Published online Jan 7, 2010. doi: 10.3748/wjg.v16.i1.56
Revised: November 23, 2009
Accepted: November 30, 2009
Published online: January 7, 2010
AIM: To assess the feasibility and utility of double balloon enteroscopy (DBE) in the management of small bowel diseases in children.
METHODS: Fourteen patients (10 males) with a median age of 12.9 years (range 8.1-16.7) underwent DBE; 5 for Peutz-Jeghers syndrome (PJ syndrome), 2 for chronic abdominal pain, 4 for obscure gastrointestinal (GI) bleeding, 2 with angiomatous malformations (1 blue rubber bleb nevus syndrome) having persistent GI bleeding, and 1 with Cowden’s syndrome with multiple polyps and previous intussusception. Eleven procedures were performed under general anesthesia and 3 with deep sedation.
RESULTS: The entire small bowel was examined in 6 patients, and a length between 200 cm and 320 cm distal to pylorus in the remaining 8. Seven patients had both antegrade (trans-oral) and retrograde (trans-anal and via ileostomy) examinations. One patient underwent DBE with planned laparoscopic assistance. The remaining 6 had trans-oral examination only. The median examination time was 118 min (range 95-195). No complications were encountered. Polyps were detected and successfully removed in all 5 patients with PJ syndrome, in a patient with tubulo-villous adenoma of the duodenum, in a patient with significant anemia and occult bleeding, and in a patient with Cowden’s syndrome. A diagnosis was made in a patient with multiple angiomata not amenable to endotherapy, and in 1 with a discrete angioma which was treated with argon plasma coagulation. The source of bleeding was identified in a further patient with varices. DBE was normal or revealed minor mucosal friability in the remaining 3 patients. Hence a diagnostic yield of 11/14 with therapeutic success in 9/14 was achieved.
CONCLUSION: Double balloon enteroscopy can be a useful diagnostic and therapeutic tool for small bowel disease in children, allowing endo-therapeutic intervention beyond the reach of the conventional endoscope.
- Citation: Thomson M, Venkatesh K, Elmalik K, Veer WVD, Jaacobs M. Double balloon enteroscopy in children: Diagnosis, treatment, and safety. World J Gastroenterol 2010; 16(1): 56-62
- URL: https://www.wjgnet.com/1007-9327/full/v16/i1/56.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i1.56
The advent of flexible fiberoptic endoscopes transformed the diagnosis and management of gastrointestinal (GI) disorders in adults and children, allowing direct visualization with targeted mucosal biopsies. Furthermore, endo-therapeutic procedures have now been possible throughout the upper GI tract and ileo-colon. However, the small bowel distal to the ligament of Trietz is inaccessible to conventional GI endoscopes. Recently, push enteroscopy, allowing the therapeutic endoscopist access up to 70-100 cm beyond the pylorus[1-4], intra-operative enteroscopy techniques which are relatively invasive[5,6], and wireless video capsule endoscopy (WCE) which affords excellent diagnostic yield combined with lack of morbidity but is non-therapeutic[7-9], have been performed.
Double balloon enteroscopy (DBE) is a more recent modality which enables high resolution endoscopic imaging of the entire small bowel, allowing interventional endo-therapy (e.g. non-variceal hemostasis, snare polypectomy and pneumatic balloon stricture dilatation)[10-12]. It is clear that this technology could allow treatment of lesions, possibly identified by WCE or other less invasive investigations such as magnetic resonance imaging (MRI) enteroclysis, in parts of the small bowel inaccessible to standard endoscopy, and hence may preclude the need for formal surgical approaches in such children. We present the first pediatric-only experience of DBE, although 2 predominantly adult series have included a few children with an age range up to 20 years and no specification as to those under 16 years[13,14].
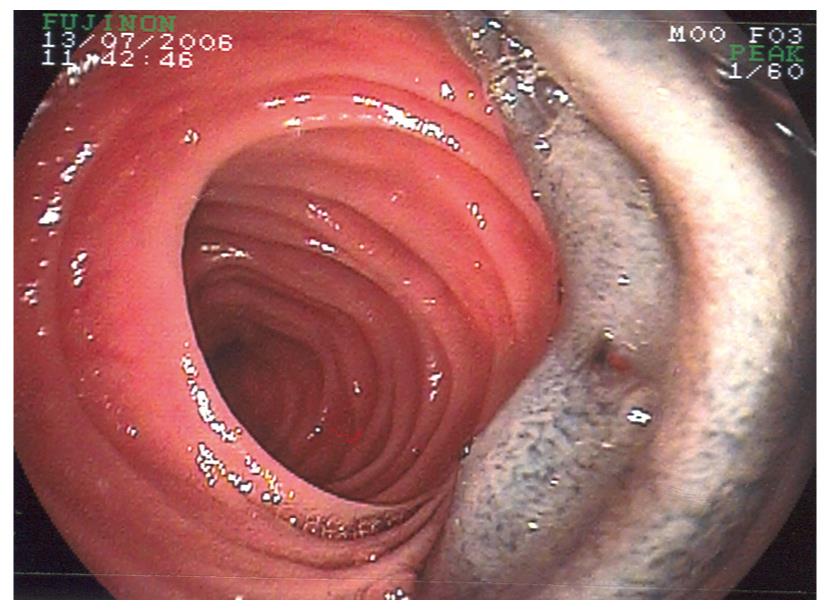
We prospectively collected the following data on all consecutively enrolled children between January 2004 and December 2007. All had undergone upper endoscopy, ileo-colonoscopy, and most had had WCE. Various imaging techniques had been employed but none had undergone virtual CT. The double balloon enteroscopy system (Fujinon; Fujinon Inc., Japan) (Figure 1) consists of a high resolution video enteroscope (EN-450P5/20) with a flexible over-tube (TS-12140), as well described elsewhere. The enteroscope has a working length of 200 cm and an outer diameter of 8.5 mm. The flexible over-tube has a length of 140 cm and outer diameter of 12 mm. Two enteroscopes are available, currently with 2.2 mm and 2.8 mm working channels, allowing therapeutic intervention. The enteroscopes and over-tube have balloons fitted at the distal tip of each, which are sequentially inflated and deflated with air from a pressure controlled pump system with a maximum inflatable pressure of 45 mmHg.
Specifics of the procedure are not provided here as these have been well documented elsewhere and do not differ in pediatric practice compared to that in adults. Patients received bowel preparation as for colonoscopy in anticipation of a trans-anal approach. For bowel preparation, Senokot 1-2 mg/kg (max 30 mg) and sodium picosulphate 2.5-10 g (depending on age) were given on the evening prior to the day of procedure with sodium picosulphate repeated on the morning of the procedure.
The preference was for general anesthesia but moderate conscious sedation was employed in the older patients in one centre. If the terminal ileum (TI) was not reached then the most distal part of the small bowel negotiated was “tattooed” in the sub-mucosal plane with an endo-needle (Figure 2). The DBE could then be repeated via the trans-anal route and retrograde movement from the TI proximally was attempted to attain the marked area. No external compression, fluoroscopy, or other aides were necessary or useful in aiding intubation. No anti-spasmodics were employed, and air rather than carbon dioxide was insufflated. If lesions were encountered it was usual practice to treat as they were encountered rather than on withdrawal in case the lesions were not then found again. Potential adverse events such as pancreatitis, perforation, or bowel damage due to traction or torsion around the mesentery have been reported in the adult literature. Two of the authors (Thomson M and Jaacobs M) performed all of the DBE having attained competence in DBE in adult patients first. Training and learning curves for this procedure are, it is estimated by the procedurists, similar to that encountered in ileo-colonoscopy, and clearly it is not yet apparent in pediatric practice how many DBE procedures are necessary in order to attain a high degree of competence.
Fourteen patients (10 males), median age 12.9 years (range 8.1-16.7), median weight 39.6 kg (range 24.1-67.3) underwent DBE. Indications: patients 1-5 for Peutz-Jeghers syndrome (PJS); patients 6-7 for recurrent abdominal pain; patients 8, 9, 10 and 13 for obscure GI bleeding. Patients 11 and 12 had, respectively; blue rubber bleb nevus syndrome and an angioma identified by WCE, and had transfusion-dependent persistent GI bleeding. Patient 14 had Cowden’s syndrome with previous episode of intussusceptions. Thirteen patients had undergone WCE and 1 MRI enteroclysis (Table 1).
| 1 | WCE: ?small sessile polyp in mid-small bowel |
| 2 | Intra-operative enteroscopy with polypectomy |
| MRI enteroclysis: normal | |
| 3 | OGD: polyps in stomach and duodenum |
| MRI: 3 big polyps in small bowel | |
| 4 | WCE: multiple polyps in mid-small bowel |
| 5 | WCE: possible polyp in ileum |
| 6 | WCE: ?polyps seen in small bowel, ?intermittent intussusception Colonoscopy: polyp in rectum |
| 7 | WCE: normal, Abdominal Ultrasound: enlarged spleen |
| 8 | WCE: no source of bleeding |
| 9 | OGD, WCE: lymphonodular hyperplasia in duodenum of little clinical significance |
| 10 | OGD, WCE: no positive findings |
| 11 | OGD, Ileo-colonoscopy, WCE: multiple blue rubber bleb nevus lesions throughout bowel |
| 12 | WCE: angioma in small bowel |
| 13 | WCE: polyp in mid-small bowel |
| 14 | WCE: multiple polyps seen throughout the small bowel including a lymphangitic polyp |
Eleven patients received general anesthesia and 3 procedures were performed under sedation with fentanyl and midazolam. Thirteen patients had trans-oral DBE, of whom 6 patients also had trans-anal, and 1 patient had trans-stomal DBE through an ileostomy. One patient underwent intra-operative DBE.
The results of this investigation suggest that DBE is both useful and feasible in children with small bowel disease. The entire small bowel was examined in 6 patients, either trans-oral alone or with both trans-oral and trans-anal DBE. When TI was not attained, trans-oral progression was assessed as approximately 200 to 320 cm beyond the pylorus (Table 2), based on the assumption that each set of maneuvers to advance the enteroscope traversed around 30 cm of bowel with diminishing distance the more attempts at advancement were made. No fluoroscopy was used hence these estimates of distance attained are presumptive. The median examination time was 118 min (range 95-195). No complications of DBE were encountered, and mild post-procedure abdominal discomfort, as occurs secondary to bowel insufflation in some ileo-colonoscopies, was temporary and controlled easily with simple analgesia.
| Patient No. | Age/Sex | Indication | Approach | Complete /incomplete | Findings |
| 1 | 13/M | PJS | Oral + anal | Complete | Rectal polyp |
| 2 | 12/M | PJS | Oral | 320 cm1 | Small polyp in jejunum |
| 3 | 16/M | PJS | Intra-operative | Complete | 3 small polyps removed endoscopically and 3 large polyps removed surgically |
| 4 | 11/M | PJS | Oral | 250 cm1 | Multiple polyps in mid-small bowel |
| 5 | 9/M | PJS | Oral | Incomplete | Mid-small bowel polyp |
| 6 | 10/F | Chronic abdominal pain | Oral + anal | Up to 200 cm1 trans-orally, 35 cm TI proximal to ICV trans-anally | Normal |
| 7 | 16/M | Chronic abdominal pain with family history of colorectal carcinoma | Oral + anal | Complete | Tubulo-villous adenoma in duodenum; Lymphoid aggregates in ileum |
| 8 | 11/M | Upper GI bleeds/possible vascular malformation | Oral | 300 cm1 | Grade 1 esophageal varices; no source found in small bowel |
| 9 | 16/M | Occult bleeding | Oral | 200 cm1 | No source found |
| 10 | 8/M | Occult bleeding | Oral + via ileal stoma | Complete | Increased friable mucosa throughout the small bowel |
| 11 | 12/F | Blue rubber bleb syndrome with persistant GI bleeding | Oral + anal | 2001 cm trans-orally, 50 cm proximal to ICV trans-anally | Numerous angiomas throughout small bowel not amenable to therapy |
| 12 | 9/F | Angioma | Oral | Incomplete | Angioma identified: APC applied |
| 13 | 16/M | Occult bleeding with significant anemia | Oral + anal | Complete | Polyp 40 cm from TI: removed |
| 14 | 12/F | Cowden’s syndrome | Oral + anal | Complete | Multiple polyps: 2 snare polypectomies; Meckel’s diverticulum found |
Patients 1-5 were known to have PJS. Patient 1 had not undergone WCE, and both trans-oral and trans-anal DBE allowed the whole small bowel to be visualized. No polyps were found in the small bowel; however a rectal polyp was resected which confirmed PJS on histology. Patient 2 had undergone previous intra-operative enteroscopy with polypectomy, and DBE revealed a presumably new small polyp in the jejunum which was removed. Patient 3 underwent laparoscopic-assisted DBE and 7 polyps were removed. The postoperative period was complicated by pelvic abscess requiring surgical drainage, but no intestinal perforation had occurred, hence the reason for the laparoscopic complication was unclear. Patient 4 had multiple sessile polyps in the jejunum and patient 5 had one PJS polyp removed from the mid jejunum. It is therefore suggested that PJS patients undergo WCE prior to DBE and if no polyps are found, then no DBE should take place.
Patients 6 and 7 had recurrent abdominal pain as the main presenting complaint with multiple negative investigations. Patient 6 had a family history of PJS and WCE had suggested a polyp in the mid-ileum. However, trans-oral (200 cm post-pylorus) and trans-anal (35 cm proximal to ileo-cecal valve) DBE failed to identify a polyp. Patient 7 presented with recurrent abdominal pain over 3.5 years (repeated upper GI endoscopy and ileocolonoscopy were inconclusive), and WCE had suggested proximal jejunal polyps. Trans-oral DBE demonstrated thickened folds in the proximal jejunum, which on histology proved to be a tubulo-villous adenoma. Surveillance enteroscopy after 1 year identified progression to intra-mucosal carcinoma. Surgical excision of the affected bowel and pancreas has proved curative. Clearly, this finding is very uncommon and the literature does not suggest an incidence in this age group. Of course, it is not suggested that all patients with recurrent abdominal pain undergo DBE. Clear clinical indication and warning signs such as a family history of polyp syndromes, in spite of negative WCE, are reasonable pointers towards DBE.
Patients 8-13 were investigated for GI bleeding. Patient 8 was transfusion-dependent and DBE was non-contributory since esophago-gastric varices were identified which could also have been identified and treated by standard upper GI endoscopy, although prior to transfer to our unit this procedure had not identified the varices. Mid-small bowel varices were not found at DBE. Patient 9 was investigated for obscure GI bleeding and trans-oral DBE did not reveal a bleeding site. Subsequently a Meckel’s scan, initially negative, was repeated, found to be positive and surgical resection occurred. Had trans-anal DBE been performed this may have identified the Meckel’s diverticulum, but this was not attempted due to a technical failure of the system and remains conjectural. The technical failure was due to the distal balloon bursting and is not considered as a dangerous adverse event.
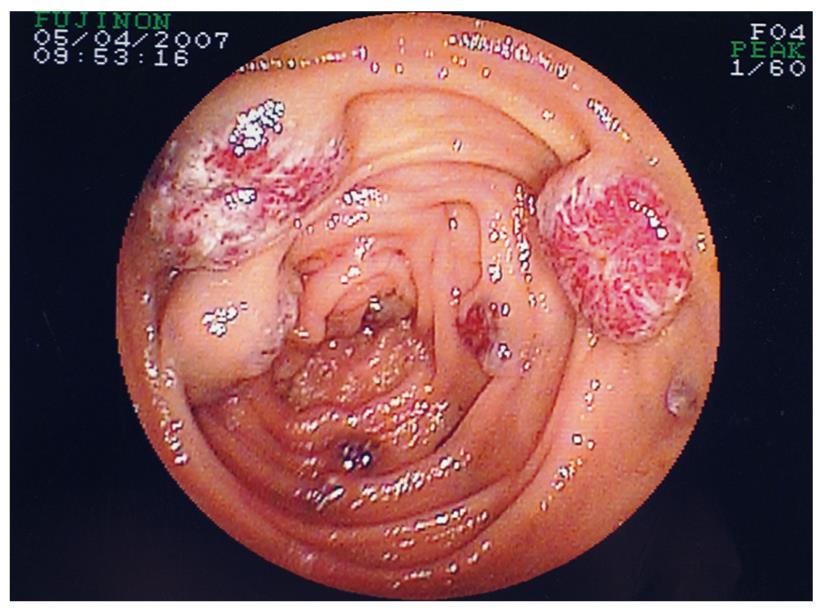
Patient 10 had intestinal aganglionosis, an ileostomy and a gastrostomy, and presented with a 3-year history of transfusion-dependent obscure GI bleeding. DBE identified very friable small bowel mucosa with contact bleeding, but no histological diagnosis was concluded with normal biopsies obtained. Patient 11 had transfusion-dependent recurrent GI bleeding due to multiple lesions consistent with blue rubber bleb nevus syndrome, identified in the colon at colonoscopy, and throughout the small bowel at DBE (Figure 3). Argon plasma coagulation (APC) was used in order to ablate some of the lesions and transfusion requirement diminished. The extensive nature of the lesions precluded definitive surgery and further DBE is planned, but has not occurred to date, therefore post-APC images are not available. However, transfusion dependency in both patients 10 and 11 had ceased.
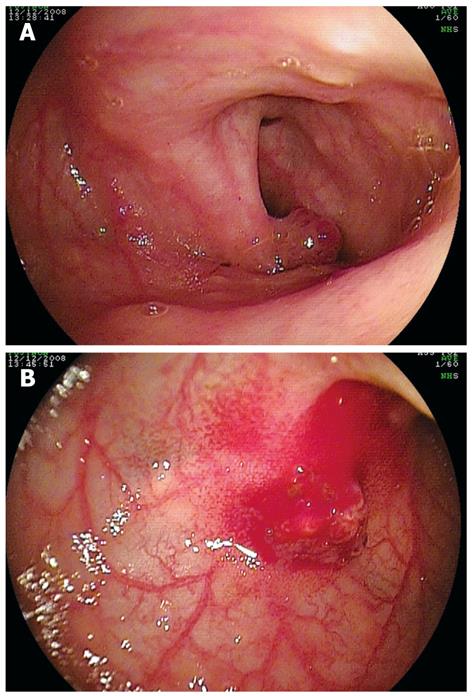
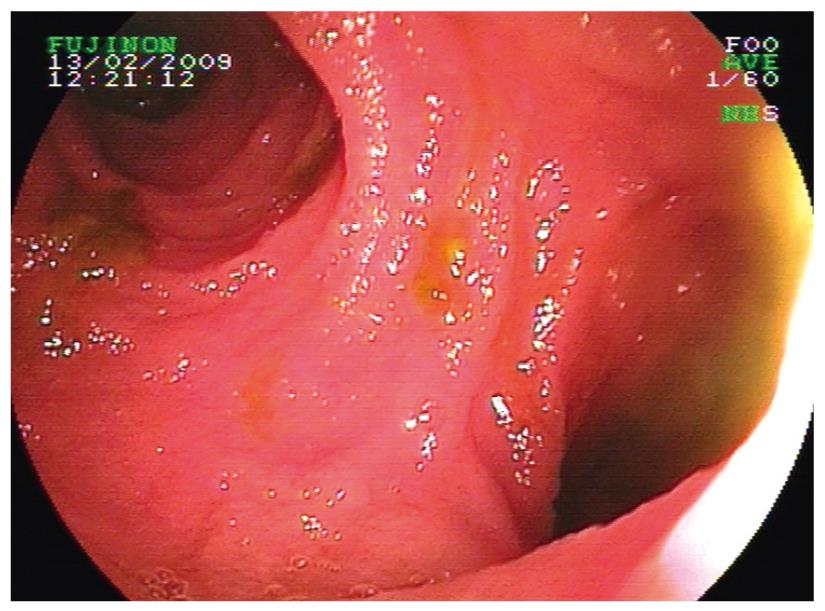
Patient 12 had angioma detected in the mid-small bowel on WCE. This was identified with DBE, and APC was applied. Patient 13 presented with occult bleeding and significant anemia, and a polyp was detected in the small bowel on WCE. At DBE, a 4 mm polypoid structure was found (Figure 4A) and removed (Figure 4B). Patient 14 had Cowden’s syndrome with a history of intussusception. DBE revealed presence of multiple sessile polyps and polypectomies were performed on 2 polyps. Incidentally, a Meckel’s diverticulum was found (Figure 5) in this patient.
No patients referred and considered for DBE were rejected, i.e. there seems no reason not to consider this minimally invasive approach. No complications occurred in the 13 patients who had DBE alone without intra-operative assistance. Significant post-procedure abdominal pain was not encountered, and only paracetamol was needed to counter minor abdominal discomfort, except in the individual who had undergone laparoscopy. All patients were in-patients although it is anticipated that day case procedures are viable. No evidence of pancreatitis, perforation or bowel damage was encountered, and the intra-abdominal abscess could have been the result of a micro-perforation rather than bowel damage due to the laparoscope, although the authors consider this unlikely. All patients were allowed home with no evidence of significant complications or discomfort within 24 h of the DBE being performed. All of those who did not undergo polypectomy were allowed home on the same day as the procedure. The longest duration at 195 min has to be considered as a long endoscopic procedure, but this has to be counter-balanced by the relative lack of invasiveness of the technique.
Flexible GI endoscopy is sufficient for diagnostic and therapeutic procedures in the vast majority of pediatric cases, and in adult patients with obscure GI bleeding this procedure is known to determine the source in up to 90% of cases. However, in the small number of cases where the pathology is confined to the small bowel beyond the reach of conventional endoscopy, WCE and DBE have been recently employed. In our series the entire small bowel was examined in 6/14 patients in whom trans-oral and trans-anal approaches were combined. One cannot claim that DBE diagnosed the disease in this series of patients, but it can be considered that it had a very important role in treatment. In all patients with PJ syndrome polyps were detected. Prior to the advent of these technologies, modalities such as push enteroscopy (PE) have had limited pediatric exposure due to safety concerns. In children, 80% of all mucosal lesions identified and biopsied by PE, and 20% of therapeutic procedures performed, were beyond the reach of a standard GI endoscope[13]. Small bowel series, angiography, scintigraphy and enteroclysis have been used with variable results in the evaluation of adult patients with obscure GI bleeding[15,16]. WCE has recently attained the position of investigation of first choice for such diagnoses, while intra-operative enteroscopy, despite its invasive quality, has been the mainstay in the subsequent treatment of obscure GI bleeding in children and adults[5,17,18].
Wireless capsule endoscopy (WCE) has been compared favorably with intra-operative enteroscopy for the diagnosis of obscure bleeding in adults, with 95% sensitivity and 75% specificity[19]. WCE has been found to be diagnostically superior to PE[20,21] and barium follow through/CT scan in obscure GI bleeding, and has been recently evaluated in children[22]. WCE is, however, non-therapeutic by its nature, and since the imperative in pediatric gastroenterology is the drive to diagnosis by mucosal histology, this is a shortcoming of WCE.
Trans-oral and, if necessary, subsequent trans-anal DBE allow therapeutic interventions such as polypectomy, hemostasis, balloon dilatation and placement of stents for the whole of the small bowel[23]. In a prospective comparative study between WCE and DBE in patients with obscure GI bleeding, the diagnostic rate was 80% for WCE and 60% for DBE; however, 51% of the patients had therapeutic intervention using argon plasma, underlining the therapeutic utility of DBE[24]. In a recent large retrospective analysis of 152 patients undergoing DBE for obscure GI bleeding in adults, 75% had the potential source of bleeding detected and, for 83% of patients, management was changed as a direct result of DBE[25]. Yamamoto has described full small intestinal examination in 86% of adults using DBE[23]. DBE in this series of children had a diagnostic yield of 11/14, and therapeutic success in 9/14 was achieved. Clearly if a regional or national small bowel diagnostic and therapeutic centre is contemplated then the duality of WCE and DBE is mandatory to achieve the goals of diagnosis and treatment without operative intervention, which should be the goal of a pediatric endoscopic centre of excellence. Hence, with the results of our prospective DBE study a case could be made for the discontinuation of push enteroscopy in the investigation and treatment of children with suspected small bowel pathology.
Complications have been reported in the literature with DBE, including intestinal perforation[26,27], pancreatitis[28] and paralytic ileus[29]. However, the only complication in our group of children occurred secondary to surgical intervention in the child who underwent intra-operative DBE.
Training remains an issue with no clear resolution attempted by this small series which included only two experienced endoscopists.
In conclusion, double balloon enteroscopy is a useful diagnostic and therapeutic tool for the investigation of small bowel disease. It is useful in conjunction with WCE for optimizing diagnostic potential in the small bowel and offering a therapeutic option. It is also of benefit in situations where diagnosis has not been reached by other investigative modalities, and particularly in those lesions amenable for therapeutic intervention endoscopically, but not reachable by the conventional endoscope.
The small bowel distal to the ligament of Trietz is inaccessible to conventional gastrointestinal (GI) endoscopes. Several techniques such as push enteroscopy, intra-operative enteroscopy techniques and wireless video capsule endoscopy have been developed. All these procedures have some limitations. Double balloon enteroscopy (DBE) is a recently developed tool which enables high resolution endoscopic imaging of the entire small bowel and allows interventional endo-therapy.
Small bowel is a particular area of the GI tract difficult to be imaged completely by conventional endoscope. DBE enables high resolution endoscopic imaging of the entire small bowel. In addition to diagnosis, DBE permits interventional endo-therapy.
DBE has found application in the investigation of obscure GI bleeding in adults. This is the first study to assess the usefulness, safety and diagnostic potential of DBE in children.
DBE is a valuable diagnostic and therapeutic tool for the investigation of small bowel disease both in adults and in children. DBE is useful in conjunction with other investigative modalities in optimizing diagnostic potential in the small bowel and offering a therapeutic option.
This is a good introductory study on DBE in children.
| 1. | Chong J, Tagle M, Barkin JS, Reiner DK. Small bowel push-type fiberoptic enteroscopy for patients with occult gastrointestinal bleeding or suspected small bowel pathology. Am J Gastroenterol. 1994;89:2143-2146. |
| 2. | Foutch PG, Sawyer R, Sanowski RA. Push-enteroscopy for diagnosis of patients with gastrointestinal bleeding of obscure origin. Gastrointest Endosc. 1990;36:337-341. |
| 3. | Pennazio M, Arrigoni A, Risio M, Spandre M, Rossini FP. Clinical evaluation of push-type enteroscopy. Endoscopy. 1995;27:164-170. |
| 4. | Linder J, Cheruvattath R, Truss C, Wilcox CM. Diagnostic yield and clinical implications of push enteroscopy: results from a nonspecialized center. J Clin Gastroenterol. 2002;35:383-386. |
| 5. | Lau WY. Intraoperative enteroscopy--indications and limitations. Gastrointest Endosc. 1990;36:268-271. |
| 6. | Apelgren KN, Vargish T, Al-Kawas F. Principles for use of intraoperative enteroscopy for hemorrhage from the small bowel. Am Surg. 1988;54:85-88. |
| 7. | Gong F, Swain P, Mills T. Wireless endoscopy. Gastrointest Endosc. 2000;51:725-729. |
| 8. | Appleyard M, Fireman Z, Glukhovsky A, Jacob H, Shreiver R, Kadirkamanathan S, Lavy A, Lewkowicz S, Scapa E, Shofti R. A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small-bowel lesions. Gastroenterology. 2000;119:1431-1438. |
| 9. | Mata A, Bordas JM, Feu F, Gines A, Pellise M, Fernandez-Esparrach G, Balaguer F, Pique JM, Llach J. Wireless capsule endoscopy in patients with obscure gastrointestinal bleeding: a comparative study with push enteroscopy. Aliment Pharmacol Ther. 2004;20:189-194. |
| 10. | Fukumoto A, Tanaka S, Yamamoto H, Yao T, Matsui T, Iida M, Goto H, Sakamoto C, Chiba T, Sugano K. Diagnosis and treatment of small-bowel stricture by double balloon endoscopy. Gastrointest Endosc. 2007;66:S108-S112. |
| 11. | May A, Nachbar L, Wardak A, Yamamoto H, Ell C. Double-balloon enteroscopy: preliminary experience in patients with obscure gastrointestinal bleeding or chronic abdominal pain. Endoscopy. 2003;35:985-991. |
| 12. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. |
| 13. | Darbari A, Kalloo AN, Cuffari C. Diagnostic yield, safety, and efficacy of push enteroscopy in pediatrics. Gastrointest Endosc. 2006;64:224-228. |
| 14. | Leung YK. Double balloon endoscopy in pediatric patients. Gastrointest Endosc. 2007;66:S54-S56. |
| 15. | Jain TP, Gulati MS, Makharia GK, Bandhu S, Garg PK. CT enteroclysis in the diagnosis of obscure gastrointestinal bleeding: initial results. Clin Radiol. 2007;62:660-667. |
| 16. | Brunnler T, Klebl F, Mundorff S, Eilles C, Reng M, von Korn H, Scholmerich J, Langgartner J, Grune S. Significance of scintigraphy for the localisation of obscure gastrointestinal bleedings. World J Gastroenterol. 2008;14:5015-5019. |
| 17. | Douard R, Wind P, Panis Y, Marteau P, Bouhnik Y, Cellier C, Cugnenc P, Valleur P. Intraoperative enteroscopy for diagnosis and management of unexplained gastrointestinal bleeding. Am J Surg. 2000;180:181-184. |
| 18. | Jakobs R, Hartmann D, Benz C, Schilling D, Weickert U, Eickhoff A, Schoenleben K, Riemann JF. Diagnosis of obscure gastrointestinal bleeding by intra-operative enteroscopy in 81 consecutive patients. World J Gastroenterol. 2006;12:313-316. |
| 19. | Hartmann D, Schmidt H, Bolz G, Schilling D, Kinzel F, Eickhoff A, Huschner W, Moller K, Jakobs R, Reitzig P. A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc. 2005;61:826-832. |
| 20. | Mylonaki M, Fritscher-Ravens A, Swain P. Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut. 2003;52:1122-1126. |
| 21. | Leighton JA, Sharma VK, Hentz JG, Musil D, Malikowski MJ, McWane TL, Fleischer DE. Capsule endoscopy versus push enteroscopy for evaluation of obscure gastrointestinal bleeding with 1-year outcomes. Dig Dis Sci. 2006;51:891-899. |
| 22. | Thomson M, Fritscher-Ravens A, Mylonaki M, Swain P, Eltumi M, Heuschkel R, Murch S, McAlindon M, Furman M. Wireless capsule endoscopy in children: a study to assess diagnostic yield in small bowel disease in paediatric patients. J Pediatr Gastroenterol Nutr. 2007;44:192-197. |
| 23. | Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016. |
| 24. | Hadithi M, Heine GD, Jacobs MA, van Bodegraven AA, Mulder CJ. A prospective study comparing video capsule endoscopy with double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:52-57. |
| 25. | Sun B, Rajan E, Cheng S, Shen R, Zhang C, Zhang S, Wu Y, Zhong J. Diagnostic yield and therapeutic impact of double-balloon enteroscopy in a large cohort of patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006;101:2011-2015. |
| 26. | Ohmiya N, Yano T, Yamamoto H, Arakawa D, Nakamura M, Honda W, Itoh A, Hirooka Y, Niwa Y, Maeda O. Diagnosis and treatment of obscure GI bleeding at double balloon endoscopy. Gastrointest Endosc. 2007;66:S72-S77. |
| 27. | Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007;66:304-309. |
| 28. | Honda K, Mizutani T, Nakamura K, Higuchi N, Kanayama K, Sumida Y, Yoshinaga S, Itaba S, Akiho H, Kawabe K. Acute pancreatitis associated with peroral double-balloon enteroscopy: a case report. World J Gastroenterol. 2006;12:1802-1804. |
Peer reviewer: William Dickey, Altnagelvin Hospital, Londonderry, BT47 6SB, Northern Ireland, United Kingdom
S- Editor Tian L L- Editor Logan S E- Editor Ma WH