Published online Jan 7, 2010. doi: 10.3748/wjg.v16.i1.42
Revised: October 26, 2009
Accepted: November 2, 2009
Published online: January 7, 2010
AIM: To determine the association between Helicobacter pylori (H. pylori) and globus sensation (GS) in the patients with cervical inlet patch.
METHODS: Sixty-eight patients with esophageal inlet patches were identified from 6760 consecutive patients undergoing upper gastrointestinal endoscopy prospectively. In these 68 patients with cervical inlet patches, symptoms of globus sensation (lump in the throat), hoarseness, sore throat, frequent clearing of the throat, cough, dysphagia, odynophagia of at least 3 mo duration was questioned prior to endoscopy.
RESULTS: Cervical heterotopic gastric mucosa (CHGM) was found in 68 of 6760 patients. The endoscopic prevalence of CHGM was determined to be 1%. H. pylori was identified in 16 (23.5%) of 68 patients with inlet patch. Fifty-three patients were classified as CHGM II. This group included 48 patients with globus sensation, 4 patients with chronic cough and 1 patient with hoarseness. All the patients who were H. pylori (+) in cervical inlet patches had globus sensation.
CONCLUSION: Often patients with CHGM have a long history of troublesome throat symptoms. We speculate that disturbances in globus sensation are like non-ulcer dyspepsia.
-
Citation: Alagozlu H, Simsek Z, Unal S, Cindoruk M, Dumlu S, Dursun A. Is there an association between
Helicobacter pylori in the inlet patch and globus sensation? World J Gastroenterol 2010; 16(1): 42-47 - URL: https://www.wjgnet.com/1007-9327/full/v16/i1/42.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i1.42
Islands of heterotopic gastric mucosa are found throughout the gastrointestinal tract, the most common site being the cervical esophagus. Cervical heterotopic gastric mucosa (CHGM) or cervical inlet patches are commonly seen during withdrawal of the gastroscope. Patients with CHGM have various laryngeal and oropharyngeal symptoms, ranging from asymptomatic to protracted symptoms such as globus sensation and chronic cough due to acid secretion from the inlet patch[1-4].
Globus sensation (GS) is caused mainly by cervical disturbances. The usual complaint in patients with globus sensation or globus pharyngeus is the sensation of a ball or lump in the throat, generally not accompanied by dysphagia. Globus sensation is felt medially deep in the throat during dry swallowing (empty swallow), and almost never while drinking or eating. It is not painful, and there is no obstruction of food[2,3].
Helicobacter pylori (H. pylori) produces chronic inflammation in the CHGM (as in non-ulcer dyspepsia). H. pylori infection plays a role in altered gastric perception in non-ulcer dyspepsia. H. pylori in CHGM may cause altered cervical perception such as globus sensation. In this prospective study, we aimed to determine the association between H. pylori and globus sensation in patients with CHGM.
Over a one-year period, between 2005 and 2006, the number of patients with cervical inlet patches from a total of 6760 consecutive patients undergoing upper gastrointestinal endoscopy at the Hospital of the Gazi University in Ankara, Turkey were identified. Patients were referred for endoscopy for a variety of reasons, primarily for evaluation of dyspepsia. In these patients, symptoms of globus sensation (lump in the throat), hoarseness, sore throat, frequent clearing of the throat, cough, dysphagia and odynophagia of at least 3 mo duration were questioned prior to endoscopy.
The investigation conformed to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all subjects and the study was approved by the ethical review committee of Gazi University in Ankara, Turkey.
After an overnight fast, a routine esophagogastroduodenoscopy was performed with a standard endoscope using topical anesthesia with or without conscious sedation, depending on patient preference. Conscious sedation was performed with midazolam (2-5 mg). During all procedures the esophagus was carefully surveyed and special attention was paid to the area of the upper esophageal sphincter. This region was best examined when slowly withdrawing the endoscope, with repeated short inflations while rotating the instrument.
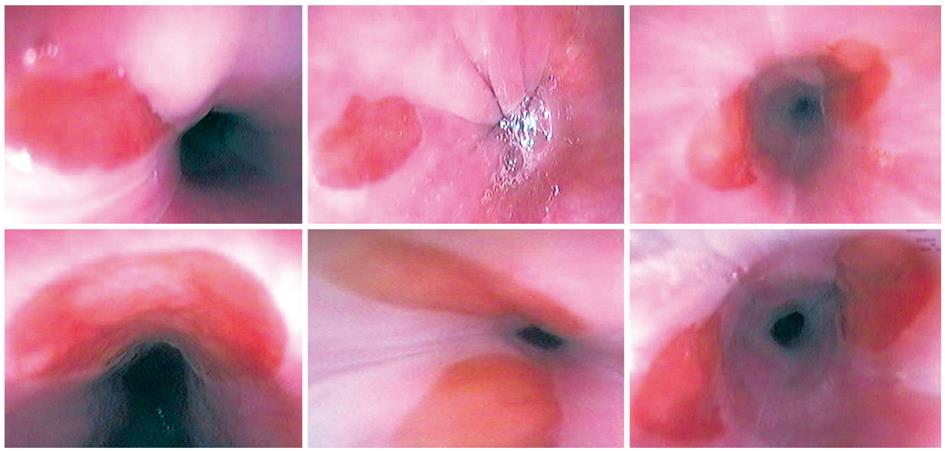
Heterotopic gastric mucosa was defined as patches covered with salmon-red mucosa distinguishable from surrounding greyish-pearly colored esophageal mucosa by well-defined margins (Figure 1). The size of the patches was determined by the top span of the fully open biopsy forceps. In all subjects, the distance between the patch and the frontal incisor was recorded and the patch size measured under the guidance of the open biopsy forceps.
pH monitoring was performed in our laboratory in the patients with inlet patch. A 2.1-mm diameter dual-electrode antimony pH catheter (pHersaflex ambulatory catheter, MMS) was placed transnasally after an overnight fast. Recording sites were fixed on the catheter, with a distance of 15 cm to measure proximal and distal pH. The distal electrode was placed 5 cm above the manometrically defined lower esophageal sphincter. The proximal electrode was placed at 16-21 cm, which corresponded approximately with the endoscopic finding of inlet patches. The pH values from both intraesophageal electrodes were recorded continuously on an ambulatory Mark III Digitrapper (Synectics Medical Inc.). Abnormal distal esophageal reflux and proximal reflux were defined as the percentage of esophageal total acid exposure (pH < 4) of ≥ 4.2% and ≥ 1%, respectively[5].
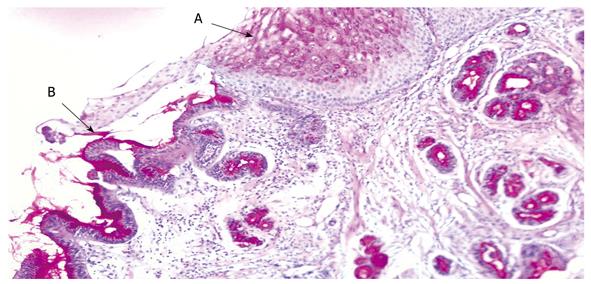
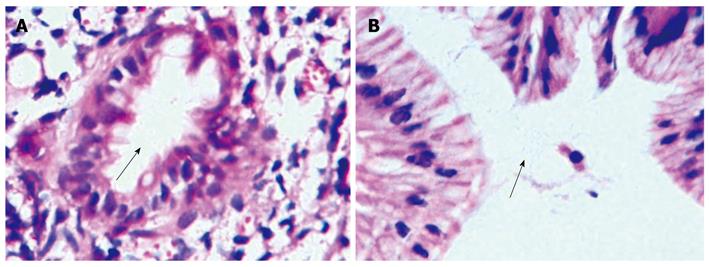
A minimum of two biopsies were obtained from the CHGM (Figure 2) and antral gastric mucosa. The samples were taken using large cup and side-opening forceps. Pathology and/or the rapid urease test were performed to determine the presence of H. pylori in all patients (Figure 3). The presence of H. pylori was identified using hematoxylin-eosin, cresyl violet, giemsa and silver stain.
All statistical analyses were carried out using the Statistical Package for Social Sciences (SPSS) 13.0 software for Windows XP. Categorical variables were compared with the chi-squared test or Fisher’s exact test, and continuous variables were compared using Student’s t-test and univariate analysis. A P value of less than 0.05 was considered to be statistically significant.
CHGM patches were found in 68 of 6760 patients (3173 female, 3587 male). The endoscopic prevalence of CHGM was determined to be 1%. Demographic characteristics of the patients with and without patches are shown in Table 1. The female/male ratio was 1.12 in the 68 patients with patches and 0.88 in those without. There was no significant difference between the mean age of the patients with CHGM with and without H. pylori (P > 0.05). Female patients with inlet patches had higher colonization of H. pylori than male patients (P < 0.05).
| HGM (+) | HGM (-) | |
| n | 68 | 6692 |
| Sex | ||
| Female (%) | 36 (53.1) | 3173 (47.4) |
| Male (%) | 32 (46.9) | 3519 (52.6) |
| Female/male | 1.12 | 0.88 |
| Age range (yr) | (17-56) | (16-90) |
| Mean age (± SE) | 37.29 ± 1.85a | 47.5 ± 0.3 |
| Endoscopic Barrett’s esophagus | 9 (13.2)a | 166 (2.4) |
| Erosive esophagitis | 7 (10.3) | 636 (9.5) |
| Globus (%) | 48 (70.6)a | 0 |
| Dyspepsia(%) | 60 (88.2) | 6550 (97.8) |
The size of the inlet patches varied between 5 and 32 mm and occupied between 10% and 30% of the circumference. Five patients had “double” patches. Symptoms are shown in Tables 1 and 2. Five patients had distal reflux and 1 patient had both proximal and distal reflux. There were no patients with only proximal reflux. The other patients were normal.
| Clinicopathologic | Number (%) | Symptoms/findings |
| HGM I | 12 (17.6) | Asymptomatic |
| HGM II | 53 (77.9) | 48 GS, 4 cough, 1 hoarseness |
| HGM III | 2 (2. 9) | 1 ulcus , 1 polyp |
| HGM IV | 0 | |
| HGM V | 1 (1.4) | Esophageal adenocarcinoma |
H. pylori:H. pylori was identified in the CHGM in 23.5% of patients (16/68) (Table 3) and gastric H. pylori infection was positive in all (16/16) of these patients (Table 4, Figure 3). Colonization of H. pylori was most common in fundic-type mucosa (81.2%). All the patients who were H. pylori (+) in the cervical inlet patches had globus sensation (P < 0.05).
| Histological characteristics | n |
| Type of patch | |
| Fundic | 44 |
| Antral | 15 |
| Foveolar epithelium | 8 |
| Complete IM | 1 |
| Chronic inflammation | 68 |
| IM (+), Hp (-) | 6 (3 antral-type, 2 fundic-type, 1 complete IM) |
| IM (-), Hp (+) | 15 (3 antral-type, 12 fundic-type) |
| IM (+), Hp (+) | 1 (1 fundic-type) |
| IM (-), Hp (-) | 46 (9 antral-type, 29 fundic-type, 8 foveolar epithelium) |
Mucosal type: In the inlet patch, fundic-type mucosa was the most common histologic type (44/68), followed by antral-type mucosa (15/68) (Table 3). Eight specimens of the inlet patch contained only foveolar epithelium and were therefore considered too superficial to be classified. In one patient, mucosal type was unremarkable because one specimen had complete replacement of the underlying mucosa with intestinal metaplasia.
Intestinal metaplasia: Intestinal metaplasia was identified in the inlet patch in seven patients (10.3%). One patient had complete replacement of the underlying mucosa with intestinal metaplasia. Both H. pylori and intestinal metaplasia were observed in the inlet patch mucosa in one patient. In the remaining five patients, intestinal metaplasia was present, but no H. pylori. The type of mucosa was evaluated in these patients; three had antral-type and three fundic-type.
Endoscopic esophagitis (7 patients), duodenal ulcer (3 patients), hiatal hernia (9 patients) and endoscopic Barrett’s esophagus (9 patients) accompanied CHGM.
Clinicopathologic classification was performed according to the classification reported by von Rahden et al[1] in patients with CHGM. (1) Asymptomatic carriers of esophageal CHGM were classified as CHGM I. Twelve patients were classified with CHGM I in our study. (2) Symptomatic individuals with esophageal CHGM complaining of globus sensation, cough, hoarseness or “extraesophageal manifestations” were classified as CHGM II without morphologic changes. Fifty-three patients were classified with CHGM II. This group included 48 patients with globus sensation, 4 patients with chronic cough and 1 patient with hoarseness. There were 48 (70.5%) patients with globus sensation out of the 68 patients with CHGM II. Symptoms of globus sensation were obvious attractive in these patients. (3) A smaller group of patients with additional morphologic changes (inlet patch complications) were classified as CHGM III. This group included one patient with ulcus and polyp in the inlet patch. (4) If dysplasia was present, this was classified as CHGM IV. None of the patients belonged to this category. (5) If the diagnosis was invasive cancer and originated within the inlet patch, this was classified as CHGM V. One patient who had adenocarcinoma in the CHGM was classified as CHGM V.
The usual endoscopic appearance of CHGM is a salmon-rose-colored mucosal patch with a sharp border or edge in the upper esophagus. The patches vary in diameter from 1 to 20 mm or more. Inlet patches are recognized endoscopically as 1 or 2 patches mostly in the lateral walls between the level of the cartilage and the fifth tracheal ring, and are seen as sharply demarcated, salmon-rose-colored oval or round patches.
The prevalence of CHGM varied between 0.29% and 2.27% in one prospective study[6]. Akbayir et al[7] reported a prevalence of 1.67% and Tang et al[8] reported a prevalence of 1.1%. In our prospective study, over a period of 1 year, 68 cases (1%) of CHGM were documented and confirmed by histology.
Microscopically, gastric mucosa containing either cardiac, antral and potentially acid-secreting fundic mucosa can be found. In general, CHGM is uniformly of the fundic-type, containing both parietal and chief cells. Less frequently, histopathologic examination of CHGM shows an “antral pattern”, defined by the absence of chief cells and only a few parietal cells[1,8,9]. In our series, fundic-type mucosa was found in 44 of 68 patients examined histologically.
H. pylori is a well known pathogenic micro-organism responsible for chronic inflammation. Ectopic gastric mucosa of the inlet patch is an ideal location for H. pylori colonization[10]. Borhan-Manesh et al[11] found H. pylori in the inlet patch in 35% of patients in a subset with gastric H. pylori. Among our 68 patients with inflamed inlet patches, 16 were positive for H. pylori (23.5%), and all of these 16 patients also had H. pylori in the antrum. Coinfection of H. pylori in the inlet patch and gastric antrum has also been reported by others[10]. Since the infection by H. pylori is through the oral route, inlet patches may be important sites of H. pylori infection in the upper gastrointestinal tract because of its more proximal location. The inlet patches may function as reservoirs for H. pylori. Inlet patch colonization by H. pylori can occur during ingestion of food, and the presence of gastric H. pylori may play a role in the development of inlet patches. We searched but did not find any follow-up studies on antibiotic therapy for H. pylori and its impact on infection of inlet patches in the literature. We believe that the elimination of H. pylori in both the inlet patch and antrum is very important in the treatment of patients with coinfection.
In this study, the female/male ratio in the H. pylori (+) CHGM group was higher than that in the H. pylori (-) CHGM group. Females had higher inlet patch colonization with H. pylori than males (P < 0.05). The mechanism of H. pylori colonization in the inlet patch is unclear.
A clinicopathological classification of CHGM as proposed by von Rahden et al[1] was carried out on all 68 patients, 53 of whom were classified as CHGM II; 48 had globus sensation, 4 has cough, one had hoarseness. Theoretically, laryngeal and oropharyngeal symptoms should be common due to acid secretion from the inlet patch. Several studies have reported cases of esophageal inlet patch presenting with various laryngeal and oropharyngeal symptoms, ranging from asymptomatic to protracted symptoms such as chronic cough and globus sensation[4,12-14]. In addition, CHGM can cause stricture, ulcer, perforation, web or polyp in the esophagus because of the its capability to secrete acid[15,16]. In our two patients with CHGM III, one had a hyperplastic polyp and one had an ulcus.
There are also reports that CHGM is associated with an increased risk for Barrett’s esophagus, suggesting a possible link. Traditionally, Barrett’s esophagus is considered a distinct entity from esophageal inlet patch. Barrett’s esophagus is an acquired precancerous lesion and the cell origin probably involves multipotential undifferentiated cells. Inlet patch is considered to be congenital. Up to half of all patients with cervical inlet patch have concurrent Barrett’s esophagus in some reports[17]. They have the same mucin core protein expression and cytokeratin pattern, suggesting a pathogenetic link between these two diseases[18]. Similarly, in the current study, the presence of CHGM in the upper esophagus was common and closely related to Barrett’s esophagus (13.2%) compared with those without inlet patches (P < 0.05). Gastro-esophageal diseases in patients with cervical inlet patch was not statistically significant when compared with those without patches (P > 0.05). We speculated that the acid secretion from the inlet patches did not contribute to the pathogenesis of Barrett’s esophagus. However, the patients with inlet patches were inherently predisposed to developing columnar metaplasia in the distal esophagus.
The usual complaint in GS is that of a ball or lump in the throat generally not accompanied by dysphagia. This sensation is often more pronounced when taking an “empty swallow”. In our study, all the patients with H. pylori in cervical inlet patches had globus sensation.
CHGM should be looked for, particularly in patients with GS. H. pylori positivity in the CHGM correlated with GS symptoms in our study. There were no reports on a link between H. pylori positivity in patients with CHGM and “globus sensation” or “globus pharyngeus” in our review of the English literature. Therefore, we were not able to compare our data.
H. pylori infection plays a role in causing symptoms in patients fulfilling the criteria for non-ulcer dyspepsia. There is agreement that H. pylori infection causes changes in gastric physiology. In addition, H. pylori infection plays a role in altered gastric perception in non-ulcer dyspepsia. We speculate that the disturbances in globus sensation are like non-ulcer dyspepsia. H. pylori produces chronic inflammation in the CHGM (as in non-ulcer dyspepsia). It could be speculated that globus sensation is a non-ulcer dyspepsia of CHGM. H. pylori is a potential cause of GS in patients with CHGM.
There are no follow-up studies on antibiotic therapy for H. pylori and its impact on infection of inlet patches in the literature. Additional studies are needed to understand the fundamental mechanisms leading to globus sensation in CHGM. These patients might benefit from H. pylori eradication therapy to alleviate this potentially aggravating factor. Based on these important findings, we expect to see more studies on inlet patches in the near future.
Cervical heterotopic gastric mucosa (CHGM) or cervical inlet patches are commonly seen on the cervical esophagus during withdrawal of the gastroscope. Patients with inlet patches have various laryngeal and oropharyngeal symptoms, ranging from asymptomatic to protracted symptoms such as globus sensation and chronic cough due to acid secretion from the inlet patch. Globus sensation has a largely unknown etiology.
All the patients who were Helicobacter pylori (H. pylori) (+) in the cervical inlet patches had globus sensation. H. pylori produces chronic inflammation in the CHGM (as in non-ulcer dyspepsia). It could be speculated that globus sensation is a non-ulcer dyspepsia of CHGM. H. pylori is a potential cause of GS in patients with CHGM.
The authors showed a causal association between H. pylori infection and the symptoms of globus sensation in these patients Inlet patches may function as reservoirs for H. pylori. Inlet patch colonization by H. pylori can occur during ingestion of food and the presence of gastric H. pylori might play a role in the development of inlet patches.
Patients might benefit from H. pylori eradication therapy to alleviate this potentially aggravating factor. Based on these important findings, we expect to see more studies on inlet patches in the near future.
CHGM in the cervical esophagus appears to result from incomplete replacement of the original columnar epithelium by stratified squamous epithelium in the embryonic period. Islands of heterotopic gastric mucosa are found throughout the gastrointestinal tract, the most common site being the cervical esophagus. Macroscopically, visible islands of CHGM, referred as “inlet patches” are often detected during endoscopic examination.
The manuscript presented interesting data on heterotopic gastric mucosa on the cervical esophagus. They proved the link between presence of inlet patch and globus sensation in these patients. Interestingly, they showed a causal association between H. pylori infection and the symptom of globus sensation in these patients.
| 1. | von Rahden BH, Stein HJ, Becker K, Liebermann-Meffert D, Siewert JR. Heterotopic gastric mucosa of the esophagus: literature-review and proposal of a clinicopathologic classification. Am J Gastroenterol. 2004;99:543-551. |
| 2. | Lancaster JL, Gosh S, Sethi R, Tripathi S. Can heterotopic gastric mucosa present as globus pharyngeus? J Laryngol Otol. 2006;120:575-578. |
| 3. | Alaani A, Jassar P, Warfield AT, Gouldesbrough DR, Smith I. Heterotopic gastric mucosa in the cervical oesophagus (inlet patch) and globus pharyngeus--an under-recognised association. J Laryngol Otol. 2007;121:885-888. |
| 4. | Meining A, Bajbouj M, Preeg M, Reichenberger J, Kassem AM, Huber W, Brockmeyer SJ, Hannig C, Hofler H, Prinz C. Argon plasma ablation of gastric inlet patches in the cervical esophagus may alleviate globus sensation: a pilot trial. Endoscopy. 2006;38:566-570. |
| 5. | Dobhan R, Castell DO. Normal and abnormal proximal esophageal acid exposure: results of ambulatory dual-probe pH monitoring. Am J Gastroenterol. 1993;88:25-29. |
| 6. | Maconi G, Pace F, Vago L, Carsana L, Bargiggia S, Bianchi Porro G. Prevalence and clinical features of heterotopic gastric mucosa in the upper oesophagus (inlet patch). Eur J Gastroenterol Hepatol. 2000;12:745-749. |
| 7. | Akbayir N, Alkim C, Erdem L, Sokmen HM, Sungun A, Basak T, Turgut S, Mungan Z. Heterotopic gastric mucosa in the cervical esophagus (inlet patch): endoscopic prevalence, histological and clinical characteristics. J Gastroenterol Hepatol. 2004;19:891-896. |
| 8. | Tang P, McKinley MJ, Sporrer M, Kahn E. Inlet patch: prevalence, histologic type, and association with esophagitis, Barrett esophagus, and antritis. Arch Pathol Lab Med. 2004;128:444-447. |
| 9. | Jabbari M, Goresky CA, Lough J, Yaffe C, Daly D, Côté C. The inlet patch: heterotopic gastric mucosa in the upper esophagus. Gastroenterology. 1985;89:352-356. |
| 10. | Gutierrez O, Akamatsu T, Cardona H, Graham DY, El-Zimaity HM. Helicobacter pylori and hetertopic gastric mucosa in the upper esophagus (the inlet patch). Am J Gastroenterol. 2003;98:1266-1270. |
| 11. | Borhan-Manesh F, Farnum JB. Study of Helicobacter pylori colonization of patches of heterotopic gastric mucosa (HGM) at the upper esophagus. Dig Dis Sci. 1993;38:142-146. |
| 12. | Chevalier JM, Brossard E, Monnier P. Globus sensation and gastroesophageal reflux. Eur Arch Otorhinolaryngol. 2003;260:273-276. |
| 13. | Tokashiki R, Yamaguchi H, Nakamura K, Suzuki M. Globus sensation caused by gastroesophageal reflux disease. Auris Nasus Larynx. 2002;29:347-351. |
| 14. | Akbayir N, Sokmen HM, Calis AB, Bolukbas C, Erdem L, Alkim C, Sakiz D, Mungan Z. Heterotopic gastric mucosa in the cervical esophagus: could this play a role in the pathogenesis of laryngopharyngeal reflux in a subgroup of patients with posterior laryngitis? Scand J Gastroenterol. 2005;40:1149-1156. |
| 15. | Ward EM, Achem SR. Gastric heterotopia in the proximal esophagus complicated by stricture. Gastrointest Endosc. 2003;57:131-133. |
| 16. | Jerome-Zapadka KM, Clarke MR, Sekas G. Recurrent upper esophageal webs in association with heterotopic gastric mucosa: case report and literature review. Am J Gastroenterol. 1994;89:421-424. |
| 17. | Avidan B, Sonnenberg A, Chejfec G, Schnell TG, Sontag SJ. Is there a link between cervical inlet patch and Barrett’s esophagus? Gastrointest Endosc. 2001;53:717-721. |
| 18. | Lauwers GY, Mino M, Ban S, Forcione D, Eatherton DE, Shimizu M, Sevestre H. Cytokeratins 7 and 20 and mucin core protein expression in esophageal cervical inlet patch. Am J Surg Pathol. 2005;29:437-442. |
Peer reviewers: Dr. Gert De Hertogh, Morphology and Molecular Pathology, University Hospitals KULeuven, Minderbroedersstraat 12, Leuven 3000, Belgium; Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH