Published online Jan 7, 2010. doi: 10.3748/wjg.v16.i1.21
Revised: October 14, 2009
Accepted: October 21, 2009
Published online: January 7, 2010
AIM: To investigate possible differences in dendritic cells (DC) within intestinal tissue of mice before and after induction of colitis.
METHODS: Mucosal DC derived from intestinal tissue, as well as from mesenteric lymph nodes and spleen, were analyzed by fluorescence activated cell sorting (FACS) analysis. Supernatants of these cells were analyzed for secretion of different pro- and anti-inflammatory cytokines. Immunohistochemistry and immunofluorescence were performed on cryosections of mucosal tissue derived from animals with colitis as well as from healthy mice.
RESULTS: It was shown that DC derived from healthy intestinal lamina propria (LP) represented an immature phenotype as characterized by low-level expression of costimulatory cytokines. In contrast to DC from spleen and mesenteric lymph nodes (MLN) that secreted proinflammatory cytokines, LP-DC produced high levels of the anti-inflammatory cytokine IL-10. After induction of murine colitis in a CD4+CD62L+ transfer model or in chronic dextran sulfate sodium-colitis, a marked increase of activated CD80+ DC could be observed within the inflamed colonic tissue. Interestingly, in contrast to splenic DC, a significant population of DC within MLN and colonic LP expressed the mucosal integrin CD103 which was lost during colitis.
CONCLUSION: The constitutive secretion of anti-inflammatory cytokines by immature DC within the intestinal LP might regulate the homeostatic balance between mucosal immunity and tolerance. CD103+ DC could mediate this important function.
- Citation: Strauch UG, Grunwald N, Obermeier F, Gürster S, Rath HC. Loss of CD103+ intestinal dendritic cells during colonic inflammation. World J Gastroenterol 2010; 16(1): 21-29
- URL: https://www.wjgnet.com/1007-9327/full/v16/i1/21.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i1.21
The intestinal mucosa is continuously challenged by innocuous antigens and potentially harmful pathogens. Therefore, the local immune system has to mount an efficient response towards pathogenic bacteria but must keep the immunological balance during exposure to commensal antigens. Dendritic cells (DC) are most likely involved within this dual functionality. However, so far only limited data are available regarding intestinal DC (reviewed in[1,2]). Mucosal DC are not only found within Peyer’s patches (PP) and mesenteric lymph nodes (MLN) but are also located within smaller isolated lymphoid follicles and within the lamina propria, distributed throughout the wall of the small and large intestine[3-5]. Unusual phenotypic subsets of DC have been described within MLN and PP[6-9] that preferentially stimulate antigen-specific CD4+ T cells to produce IL-10 and/or TGF-β1[9,10]. This cytokine pattern is similar to that of TR1 or TH3 regulatory T cells which have been identified in gut-associated lymphoid tissue of mice fed tolerogenic doses of proteins, and are thought to play an important role in oral tolerance[11].
Considerably more is known about DC in PP and MLN than about DC within the lamina propria of the gut. However, these cells are ideally situated to pick up any material that is transported between or through epithelial cells and have been shown to sample luminal antigens directly by sending dendrites outside the epithelium[12]. It is thought that DC as mobile cells migrate to MLN after antigen-uptake and interact with naïve T cells mainly within lymphatic organs, rather than in the mucosa itself. This rapid and constitutive trafficking of DC from lamina propria to MLN is increased by the presence of inflammatory stimuli[13]. As shown recently, interaction of mucosal DC with T cells generates gut-tropic CD8+ effector T cells that express CCR9 and α4β7[14]. Additionally, DC expressing the mucosal integrin CD103 promote the development of regulatory Foxp3+ T cells through a TGF-β and retinoic acid-dependent mechanism[15]. Together, these findings indicate that lamina propria DC (LP-DC) might be more important for the surveillance of the intestinal milieu and the shaping of intestinal immune responses than previously thought.
Animal model systems of colitis have been used extensively in an effort to determine possible mechanisms that contribute to the initiation and perpetuation of colitis in humans[16]. One model in particular which has been well characterized involves the transfer of CD4+CD45RBhi T cells. Here, transfer of naïve (CD4+CD45RBhi or CD4+CD62L+) T cells into immunodeficient SCID mice induces chronic intestinal inflammation[17,18]. Key factors that drive the pathogenic TH1-biased mucosal T cell responses within this model are still unknown[19]. However, as described for various other experimental colitis models[20-25], antigen presentation of bacterial antigens plays a role, as only mild colitis develops when lymphocytes are transferred into animals with a restricted enteric flora and no intestinal inflammation is observed after transfer into germ-free mice[26]. Additionally, intestinal bacterial antigens and their presentation were shown to be crucial for the generation and expansion of regulatory T cells in a healthy individual[18], and disruption of the interaction of mucosal DC with activated T lymphocytes by administration of a receptor-blocking antibody against OX40L was shown to ameliorate colitis[27]. Overall, the data indicate that antigen presentation, presumably via mucosal DC, plays a role in the pathogenesis of chronic intestinal inflammation. However, the properties of these cells during intestinal inflammation are only beginning to be explored.
The aim of our study was to investigate differences between intestinal DC populations under healthy conditions and after induction of colitis.
Balb/c mice and scid mice (C.B.-17 SCID) (H2d) were obtained from Charles River (Germany). Animals were housed under conventional animal facility conditions and were generally used at 6-8 wk of age weighing 20-22 g. The animal studies were approved by the local institutional Review Board.
The following experimental monoclonal antibodies (mAbs) were purchased from BD Pharmingen (Heidelberg, Germany): anti-CD8, anti-MHC-II, anti-B220, anti-CD11b, anti-CD3, anti-CD11c, anti-CD80, anti-CD86, anti-CD40, anti-CD103 (αEβ7), anti-CD16/CD32. Directly PE- or FITC-conjugated mAbs were used for FACS analysis. For immunofluorescent staining directly FITC-conjugated anti-CD103 mAb was used in addition to Alexa 546-conjugated tyramide (Invitrogen, Germany).
DC were isolated from spleen, MLN and intestinal lamina propria by enzymatic digestion of tissue using collagenase I (Worthington, UK), hyaluronidase and DNase I (both from Sigma-Aldrich, Germany) followed by immunomagnetic selection with anti-CD11c coated microbeads (Miltenyi Biotech, Germany). For intestinal DC, mononuclear lamina propria cells were isolated from digested tissue as described previously[28], followed by enrichment of CD11c+ DC using microbeads. Purity was generally > 85%. Routinely, isolated cells were stained for contaminating T cells and B cells, however virtually no cells could be detected by FACS analysis in the DC preparations.
We adapted a previously described transfer model that resembles the CD4+CD45RBhigh model and uses the expression of L-selectin (CD62L) to select for naïve splenic T lymphocytes[18]. Briefly, CD4+ T cells were purified from spleen mononuclear cells of healthy mice by negative depletion of other cell types using anti-CD8, anti-MHC-II, anti-B220 and anti-CD11b mAbs and anti-rat-IgG immunomagnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). The resulting CD4+ lymphocytes were separated further into CD62L+ and CD62L- T cells by CD62L-conjugated microbeads (Miltenyi Biotech). Recipient SCID mice were reconstituted with 0.25 × 106 CD4+CD62L+ lymphocytes in 200 μL of sterile PBS by intraperitoneal injection. Colitis activity was monitored by changes in weight over time and by histological analysis.
For chronic DSS-colitis, dextran sodium sulfate (DSS; MW 40 000) was purchased from ICN (Eschwege, Germany) and intestinal inflammation was induced by feeding 3% DSS over 7 d followed by a period of 10 d of water without DSS. Mice received 4 cycles of DSS treatment and animals were sacrificed on day 8 after completion of the 4th cycle[29].
Dendritic cells from different organs were isolated as described above and 2 × 105 cells/well were incubated in 200 μL complete medium (RPMI-1640, 10% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, all from GIBCP-BRL, Eggenstein, Germany; and 3 × 10-5 mol/L β-mercaptoethanol, Sigma) for 24 h. Cells were partly stimulated with 5 μg/mL CpG-ODN (Metabion, Martinsried, Germany) or with 1 μg/mL Salmonella typhimurium-derived lipopolysaccharide (LPS) (Sigma, Deisenhofen, Germany). Cytokine levels were measured in the supernatant by ELISA (all from Endogene, Woburn, MA, USA), according to the manufacturer’s instructions.
Samples were analyzed using two-color staining as described previously[18]. Briefly, isolated DC were preincubated with 20 μg/mL of anti-CD16/CD32 and 10% FCS to block Fc-Receptors and stained with both FITC- and PE-conjugated mAbs. The cells were washed and analyzed by FACS using an EPICS-XL MCL Coulter.
Tissue samples were snap-frozen in liquid nitrogen, embedded in OCT resin and 5 to 10-μm cryostat-sections cut. For immunohistochemistry, primary antibody application was followed by biotinylated polyclonal anti-rat IgG or anti-hamster IgG (both Dianova, Germany) as secondary antibody. Tissue was stained using the ABC (avidin/biotin complex)-immunoperoxidase kit according to the manufacturer’s instructions (Vector Laboratories) and developed with AEC. Sections were counterstained with hematoxylin. For immunofluorescence, sections were incubated with APC-conjugated anti-CD11c and with FITC-labeled anti-CD103 mAbs. The anti-CD11c mAb was visualized by applying horseradish peroxidase-labeled streptavidin followed by Alexa 546-conjugated tyramide, according to the manufacturer’s recommendations (Invitrogen, Germany). Slides were counterstained with DAPI.
Statistical analysis was performed using the two-tailed Mann-Whitney U test. Differences were considered statistically significant when P < 0.05.
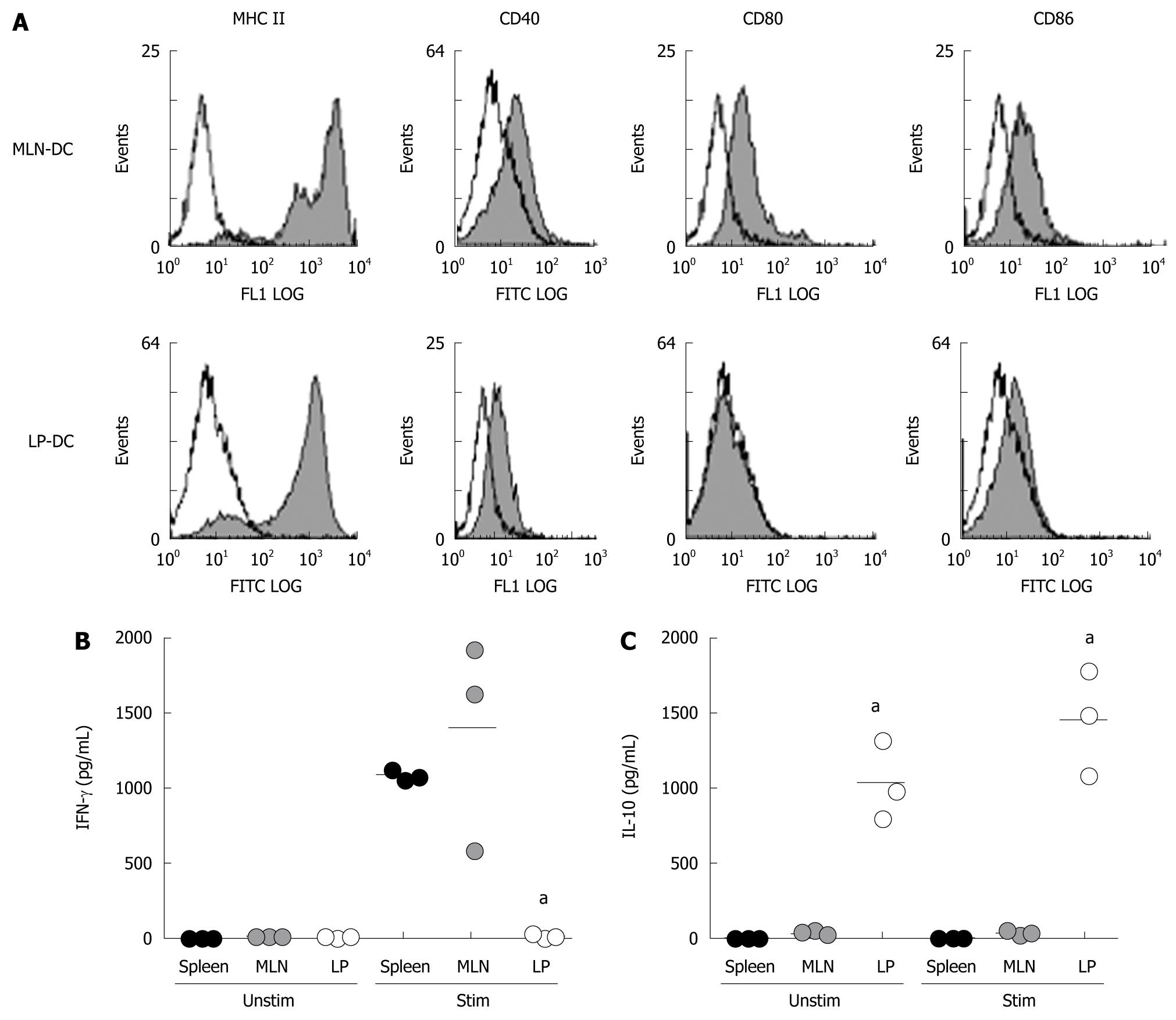
CD11c+ DC are found within the lamina propria (LP) of the small and large intestine of healthy mice. Whereas a dense network of cells underlining the epithelium can be detected by immunohistochemistry within the mucosa of the small intestine, only a few scattered cells are found within the colonic LP. To compare the phenotype of DC derived from colonic LP and mesenteric lymph nodes (MLN), CD11c+ DC were isolated from intestinal tissue and MLN of healthy mice. As demonstrated in Figure 1A, no significant levels of costimulatory molecules (CD40, CD80 and CD86) could be detected on the cell surface of freshly isolated DC from the intestinal LP or MLN. This suggests that DC within mucosal tissues demonstrate a rather immature phenotype as compared to splenic DC which show low levels of costimulatory molecules (data not shown). Additionally, isolated primary DC were incubated either unstimulated or in the presence of CpG and secretion of cytokines was detected by ELISA. As shown, MLN-DC and splenic DC differed markedly from LP-DC with regard to their cytokine profile. Unstimulated MLN-DC or splenic DC did not secrete significant amounts of pro- or anti-inflammatory cytokines, however, LP-DC dramatically produced 30-fold higher levels of the anti-inflammatory cytokine IL-10 (MLN-DC: 35.4 ± 5.0; splenic-DC: 12.8 ± 0.7, LP-DC: 1035 ± 270 pg/mL, P = 0.0235, Figure 1B). In contrast, large amounts of IFN-γ and IL-12 were secreted by MLN-DC and splenic-DC after stimulation with CpG, whereas LP-DC did not produce significant amounts of proinflammatory cytokines (MLN-DC: 1398 ± 407, splenic-DC: 1087 ± 30, LP-DC: 24 ± 11 pg/mL, P = 0.0009). The differences seen were independent from the stimulatory agent used as similar results were detected by using LPS to stimulate primary DC.
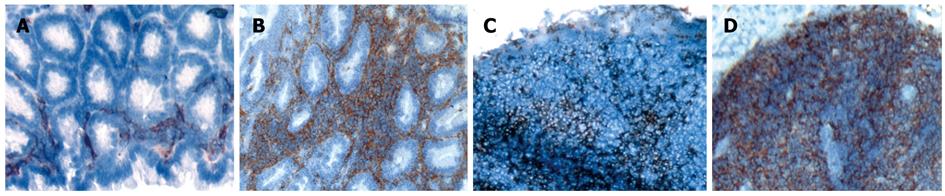
As shown in Figure 2A, CD11c+ DC were diffusely distributed throughout the non-inflamed colonic LP and were rarely detected in the submucosa. Chronic colitis was either induced by adoptive transfer of splenic CD4+CD62L+ T lymphocytes from donor mice into immunodeficient SCID recipients or by cyclic administration of DSS in the drinking water of animals[18,29]. As analyzed by immunohistochemistry using an anti-CD11c antibody in both models of colitis, a dramatic increase in numbers of CD11c+ DC could be detected within the inflamed mucosa of the colon of mice (Figure 2B). MLN of mice with colitis showed a slight increase in the number of DC (Figure 2C and D), whereas no changes in infiltrating DC were seen in Peyer’s patches and the spleen (data not shown).
Infiltrating LP-DC in inflamed colonic tissue show a mature phenotype with high expression of CD80 cells and secretion of the regulatory cytokine IFN-α is decreased
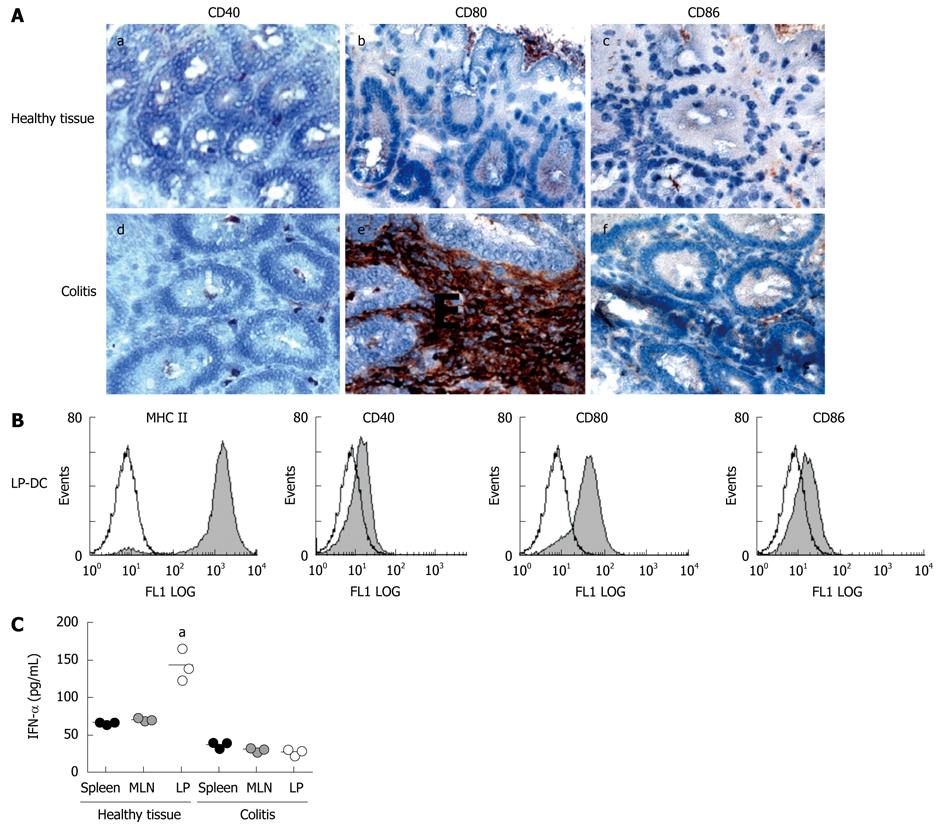
As shown above, mucosal DC in healthy tissue are immature. To investigate whether infiltrating CD11c+ DC within the inflamed colon show an activated phenotype, tissue sections were stained for the expression of costimulatory molecules. As demonstrated in Figure 3A, we were able to detect dramatically increased numbers of CD80+ DC within the colonic LP as compared to healthy mucosa. On the other hand, no significant numbers of CD40+ and CD86+ cells were detected. We confirmed the results by FACS analysis after isolation of primary DC from inflamed tissue, demonstrating that LP-DC indeed showed increased cell-surface levels of CD80, whereas no difference in expression of CD40 and CD86 could be observed (Figure 3B).
To investigate whether the cytokine profile of isolated DC from different tissues was changed in animals with colitis we measured the cytokine secretion of primary DC. Secretion of IL-10 by LP-DC was reduced to only 27% of the amount secreted by LP-DC from healthy intestine (colitic LP-DC: 277 ± 27, healthy LP-DC: 1035 ± 270 pg/mL) (data not shown). Additionally, whereas LP-DC from normal colon did not secrete any proinflammatory cytokines as demonstrated above, DC from inflamed intestine were able to produce significant amounts of IFN-γ (397 ± 163 pg/mL) and TNF-α (650 ± 91 pg/mL) (data not shown). Furthermore, IFN-α, a cytokine attributed to plasmocytoid DC with regulatory function, was secreted to a greater extent by LP-DC from healthy mice as compared to splenic or MLN-DC which produced distinctly lower amounts of this cytokines (LP-DC: 143 ± 12, MLN-DC: 71 ± 2, splenic-DC: 67 ± 2 pg/mL, P = 0.0242). However, during colitis, production of IFN-α by LP-DC was significantly reduced to 19.6% (Figure 3C, colitic LP-DC: 28 ± 4 pg/mL, P = 0.0111).
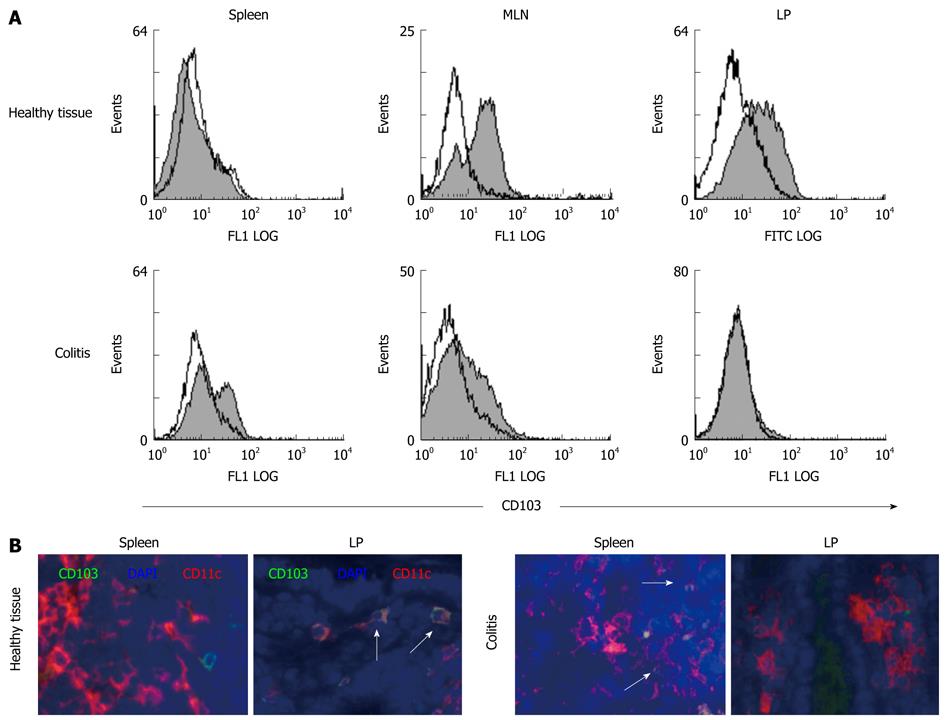
The mucosal integrin αEβ7 (CD103) is expressed on the cell surface of intraepithelial lymphocytes and mediates adhesion to epithelial cells via binding to E-cadherin[30]. Recently, it was shown that expression of CD103 characterizes an important subset of regulatory T cells[31]. Additionally, CD103+ mucosal DC were suggested to play an important role for the generation of Foxp3+ T lymphocytes within the gut[15]. As this integrin seems to play a role in regulatory immunological functions, especially in mucosal sites, we wanted to investigate whether CD103+ intestinal DC change during intestinal inflammation. As demonstrated in Figure 4, freshly isolated DC from healthy spleen did not contain a significant population of CD103+ DC. However, this was very different in mucosal sites of healthy animals. Here, a large subpopulation of MLN-DC expressed the integrin αEβ7 and even more strikingly, almost all LP-DC showed at least low levels of CD103 expression on the cell surface, demonstrating that LP-DC from healthy mucosa did not only differ dramatically in their cytokine secretion potential but also in their phenotype from DC at peripheral sites. However, when we looked for CD103 expression on DC isolated from animals with colitis we observed a dramatic loss of this population in mucosal tissues. Within the chronically inflamed colonic LP, no CD103+ DC where seen as demonstrated by FACS analysis, and numbers of integrin-positive DC in MLN were dramatically reduced. In contrast, we detected a significant subpopulation of CD103+ DC within the spleen of colitic animals (Figure 4).
Antigen-presenting cells are the key to maintaining the immunological balance between active immune responses and tolerance within the intestine and DC are most likely to participate importantly in this immunological homeostasis within the gut. However, data has recently started to be available regarding intestinal DC in normal and inflamed colon. Comparing DC populations from different tissues we were able to demonstrate that mucosal DC represent an immature phenotype as characterized by the absence of CD40, CD80 and CD86 expression. Whereas CD80 was found in low levels on the cell surface of MLN-DC, the molecule was absent on LP-DC, indicating that LP-DC represent an even more immature phenotype than MLN-DC. It is thought that DC can be divided into tolerogenic immature and immunogenic mature differentiation stages[32], as tolerance is mediated by partial- or semi-maturated DC, whereas only full DC maturation is immunogenic[1,2,33]. Therefore, our observation of phenotypically immature (or semi-mature) LP-DC within the healthy gut supports the hypothesis that intestinal DC, which sample antigens without being fully activated, induce tolerance against antigens of the regular gut flora. Additionally, we were able to show that DC from spleen and MLN secrete proinflammatory cytokines such as IFN-γ and IL-12 in response to the different strong inflammatory stimuli, CpG and LPS. In contrast, intestinal-derived CD11c+ DC constitutively produced high levels of the anti-inflammatory cytokine IL-10 and did not release significant amounts of proinflammatory mediators after stimulation. As shown previously, pulmonary DC - situated within a mucosa that is similar to the intestine exposed to antigens - produce IL-10 in response to inhalative antigens and induce the development of regulatory T cells[34]. Therefore, it can be speculated that constitutive production of the anti-inflammatory cytokine IL-10 by LP-DC is also critical for the generation of regulatory T cells and the maintenance of tolerance towards luminal antigens within the normal gut.
However, during intestinal inflammation the cellular composition within the colonic lamina propria changes. As shown, gut inflammation in different murine models of colitis was accompanied by a marked infiltration of the colonic mucosa by CD11c+ DC. LP-DC derived from the inflamed mucosa expressed high levels of CD80, a cell surface molecule thought to be involved with induction of TH1 responses[35,36], and cells resembled a phenotype of mature activated DC. Additionally, these cells produced dramatically lower levels of IL-10 and INF-α, cytokines necessary for anti-inflammatory responses[37]. Our observation is in concordance with a recent study that also demonstrated expansion of colonic LP-DC during murine colitis[5,38] and other data that showed up-regulated expression of activation markers on DC in diseased mucosal tissues from patients with inflammatory bowel disease[39]. It is likely that the infiltration of the intestinal mucosa with mature DC during intestinal inflammation leads to a continuous activation of T lymphocytes and a sustained overproduction of proinflammatory mediators within the lamina propria, which perpetuates colitis.
Additionally, we were able to show that LP-DC from normal colonic mucosa and MLN-DC contain a significant subpopulation of CD103+ (αEβ7) DC, whereas splenic-DC were negative for the mucosal adhesion molecule. This observation suggests the localization of specific DC subpopulations within the intestinal lamina propria. So far, it is known that intraepithelial lymphocytes express the integrin αEβ7 which interacts with epithelial E-cadherin and is thought to mediate localization of T cells within the epithelial layer[30]. Additionally, it seems to characterize a specific subgroup of lymphocytes with regulatory function[31] and a recent study was able to show that CD103+ DC promote expression of the gut-homing receptor CCR9 on T cells[14,40], as well as generation of Foxp3+ T lymphocytes with TGF-β and retinoic-acid as cofactors[15]. Because in our study almost all intestinal LP-DC within the healthy mucosa express this integrin and show a tolerogenic phenotype and function, we hypothesize that CD103 mediates homing for tolerogenic DC into the intestinal mucosa or enables as adhesion molecule the crosstalk with other lamina propria cells, thereby influencing the balance between effector and regulatory T cell activity in the intestine. As we were not able to identify the CD103+ LP-DC during colitis when tolerance is lost, this subgroup of DC could help to maintain the immunological balance within the normal intestinal mucosa. Surprisingly, during inflammation, CD103+ DC were found within the spleen, suggesting that intestinal CD103+ DC might migrate to systemic lymphatic tissues.
Overall, our results indicate that the specific localization of particular CD103+ DC subpopulations within the intestinal mucosa may be an important mechanism of the immune system to determine between active immune responses and tolerance towards luminal antigens. Additionally, the constitutive secretion of anti-inflammatory cytokines by intestinal DC might regulate the homeostatic balance under healthy conditions. After induction of colitis, loss of CD103+ intestinal DC and infiltration of mature DC that express the costimulatory molecule CD80 within the colonic mucosa would lead to a dysregulation of this balance. Antigen presentation via activated DC could be involved in the onset or/and chronification of colitis. Interrupting the activation of intestinal DC in vivo and promoting the preservation of presumably tolerogenic CD103+ DC within the colonic mucosa may be key approaches to control the pathogenesis of inflammatory bowel disease.
Within the gut mucosa the immune system has the task of distinguishing between commensal bacteria and foreign antigens, to maintain tolerance or to mount an inflammatory response. Dendritic cells are very important for this process. However, even if in the last few years some important insights have been made, much still is unknown about these cells, especially about the changes they undergo during intestinal inflammation.
Previous data indicate that antigen presentation, presumably via mucosal dendritic cells, plays a role in the pathogenesis of chronic intestinal inflammation. However, the properties of these cells during intestinal inflammation are only beginning to be explored.
Dendritic cells in normal intestinal lamina propria showed an immature phenotype and produced high levels of the anti-inflammatory cytokine IL-10 whereas dendritic cells in the spleen and local lymph nodes secreted the proinflammatory cytokine IFN-γ. Furthermore, the studies in mouse models of colitis showed that the development of colitis was associated with a marked increase of activated dendritic cells within the inflamed colonic tissue and the loss of CD103+ dendritic cells in the colonic mucosa and local lymph nodes but not in spleen, suggesting that CD103+ dendritic cells could play important roles in the regulation of homeostatic balance between mucosal immunity and tolerance in the gastrointestinal tract.
Overall, these results indicate that the specific localization of particular CD103+ dendritic cell subpopulations within the intestinal mucosa may be an important mechanism of the immune system in determining between active immune response and tolerance towards luminal antigens. Additionally, the constitutive secretion of anti-inflammatory cytokines by intestinal DC might regulate the homeostatic balance under healthy conditions. Interrupting the activation of intestinal dendritic cells in vivo and promoting the preservation of presumably tolerogenic CD103+ dendritic cells within the colonic mucosa may be key approaches to control the pathogenesis of inflammatory bowel disease.
This is a well prepared manuscript and the experiments described were well designed, controlled and executed. The loss of CD103+ CD11c+ DCs in progression of colitis is a unique and novel finding which seems to coincide with other groups’ findings relating to regulatory T cells. Overall the results could contribute to our understanding of the immunopathogenesis of human inflammatory bowel diseases.
| 1. | Bilsborough J, Viney JL. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology. 2004;127:300-309. |
| 2. | Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331-341. |
| 3. | Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57-64. |
| 4. | Leithäuser F, Trobonjaca Z, Möller P, Reimann J. Clustering of colonic lamina propria CD4(+) T cells to subepithelial dendritic cell aggregates precedes the development of colitis in a murine adoptive transfer model. Lab Invest. 2001;81:1339-4139. |
| 5. | Krajina T, Leithäuser F, Möller P, Trobonjaca Z, Reimann J. Colonic lamina propria dendritic cells in mice with CD4+ T cell-induced colitis. Eur J Immunol. 2003;33:1073-1083. |
| 6. | Anjuère F, Martín P, Ferrero I, Fraga ML, del Hoyo GM, Wright N, Ardavín C. Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse. Blood. 1999;93:590-598. |
| 7. | Ruedl C, Rieser C, Böck G, Wick G, Wolf H. Phenotypic and functional characterization of CD11c+ dendritic cell population in mouse Peyer’s patches. Eur J Immunol. 1996;26:1801-1806. |
| 8. | Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381-1394. |
| 9. | Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer’s patch dendritic cells. J Immunol. 2001;166:4884-4890. |
| 10. | Alpan O, Rudomen G, Matzinger P. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J Immunol. 2001;166:4843-4852. |
| 11. | Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737-742. |
| 12. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. |
| 13. | Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435-444. |
| 14. | Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Förster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063-1073. |
| 15. | Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757-1764. |
| 16. | Hoffmann JC, Pawlowski NN, Kühl AA, Höhne W, Zeitz M. Animal models of inflammatory bowel disease: an overview. Pathobiology. 2002;70:121-130. |
| 17. | Leach MW, Bean AG, Mauze S, Coffman RL, Powrie F. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T cells. Am J Pathol. 1996;148:1503-1515. |
| 18. | Strauch UG, Obermeier F, Grunwald N, Gürster S, Dunger N, Schultz M, Griese DP, Mähler M, Schölmerich J, Rath HC. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54:1546-1552. |
| 19. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. |
| 20. | Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945-953. |
| 21. | Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253-261. |
| 22. | Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernández-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359-2364. |
| 23. | Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91-97. |
| 24. | Schultz M, Tonkonogy SL, Sellon RK, Veltkamp C, Godfrey VL, Kwon J, Grenther WB, Balish E, Horak I, Sartor RB. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am J Physiol. 1999;276:G1461-G1472. |
| 25. | Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224-5231. |
| 26. | Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464-3473. |
| 27. | Malmström V, Shipton D, Singh B, Al-Shamkhani A, Puklavec MJ, Barclay AN, Powrie F. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol. 2001;166:6972-6981. |
| 28. | Schön MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641-6649. |
| 29. | Obermeier F, Dunger N, Strauch UG, Grunwald N, Herfarth H, Schölmerich J, Falk W. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217-224. |
| 30. | Strauch UG, Mueller RC, Li XY, Cernadas M, Higgins JM, Binion DG, Parker CM. Integrin alpha E(CD103)beta 7 mediates adhesion to intestinal microvascular endothelial cell lines via an E-cadherin-independent interaction. J Immunol. 2001;166:3506-3514. |
| 31. | Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc Natl Acad Sci USA. 2002;99:13031-13036. |
| 32. | Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445-449. |
| 33. | Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394-400. |
| 34. | Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725-731. |
| 35. | van Rijt LS, Vos N, Willart M, Kleinjan A, Coyle AJ, Hoogsteden HC, Lambrecht BN. Essential role of dendritic cell CD80/CD86 costimulation in the induction, but not reactivation, of TH2 effector responses in a mouse model of asthma. J Allergy Clin Immunol. 2004;114:166-173. |
| 36. | Dilioglou S, Cruse JM, Lewis RE. Function of CD80 and CD86 on monocyte- and stem cell-derived dendritic cells. Exp Mol Pathol. 2003;75:217-227. |
| 37. | Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461-465. |
| 38. | Karlis J, Penttila I, Tran TB, Jones B, Nobbs S, Zola H, Flesch IE. Characterization of colonic and mesenteric lymph node dendritic cell subpopulations in a murine adoptive transfer model of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:834-847. |
Peer reviewers: Dr. Wang-Xue Chen, Institute for Biological Sciences, National Research Council Canada, 100 Sussex Drive, Room 3100, Ottawa, Ontario K1A 0R6, Canada; Hartmut Jaeschke, Professor, Liver Research Institute, University of Arizona, College of Medicine, 1501 N Campbell Ave, Room 6309, Tucson, AZ 85724, United States
S- Editor Tian L L- Editor Logan S E- Editor Ma WH