to islets in this study was not predicted especially in view of the increase in serum NGF level in the islet group though it may be a function of ambient glucose concentration[29]. Effects on islet graft function may also be related to the type of NGF receptor activated in the context of bone marrow co-transplantation[30].
NGF is induced by hypoxia[23] and also has anti-apoptotic roles[31], therefore, it has the potential to improve the function and survival of transplanted islets[12]. No apoptotic islets were detected during the observation span of this study, but renal subcapsular islet transplantation may not uniformly manifest the effects of severe ischemia seen with intraportal islet transplantation[14]. Therefore, NGF may be particularly beneficial for intraportal islet transplantation.
The lack of correlation between serum NGF and other islet parameters (blood glucose, serum insulin and GTT) remains to be further confirmed. NGF levels vary according to the condition of islets. For example, one study revealed that STZ stimulation increased NGF secretion[32] while another showed that NGF is decreased in diabetes-associated conditions[23]. On the other hand, NGF may reflect the status of islet vascularization rather than its function.
In conclusion, NGF production may underlie the beneficial effect of bone marrow co-transplantation on islet graft function. The mechanism of this potential benefit deserves further investigation.
Islet transplantation is a promising treatment for type 1 diabetes mellitus (T1DM). However, clinical islet transplantation using current protocols has not yet yielded long-term insulin-independence. One of the hurdles to overcome is the lack of a vascular network to support the newly transplanted islets. The promotion of angiogenesis is an important endeavor to prevent islet graft failure.
Nerve growth factor (NGF) is a neurotrophin that plays a crucial role in promoting growth, differentiation and function of sympathetic nerve cells. NGF levels decrease with diabetes and are correlated with neuropathy. Thus a therapeutic trial to increase NGF has been performed to improve diabetic neuropathy. Recently, some studies have reported the angiogenic effects of NGF.
NGF may have a role in islet transplantation by promoting angiogenesis and preventing hypoxia at the early post-transplant period. However, this remains to be tested and reproduced in appropriate trials.
Bone marrow cells produce NGF and promote angiogenesis. Islet co-transplantation with bone marrow is associated with improvement of islet graft function.
Islet transplantation: islet transplantation is one of the therapies for T1DM. Islets are acquired from donor pancreas with the process of islet isolation. Acquired islets are transplanted into liver. Islet transplantation is a possibility for standard treatment of T1DM in the future.
In this manuscript Sakata et al investigated whether islet transplantation in association with bone marrow transplantation improves diabetes related complications in streptozotocin induced diabetic mice. Although bone marrow cells yield similar results through vascular endothelial growth factor expression and this study is in press, their findings with NGF are still interesting and validates publication.
| 1. | Alejandro R, Barton FB, Hering BJ, Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86:1783-1788. |
| 2. | Pileggi A, Ricordi C, Alessiani M, Inverardi L. Factors influencing Islet of Langerhans graft function and monitoring. Clin Chim Acta. 2001;310:3-16. |
| 3. | Emamaullee JA, Rajotte RV, Liston P, Korneluk RG, Lakey JR, Shapiro AM, Elliott JF. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54:2541-2548. |
| 4. | Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg. 2001;25:509-515. |
| 5. | Sepúlveda P, Martinez-León J, García-Verdugo JM. Neoangiogenesis with endothelial precursors for the treatment of ischemia. Transplant Proc. 2007;39:2089-2094. |
| 6. | Milovanova TN, Bhopale VM, Sorokina EM, Moore JS, Hunt TK, Hauer-Jensen M, Velazquez OC, Thom SR. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. J Appl Physiol. 2009;106:711-728. |
| 7. | Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036-1044. |
| 8. | Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E. Bone Marrow Cell Cotransplantation With Islets Improves Their Vascularization and Function. Transplantation. 2010;Epub ahead of print. |
| 9. | Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520. |
| 10. | Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci USA. 2001;98:4160-4165. |
| 11. | Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257-2262. |
| 12. | Miao G, Mace J, Kirby M, Hopper A, Peverini R, Chinnock R, Shapiro J, Hathout E. In vitro and in vivo improvement of islet survival following treatment with nerve growth factor. Transplantation. 2006;81:519-524. |
| 13. | Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437-438. |
| 14. | Sakata N, Hayes P, Tan A, Chan NK, Mace J, Peverini R, Sowers L, Pearce WJ, Chinnock R, Obenaus A. MRI assessment of ischemic liver after intraportal islet transplantation. Transplantation. 2009;87:825-830. |
| 15. | Sakata N, Tan A, Chan N, Obenaus A, Mace J, Peverini R, Sowers L, Chinnock R, Hathout E. Efficacy comparison between intraportal and subcapsular islet transplants in a murine diabetic model. Transplant Proc. 2009;41:346-349. |
| 16. | Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102-106. |
| 17. | Sakata N, Sumi S, Gu Y, Qi M, Yamamoto C, Sunamura M, Egawa S, Unno M, Matsuno S, Inoue K. Hyperglycemia and diabetic renal change in a model of polyvinyl alcohol bioartificial pancreas transplantation. Pancreas. 2007;34:458-465. |
| 18. | Ahmed Z, Brown RA, Ljungberg C, Wiberg M, Terenghi G. Nerve growth factor enhances peripheral nerve regeneration in non-human primates. Scand J Plast Reconstr Surg Hand Surg. 1999;33:393-401. |
| 19. | Hanaoka Y, Ohi T, Furukawa S, Furukawa Y, Hayashi K, Matsukura S. Effect of 4-methylcatechol on sciatic nerve growth factor level and motor nerve conduction velocity in experimental diabetic neuropathic process in rats. Exp Neurol. 1992;115:292-296. |
| 20. | Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappalà G, Pafumi C, Bernardini R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307-1309. |
| 21. | Davidson B, Reich R, Lazarovici P, Nesland JM, Skrede M, Risberg B, Tropé CG, Flørenes VA. Expression and activation of the nerve growth factor receptor TrkA in serous ovarian carcinoma. Clin Cancer Res. 2003;9:2248-2259. |
| 22. | Seo K, Choi J, Park M, Rhee C. Angiogenesis effects of nerve growth factor (NGF) on rat corneas. J Vet Sci. 2001;2:125-130. |
| 23. | Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by Type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47:1055-1063. |
| 24. | Park MJ, Kwak HJ, Lee HC, Yoo DH, Park IC, Kim MS, Lee SH, Rhee CH, Hong SI. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol 3-kinase/Akt signaling pathway and AP-2 transcription factor. J Biol Chem. 2007;282:30485-30496. |
| 25. | Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, Manni L, Madeddu P. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of Type 1 diabetic mice. Diabetologia. 2004;47:1047-1054. |
| 26. | Li C, Stanton JA, Robertson TM, Suttie JM, Sheard PW, Harris AJ, Clark DE. Nerve growth factor mRNA expression in the regenerating antler tip of red deer (Cervus elaphus). PLoS One. 2007;2:e148. |
| 27. | Adriaenssens E, Vanhecke E, Saule P, Mougel A, Page A, Romon R, Nurcombe V, Le Bourhis X, Hondermarck H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008;68:346-351. |
| 28. | Hokari M, Kuroda S, Shichinohe H, Yano S, Hida K, Iwasaki Y. Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J Neurosci Res. 2008;86:1024-1035. |
| 29. | Rosenbaum T, Vidaltamayo R, Sánchez-Soto MC, Zentella A, Hiriart M. Pancreatic beta cells synthesize and secrete nerve growth factor. Proc Natl Acad Sci USA. 1998;95:7784-7788. |
| 30. | Raile K, Klammt J, Garten A, Laue S, Blüher M, Kralisch S, Klöting N, Kiess W. Glucose regulates expression of the nerve growth factor (NGF) receptors TrkA and p75NTR in rat islets and INS-1E beta-cells. Regul Pept. 2006;135:30-38. |
| 31. | Com E, Lagadec C, Page A, El Yazidi-Belkoura I, Slomianny C, Spencer A, Hammache D, Rudkin BB, Hondermarck H. Nerve growth factor receptor TrkA signaling in breast cancer cells involves Ku70 to prevent apoptosis. Mol Cell Proteomics. 2007;6:1842-1854. |
| 32. | Larrieta ME, Vital P, Mendoza-Rodríguez A, Cerbón M, Hiriart M. Nerve growth factor increases in pancreatic beta cells after streptozotocin-induced damage in rats. Exp Biol Med (Maywood). 2006;231:396-402. |
Peer reviewer: Dr. Salih Sanlioglu, Professor, VMD, PhD, Department of Medical Genetics, Human Gene Therapy Division of Akdeniz University Faculty of Medicine, Dumlupinar Boulevard Campus, Antalya 07058, Turkey
S- Editor Wang JL L- Editor O’Neill M E- Editor Ma WH