INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic disease of unknown etiology that is characterized by the presence of circulatory autoantibodies and inflammatory changes in liver histology[1]. Several triggers for AIH, particularly drugs and viral hepatitis, have been described, which may induce the development of autoimmunity in predisposed individuals[12]. We report a case of severe AIH preceded by varicella zoster virus (VZV) infection, which we believe, triggered the AIH. The possible pathogenic mechanism is based on the molecular mimicry hypothesis, in which viral proteins that are similar to the amino acid chains of autoantigens in the liver induce immune cross reactions that cause liver damage[3]. As far as we are aware, VZV-induced AIH has not been reported previously.
CASE REPORT
A 23-year-old man was referred to our hepatology service on September 1, 2007 with jaundice, anorexia, weight loss and malaise of 2 mo duration. The episode of jaundice was preceded by VZV infection (chicken pox), which he contracted from close contact with infected family members. Despite recovery from the skin eruption, he had persistent anorexia and intermittent right upper quadrant pain. One month later he developed increasing jaundice. He had no prior history of alcohol ingestion or substance abuse and no family history of liver disease. His past medical history was otherwise unremarkable. His initial presentation to our hospital was 2 mo following the onset of jaundice. His physical examination revealed jaundice and hepatomegaly 2 cm below the costal margin. His initial laboratory findings were as follows: hemoglobin 149 g/L, white blood cell count 9.9 × 109/L, platelets 512 × 109/L, erythrocyte sedimentation rate 39 mm/h, alanine aminotransferase (ALT) 1066 U/L, aspartate aminotransferase (AST) 755 U/L, alkaline phosphatase (ALP) 185 U/L, total bilirubin 425 &mgr;mol/L, direct bilirubin 318 &mgr;mol/L, and albumin 32 g/L. His globulins were elevated at 46 g/L and his IgG was also elevated at 20.5 g/L. The coagulation profile was normal. Anti-nuclear, anti-smooth muscle, anti-mitochondrial and anti-liver/kidney microsomes (ALKM-1) autoantibodies were negative. Perinuclear antineutrophil cytoplasmic antibodies were positive. Hepatitis B surface antigen, anti-hepatitis B core antigen IgM, anti-hepatitis C virus antibodies, anti-cytomegalovirus (CMV) IgM, anti-Epstein-Barr virus (EBV) IgM, and anti-hepatitis A virus (HAV) IgM were all negative. Anti-VZV IgG antibodies were positive. Ceruloplasmin and iron indices were normal.
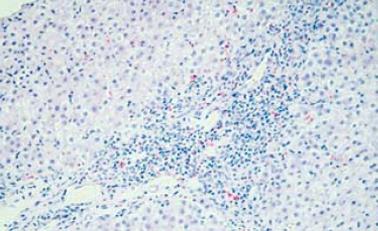
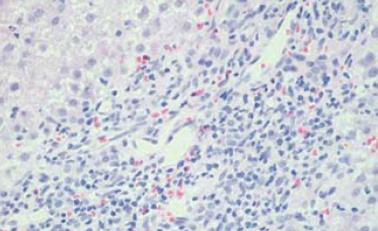
The patient refused a liver biopsy, therefore, we started him on a tapering course of prednisone for presumed AIH, at a starting dose of 60 mg. He responded to that dramatically, as he became totally asymptomatic and started gaining weight. Within 2 mo of treatment, his liver enzymes normalized. On tapering his steroids below 10 mg, his liver enzymes increased again. His prednisone dose was increased to 30 mg and 50 mg azathioprine was added, however, because of vomiting and epigastric pain, the latter was changed to mycophenolate mofetil 500 mg twice daily. Follow-up showed improvement in his liver enzymes. In August, 2008, he stopped all his medications as he was feeling well and his liver enzymes were normal. A follow-up visit in September, 2008 revealed an increase in his liver enzymes, with ALT of 501 U/L and AST of 230 U/L, and bilirubin and ALP remained normal. A liver biopsy was performed and revealed features of AIH, with interface hepatitis, predominant lymphocytic infiltration, and the presence of plasma cells. Additionally, bridging fibrosis was also noted (Figure 1 and Figure 2).
Figure 1 Portal tract expansion secondary to infiltration by inflammatory cells (interface hepatitis is seen).
(HE stain × 10).
Figure 2 Inflammatory cells comprising mainly lymphocytes with some plasma cells.
(HE stain × 20).
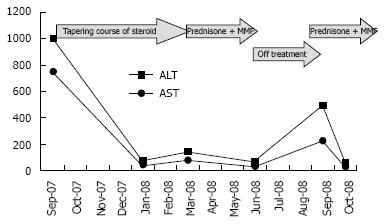
Treatment was reintroduced with improvement in the biochemical markers (Figure 3).
Figure 3 ALT and AST pattern during the course of the disease.
DISCUSSION
VZV is a double-stranded, linear DNA virus. Primary infection with VZV causes chicken pox in susceptible hosts[4]. Varicella hepatitis in immunocompetent hosts is usually self-limiting and asymptomatic, with subclinical elevation in serum transaminase levels[5]. However, severe varicella hepatitis leading to fulminant liver failure has been reported[6]. There is an increased risk of VZV infections in patients with underlying autoimmune disease. However, VZV-triggered autoimmune diseases have not been reported before. In the present case, anorexia and weight loss persisted following resolution of the varicella-induced skin eruption. One month later, the patient developed jaundice that persisted for 2 mo before the patient presented to our hospital. The patient had a post-treatment score of 17 according to the revised International Autoimmune Hepatitis Group system. The sequence of events in this patient indicated strongly that the AIH was triggered by VZV infection.
The pathogenic mechanism of AIH is unknown. Genetic predisposition is considered to play a major role, however, it is unlikely to be the single causative factor and other triggering factors may exist. There has been evidence implicating CMV, EBV, measles virus and hepatitis viruses as triggers of the disease[17–11]. It is thought that viral proteins belonging to these viruses may be similar to amino acid chains of different autoantigens in the liver, and this resemblance leads to immune cross reactions that target liver tissue. Asialoglycoprotein receptor, which is found in high density in the periportal hepatocytes, is an autoantigen that is thought to play a role in the immunological reactions in AIH[12]. A defect in suppressor-inducer T lymphocytes, specifically controlling immune responses to the asialoglycoprotein receptor, has been detected in patients following viral infection[1314].
The study of Vento et al[13] supports the concept that the pathogenesis of AIH in genetically susceptible individuals is caused by an environmental trigger that leads to an aberrant autoimmune response. They followed 53 relatives of 13 AIH patients for a total of 4 years. Three cases developed subclinical hepatitis A and two of them subsequently developed AIH after 5 mo. Prior to developing HAV, a defect in suppressor-inducer T lymphocytes specifically controlling immune responses to the asialoglycoprotein receptor was detected[13]. Following this study, multiple reports linking AIH to viral infections were published[7–11]. In addition to the molecular mimicry theory, other pathogenic mechanisms that are thought to play a role in virus-induced AIH include modification or release of sequestered cellular proteins by viruses, activation of resting T cells by inducing the release of a variety of cytokines, and polyclonal activation of lymphocytes[15].
In conclusion, VZV infection can trigger AIH that may progress to advance liver fibrosis and eventually cirrhosis in genetically predisposed individuals. Therefore, AIH should be considered in any patient with persistently altered liver enzymes following a viral infection. Similarly, patients with AIH should be screened for viral hepatitis.