Published online Feb 21, 2009. doi: 10.3748/wjg.15.871
Revised: December 14, 2008
Accepted: December 21, 2008
Published online: February 21, 2009
AIM: To investigate the efficacy and side effects of the combined therapy of oxaliplatin and capecitabine in patients with metastatic esophageal squamous cell cancer (ESCC) and the survival of the patients.
METHODS: Sixty-four patients (median age of 63 years) with histological or cytological confirmation of ESCC received oxaliplatin 120 mg/m2 intravenously on day 1 and capecitabine 1000 mg/m2 orally twice daily on days 1 to 14 in a 21-d treatment cycle as palliative chemotherapy. Each patient received at least two cycles of treatment. The efficacy, side effects and patient survival were evaluated.
RESULTS: The partial response (PR) rate was 43.8% (28/64). Stable disease (SD) rate was 47.9% (26/64), and disease progression rate was 15.6% (10/64). The clinical benefit rate (PR + SD) was 84.4%. The main toxicities were leukopenia (50.0%), nausea and vomiting (51.6%), diarrhea (50.0%), stomatitis (39.1%), polyneuropathy (37.5%) and hand-foot syndrome (37.5%). No grade 4 event in the entire cohort was found. The median progression-free survival was 4 mo, median overall survival was 10 mo (95% CI: 8.3-11.7 mo), and the 1- and 2-year survival rates were 38.1% and 8.2%, respectively. High Karnofsky index, single metastatic lesion and response to the regimen indicated respectively good prognosis.
CONCLUSION: Oxaliplatin plus capecitabine regimen is effective and tolerable in metastatic ESCC patients. The regimen has improved the survival moderately and merits further studies.
- Citation: Qin TJ, An GL, Zhao XH, Tian F, Li XH, Lian JW, Pan BR, Gu SZ. Combined treatment of oxaliplatin and capecitabine in patients with metastatic esophageal squamous cell cancer. World J Gastroenterol 2009; 15(7): 871-876
- URL: https://www.wjgnet.com/1007-9327/full/v15/i7/871.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.871
Esophageal cancer, which has the highest incidence and mortality worldwide[1–4] is one of the most common malignant tumors in China. Linzhou (formerly known as Linxian) and nearby cities, such as Anyang and Huixian in Henan Province of northern China have been well recognized as the highest incidence area for esophageal squamous cell carcinoma (ESCC) in the world; the average incidence rates for men and women are 161 and 103 per 100000, respectively[5].
Due to the lack of obvious early symptoms, the patients were often diagnosed at advanced stages, more than half of them with metastasis[6]. The recurrence and metastasis rate after treatment of esophageal cancer have the trend to ascend in recent years. In 2007, Grunberger et al[7] have confirmed that palliative chemotherapy can prolong the survival of stage IV esophageal cancer patients, relieve their symptoms and improve their quality of life. Nevertheless, no optimizing chemotherapy regimen has been developed so far, the combined regimens based on cisplatin and 5-FU has been used frequently, with an effective rate of about 25.0%-33.0%[89]. Squamous cell esophageal cancer is the most common histology in China, and the constituent ratio is different from that in Europe and America. Some experts state that there are complete differences between esophageal adenocarcinomas and squamous cell cancer, such as the treatment protocol and prognosis. Therefore, the focus must be laid on the study of palliative chemotherapy of metastatic ESCC.
Oxaliplatin is a kind of chemotherapeutic drug belonging to the third generation of platinum compounds, which has played an important role in the treatment of colon cancer and other solid tumors[1011]. Oxaliplatin’s side chain is substituted by the diamino-cyclohexane radical (DACH). Therefore, compared to cisplatin, DACH-platinum combines to DNA much faster with stronger cell toxicity, which has no cross tolerance with cisplatin and no oto-renal toxicity. Furthermore, it has a synergistic effect with 5-FU, with slight digestive tract reaction and hematotoxicity. Its common side effect is reversible peripheral nerve paresthesia. Oral capecitabine can be rapidly absorbed as an intact molecule in the gastrointestinal tract and most of a given dose of capecitabine is initially hydrolyzed in the liver by a carboxylesterase to 5’-deoxy-5-fluorocytidine (5’-DFCR) without bioactivity. Cytidine deaminase, an enzyme found in many tissues, including tumors, converts 5’-DFCR to 5’-DFUR. Certain human carcinomas express the enzyme thymidine phosphorylase in higher concentrations than the surrounding normal tissues, which potentially converts 5’-DFUR to higher concentrations of active 5-fluorouracil (5-FU) within these tumors.
This study aims to explore the efficacy and toxic reaction of the combined treatment of oxaliplatin and capecitabine in metastatic ESCC and the survival of the patients. The results will be used to supply information and instruction for clinical treatment.
From January 2003 to January 2006, 64 patients (45 males and 19 females) with histological or cytological confirmation of metastatic ESCC received oxaliplatin plus capecitabine therapy. The median age of the patients was 63 years (27cases under 60 years and 37 cases over 60 years). The metastatic sites of ESCC patients were lymph node, bone, liver, lung, membrana pleuralisa, abdominal membrane, adrenal gland, skin and soft tissue. Among these patients, 42 had single-site metastasis and 22 had multi-site metastases. Karnofsky performance status (KPS) of the patients was between 60 and 100 (60-80 in 42 patients and 90-100 in 23 patients). Before the study, 28 patients had received no chemotherapy, and 36 had received previous chemotherapy, and oxaliplatin and capecitabine treatment was excluded.
All patients were required to take pathological examinations, upper gastrointestinal tract barium meal perspective, computed tomography (CT) for neck thorax and abdomen, magnetic resonance imaging or CT for skull, emission computerized tomography for bone, blood routine test, liver-renal function test, electrocardiography (ECG) and other routine tests.
All patients received oxaliplatin and capecitabine as follows: oxaliplatin 120 mg/m2, infused on day 1; capecitabine 1000 mg/m2, taken orally twice a day on days 1-14. Before taking oxaliplatin, the patients received 5-hydroxy-tryptamine inhibitors to prevent vomiting. During the medication, the patients should keep their body warm, avoid cold drinks, and take vitamin B6 100 mg orally three times a day with capecitabine to prevent and decrease the occurrence of extremity syndrome. Blood routine and liver-renal function tests should also be performed, and abnormal tests should be managed to accomplish the chemotherapy. Patients with bone metastasis should receive the radiotherapy and diphosphonate simultaneously in the 21-day cycle treatment. Each patient received at least two cycles of chemotherapy.
After completion of two cycles of chemotherapy, all patients received overall check-up. Tumor response was assessed using Response Evaluation Criteria in Solid Tumors, such as the change of the tumor size, quantity and the appearance of new lesions. Toxicity was evaluated according to the Common Toxicity Criteria for acute and subacute toxicity reactions, and confirmed again at 4 wk after treatment. Patients benefited from the treatment complete response (CR) and partial response (PR) were given one or two more cycles of chemotherapy based on their agreement and tolerance. If the disease was progressive, they should receive other chemotherapeutic protocols, and optimized supportive treatment should be administered if the patients agree and are tolerant.
After completion of chemotherapy, all patients were followed up every 3 mo in the first year and every 6 mo in the second year by outpatient service and telephone interview till patients’ death.
Overall survival, progression-free survival, death or last follow-up results were evaluated by the Kaplan-Meier method. The life table method was used to evaluate the 1-year and 2-year survival rates. Single factor was compared by log-rank test, and multi-factor was analyzed by Cox regression proportional hazard model.
All patients were evaluated for short-term effects and toxicity. There was no CR; 28 patients (43.8%) had PR, 26 patients (40.6%) demonstrated stable disease (SD) and 10 (15.6%) patients presented with cancer progression. The effectiveness rate was 40.6% and the overall clinical benefit rate was 84.4%.
The main side effects of chemotherapy were alimentary tract reaction such as nausea, vomiting and diarrhea, and different grade bone marrow suppression. The occurrence of nausea and vomiting was 51.6%, and that of diarrhea was 50.0%. All side effects were slight or moderate. The main bone marrow suppression was leukopenia. The incidence rates of grade I, II, III and IV leucopenia were 31.3%, 15.6%, 3.1% and 0.0%, respectively. One female patient had the symptom of digit anesthesia and anodynia late in the second cycle of chemotherapy, but this relieved gradually without treatment. The others only had grade I or II nerve toxicity such as dead limb, dysesthesia, and cold sensitivity. All of these recovered soon during the intermission of chemotherapy. No severe hand-foot syndrome occurred. The incidence rate of slight and moderate hand-foot syndrome was 37.5%. The other toxicities were grade I or II tolerable oral mucositis (39.1%), liver function abnormality (1.4%) and alopecia (6.2%). There was no renal function abnormality and death related to chemotherapy (Table 1).
| Side effects | Grade | ||||
| 0 | I | II | III | IV | |
| Nausea and vomiting | 31 (48.4) | 21 (32.8) | 12 (18.8) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 32 (50.0) | 20 (31.3) | 12 (18.7) | 0 (0.0) | 0 (0.0) |
| Aspherinia | 44 (68.7) | 11 (17.2) | 9 (14.1) | 0 (0.0) | 0 (0.0) |
| Leukopoenia | 32 (50.0) | 20 (31.3) | 10 (15.6) | 2 (3.1) | 0 (0.0) |
| Thrombocytopenia | 46 (71.9) | 11 (17.2) | 7 (10.9) | 0 (0.0) | 0 (0.0) |
| Nerve toxicity | 40 (62.5) | 14 (21.9) | 9 (14.1) | 1 (1.5) | 0 (0.0) |
| Hand-foot syndrome | 40 (62.5) | 13 (20.3) | 11 (17.2) | 0 (0.0) | 0 (0.0) |
| Alopecia | 60 (93.8) | 4 (6.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mucositis of mouth | 39 (60.9) | 18 (28.1) | 7 (10.9) | 0 (0.0) | 0 (0.0) |
| Abnormal liver function | 56 (87.6) | 7 (10.9) | 1 (1.5) | 0 (0.0) | 0 (0.0) |
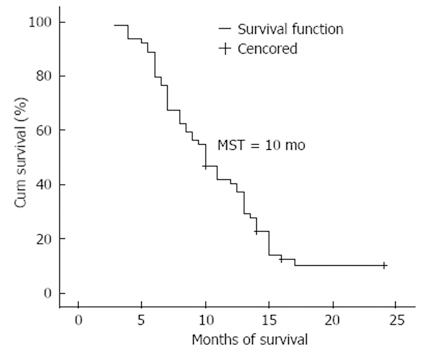
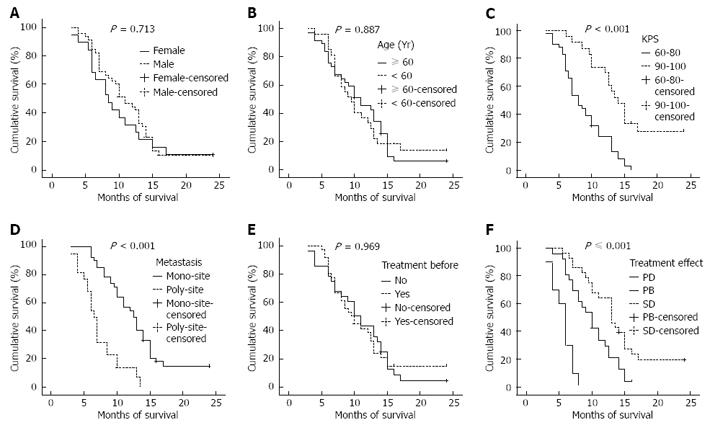
The 64 patients were all followed up either for 2 years or until death. The follow-up rate was 100.0% (Figure 1). The median progression-free survival was 4.0 mo, and the median overall survival was 10.0 mo (95% CI: 8.3-11.7 mo). The 1- and 2-year survival rates were 38.1% (24/63) and 8.2% (5/61), respectively. Kaplan-Meier monofactorial analysis indicated that there was a statistical significance between the influence of KPS index, metastasis and short-term effect and survival (P≤ 0.0001), but there was no statistical significance between the influence of sex, age and therapy and survival (P > 0.05), (Figure 2). Cox regression proportional hazard model polyfactorial analysis indicated that KPS index, the number of tumor metastasis loci and short-term effect (P < 0.001) were independent survival prognostic factors, while sex, age and former therapy (P > 0.05) were not (Tables 2 and 3).
| Prognostic factor | Number | Survival rate (%) | MST (mo) | P | |
| 1-yr | 2-yr | ||||
| Sex | 0.713 | ||||
| Male | 42 | 38.1 | 7.1 | 10.0 | |
| Female | 119 | 26.3 | 10.5 | 8.5 | |
| Age (Yr) | 0.887 | ||||
| < 60 | 26 | 30.8 | 11.5 | 9.0 | |
| ≥ 60 | 35 | 42.9 | 5.7 | 10.0 | |
| KPS | < 0.001 | ||||
| 60-80 | 40 | 22.5 | 0.0 | 8.0 | |
| 90-100 | 21 | 66.7 | 23.8 | 13.5 | |
| Metastasis | < 0.001 | ||||
| Mono-site | 40 | 52.5 | 12.5 | 12.5 | |
| Poly-site | 21 | 9.5 | 0.0 | 6.5 | |
| Prechemotherapy | 0.969 | ||||
| Yes | 27 | 40.7 | 3.7 | 10.0 | |
| No | 34 | 35.3 | 11.8 | 9.0 | |
| Therapeutic effect | |||||
| PR | 27 | 63.0 | 18.5 | 13.0 | |
| SD | 24 | 25.0 | 0.0 | 9.0 | |
| PD | 10 | 0.0 | 0.0 | 6.0 | |
| Factor | Regression coefficient | Standard error | Wald | DOF | P | Exp (β) | 95% CI |
| Sex | -0.439 | 0.361 | 1.475 | 1 | 0.225 | 0.645 | 0.318-1.309 |
| Age | -0.151 | 0.342 | 0.194 | 1 | 0.659 | 0.860 | 0.440-1.682 |
| KPS | -1.449 | 0.342 | 17.906 | 1 | < 0.001 | 0.235 | 0.120-0.459 |
| Metastasis | 1.932 | 0.390 | 24.497 | 1 | < 0.001 | 6.902 | 3.212-14.833 |
| Pre-chemotherapy | -0.235 | 0.291 | 0.653 | 1 | 0.419 | 0.790 | 0.447-1.398 |
| Short-term effect | 0.972 | 0.254 | 14.610 | 1 | < 0.001 | 2.645 | 1.606-4.354 |
It is very important to treat enteric tumors by oxaliplatin plus capecitabine. There are few reports about this protocol used in esophageal cancer[1213]. As a result of different pathological types, there have been some reports about combined treatment of oxalipatin and capecitabine for esophageal adenocarcinoma. However, there has been no report about this protocol for ESCC. Compared with other treatment of advanced ESCC, our effectiveness rate is slightly lower than that of protocol of paclitaxel and cisplatin reported by Huang et al[14], but higher than that of combined regimens based on cisplatin and 5-FU, as well as irinotecan and cisplatin, and similar to that of FOLFOX 4. The median overall survival is longer and the 1-year survival rate is a little higher in our study than the regimens based on cisplatin and 5-FU, as well as FOLFOX 4, both of which are frequently applied clinically. Moreover, our regimen has fewer side effects.
Mauer et al[10] reported that oxaliplatin and 5-FU protocol (oxaliplatin 85 mg/m2 iv, 5-FU 400 mg/m2 iv quickly and then 600 mg/m2, iv for 22 h, on day 1 and 2), has better results. The PR rate was 40.0%, the median overall survival was 7.1 mo, and 1-year survival rate was 31.0%. The main toxicities were neutrocytopenia (grade IV, 29.0%,) and peripheral neuropathy (grade II-III, 26.0%).
Huang et al[14] used paclitaxel and cisplatin regimen (paclitaxel 175 mg/m2 , iv less than 3 h on day 1; cisplatin DDP 40 mg/m2, iv on day 2 and 3; and repeated every 3 wk), with a PR rate of 55.5%. Of seven patients with severe neutrocytopenia, one patient died of grade IV neutrocytopenia.
Polee et al[15] used cisplatin, etoposide and 5-FU regimen (cisplatin DDP 80 mg/m2, iv on day 1; etoposide 125 mg/m2, iv on day 1 and 200 mg/m2 , iv on day 3 and 5; 5-FU 375 mg/m2, iv on days 1-4; folic acid 30 mg, taken orally every 4 h, on days 1-4; and the cycle was repeated every 4 wk), the PR rate was 34.0%, the median overall survival was 9.5 mo, and the 1-year survival rate was 36.0%. The main toxicities were leukopenia (grade III-IV, 16.0%), fever related to leukopenia (19.0%), thrombocytopenia (grade III-IV, 7.0%), mucositis (grade III-IV, 23.0%), nausea and vomiting (grade III, 32.0%) and diarrhea (grade III, 6.0%).
Lorenzen et al[16] used capecitabine (1000 mg/m2 taken orally twice daily on days 1-14) plus intravenous docetaxel (75 mg/m2 on day 1). The median survival was 15.8 mo (95% CI, 7.8-23.9 mo). The intent-to-treat efficacy analysis showed an overall response rate (ORR) of 46.0%.
Lee et al[17] used 60 mg/m2 of CDDP iv on day 1 and capecitabine 1250 mg/m2 taken orally twice a day on days 1-14. The ORR was 57.8% (95% CI, 43.3-72.2). The median duration of response was 4.6 (1.0-15.6) mo, follow-up of 25.7 (10.8-42.6) mo, progression time of 4.7 mo (95% CI: 2.5-7.0 mo) and the median survival time was 11.2 mo (95% CI: 8.5-13.9 mo).
Lin et al[18] used the regimen, composed of paclitaxel 35 mg/m2 1 h iv on day 1, 4, 8 and 11; cisplatin 20 mg/m2, 2 h iv on day 2, 5, 9 and 12; and 5-FU 2000 mg/m2, leucovorin 300 mg/m2 24 h iv on day 5 and 12; and repeated every 21 d. The median progression-free and overall survival rates were 6.3 and 8.9 mo, respectively.
Evans et al[19] used docetaxel and oxaliplatin on day 1 and 8 and capecitabine individually, twice daily, on day 1-10, with each cycle repeated every 21 d. The docetaxel dose ranged from 30 to 35 mg/m2, the oxaliplatin from 40 to 50 mg/m2, and the capecitabine from 750 to 850 mg/m2 twice daily. Grade 3/4 dose-limiting toxicities of diarrhea, nausea, fatigue and febrile neutropenia occurred in three of four patients at dose level 3. An intermediate dose was added (2A) and the capecitabine dose reduced to 750 mg/m2. One of 6 patients had a dose-limiting toxicity at level 2A.
Tsai et al[20] used carboplatin (area under the ROC curve AUC = 2) on day 1 and 8, docetaxel (35-40 mg/m2) on day 1 and 8, and capecitabine (500-2000 mg/m2) on days 1-10. The maximum tolerated dose of docetaxel was 40 mg/m2 on day 1 and 8; carboplatin, AUC = 2 on day 1 and 8; and capecitabine, and 1500-2000 mg/m2 on days 1-10 in a 21-day cycle. Ten of 25 patients who could be evaluated (40.0%) responded and eight of 14 patients treated at the final dose level responded (57.0%).
Lee et al[21] used two cycles of XP induction chemotherapy, consisting of capecitabine 1000 mg/m2 twice daily on days 1-14, and cisplatin 60 mg/m2 iv on day 1, every 3 wk. Patients classified as M1a and M1b (non-visceral lymph node metastases) were treated with 54 Gy radiotherapy, concurrently with weekly capecitabine 800 mg/m2 twice daily on days 1-5 and cisplatin 30 mg/m2 iv on day 1 during radiation. Patients classified as M1b (visceral metastases) were treated with chemotherapy only until disease progression or intolerance to chemotherapy. The median time of progression was 7.8 mo (95% CI, 6.0-9.5 mo) and the median overall survival was 12.0 mo (95% CI, 9.0-15.0 mo).
Evans et al[22] used a regimen comprised of docetaxel 40 mg/m2, on day 1 and 8, carboplatin (AUC = 2) on day 1 and 8, and capecitabine 2000 mg/m2, on days 1-10 in a 21-day cycle. The median survival was 8.0 mo (95% CI, 5.5-13.0 mo), and the 1-year survival rate was 36.0%.
In our study (oxaliplatin 120 mg/m2, iv on day 1; capecitabine 1000 mg/m2, taken orally twice a day on days 1-14; and repeated every 3 wk), the rate was 43.8%, the median overall survival was 10 mo, and the 1-year survival rate was 38.1%. The main toxicities were leukopenia (grade III, 31.0%) and neuro-toxicity (grade III, 1.5%).
Capecitabine can be taken orally, so the protocol has superiority in medication. The mono-factorial analysis by Kaplan-Meier indicates that patients with low KPS and multi-locus metastases benefit little from this therapy. The short-term effect indicates that the prognosis demonstrates the importance of prompt, objective and precise therapeutic effect in clinical practice. Cox regression proportional hazard model poly-factorial analysis indicates that KPS index, the number of tumor metastasis locus and short-term effect are independent survival prognostic factors.
Our results demonstrate that oxaliplatin plus capecitable regimen has the advantage of good short-term effects, convenient administration and minor side effects in metastatic ESCC. The functional status of prior treatment, the number of tumor metastasis loci and short-term effects are independent survival prognostic factors.
Esophageal cancer which has the highest incidence and mortality worldwide is one of the most common malignant tumors in China. Esophageal squamous cell cancer (ESCC) is the most common histology. It has been confirmed that palliative chemotherapy can prolong the survival of stage IVesophageal cancer patients, relieve their symptoms and improve their quality of life. Nevertheless, no optimizing chemotherapy regimen has been available so far, the combined regimens based on cisplatin and 5-fluorouracil have been used frequently, but the effectiveness rate is only about 25.0%-33.0%.
Oxaliplatin is a kind of chemotherapeutic drug belonging to the third generation of platinum compounds, which has played an important role in the treatment of colon and rectum cancer and other solid tumors. It has a synergistic effect with lesser digestive tract reaction and hematotoxicity. Capecitabine, which has milder side effects, can be taken orally and is rapidly absorbed as an intact molecule in the gastrointestinal tract. Therefore, the combined regimen of oxaliplatin and capecitabine may produce more clinical benefits.
This study explored the efficacy and toxic reaction of oxaliplatin plus capecitabine in the treatment of patients with metastatic ESCC and the survival of the patients. The partial response (PR) rate was 43.8% (28/ 64). Stable disease (SD) rate was 47.9% (26/64), and disease progression rate was 15.6% (10/64). The clinical benefit rate (PR + SD) was 84.4%. No grade IV side effect in the entire cohort was found. The results can be used to supply information and instruction for clinical treatment.
Higher response and survival rate, and lower rate of toxicity were obtained by the combined treatment in this study. Capecitabine can be taken orally, therefore, that this treatment can be used clinically.
RECIST stands for Response Evaluation Criteria in Solid Tumors. KPS stands for Karnofsky performance score.
This is the first report to examine the efficacy and toxicity of the combined therapy of oxaliplatin and capecitabin in patients with metastatic ESCC. Higher response and survival rate, and lower rate of toxicity were obtained by this treatment than the other treatment protocols reported previously. It seems that this treatment has become a candidate for phase III study. Furthermore, since this treatment can be given on an outpatient basis, this study has great value.
| 1. | Wu KS, Huo X, Zhu GH. Relationships between esophageal cancer and spatial environment factors by using Geographic Information System. Sci Total Environ. 2008;393:219-225. |
| 2. | Lambert R, Hainaut P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol. 2007;21:921-945. |
| 3. | Wu K, Li K. Association between esophageal cancer and drought in China by using Geographic Information System. Environ Int. 2007;33:603-608. |
| 4. | Wu KS, Huo X. [Comparative study on soil and vegetation characteristics from high- and low risk areas of esophageal cancer in China]. Zhonghua Liuxing Bingxue Zazhi. 2008;29:44-47. |
| 5. | Wang LD, Yang HH, Fan ZM, Lu XD, Wang JK, Liu XL, Sun Z, Jiang YN, He X, Zhou Q. Cytological screening and 15 years' follow-up (1986-2001) for early esophageal squamous cell carcinoma and precancerous lesions in a high-risk population in Anyang County, Henan Province, Northern China. Cancer Detect Prev. 2005;29:317-322. |
| 6. | Ishihara R, Tanaka H, Iishi H, Takeuchi Y, Higashino K, Uedo N, Tatsuta M, Yano M, Ishiguro S. Long-term outcome of esophageal mucosal squamous cell carcinoma without lymphovascular involvement after endoscopic resection. Cancer. 2008;112:2166-2172. |
| 7. | Grunberger B, Raderer M, Schmidinger M, Hejna M. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 2007;27:2705-2714. |
| 8. | Burkart C, Bokemeyer C, Klump B, Pereira P, Teichmann R, Hartmann JT. A phase II trial of weekly irinotecan in cisplatin-refractory esophageal cancer. Anticancer Res. 2007;27:2845-2848. |
| 9. | Zhang P, Feng FY, Wu LY, Hu Y, Liu JW, Gao YJ, Guan XQ, Nan KJ, Suo AL, Wang XW. [Phase II multicenter clinical trial of nedaplatin in the treatment of malignant tumors]. Zhonghua Zhongliu Zazhi. 2006;28:230-234. |
| 10. | Mauer AM, Kraut EH, Krauss SA, Ansari RH, Kasza K, Szeto L, Vokes EE. Phase II trial of oxaliplatin, leucovorin and fluorouracil in patients with advanced carcinoma of the esophagus. Ann Oncol. 2005;16:1320-1325. |
| 11. | Jatoi A, Murphy BR, Foster NR, Nikcevich DA, Alberts SR, Knost JA, Fitch TR, Rowland KM Jr. Oxaliplatin and capecitabine in patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction and gastric cardia: a phase II study from the North Central Cancer Treatment Group. Ann Oncol. 2006;17:29-34. |
| 12. | Bolke E, Peiper M, Budach W. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:1965; author reply 1965. |
| 13. | van Meerten E, Eskens FA, van Gameren EC, Doorn L, van der Gaast A. First-line treatment with oxaliplatin and capecitabine in patients with advanced or metastatic oesophageal cancer: a phase II study. Br J Cancer. 2007;96:1348-1352. |
| 14. | Huang J, Cai RG, Meng PJ, Zhang MJ, Cui CX, Yang L, Chu DT, Sun Y, Wang JW. [Phase II study of paclitaxel and cisplatin for advanced squamous-cell carcinoma of esophagus]. Zhonghua Zhongliu Zazhi. 2004;26:753-755. |
| 15. | Polee MB, Verweij J, Siersema PD, Tilanus HW, Splinter TA, Stoter G, Van der Gaast A. Phase I study of a weekly schedule of a fixed dose of cisplatin and escalating doses of paclitaxel in patients with advanced oesophageal cancer. Eur J Cancer. 2002;38:1495-1500. |
| 16. | Lorenzen S, Duyster J, Lersch C, von Delius S, Hennig M, Bredenkamp R, Peschel C, Lordick F. Capecitabine plus docetaxel every 3 weeks in first- and second-line metastatic oesophageal cancer: final results of a phase II trial. Br J Cancer. 2005;92:2129-2133. |
| 17. | Lee J, Im YH, Cho EY, Hong YS, Lee HR, Kim HS, Kim MJ, Kim K, Kang WK, Park K. A phase II study of capecitabine and cisplatin (XP) as first-line chemotherapy in patients with advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol. 2008;62:77-84. |
| 18. | Lin CC, Yeh KH, Yang CH, Hsu C, Tsai YC, Hsu WL, Cheng AL, Hsu CH. Multifractionated paclitaxel and cisplatin combined with 5-fluorouracil and leucovorin in patients with metastatic or recurrent esophageal squamous cell carcinoma. Anticancer Drugs. 2007;18:703-708. |
| 19. | Evans D, Miner T, Akerman P, Millis R, Jean M, Kennedy T, Safran H. A phase I study of docetaxel, oxaliplatin, and capecitabine in patients with metastatic gastroesophageal cancer. Am J Clin Oncol. 2007;30:346-349. |
| 20. | Tsai JY, Iannitti D, Berkenblit A, Akerman P, Nadeem A, Rathore R, Harrington D, Roye D, Miner T, Barnett JM. Phase I study of docetaxel, capecitabine, and carboplatin in metastatic esophagogastric cancer. Am J Clin Oncol. 2005;28:329-333. |
| 21. | Lee SS, Kim SB, Park SI, Kim YH, Ryu JS, Song HY, Shin JH, Jung HY, Lee GH, Choi KD. Capecitabine and cisplatin chemotherapy (XP) alone or sequentially combined chemoradiotherapy containing XP regimen in patients with three different settings of stage IV esophageal cancer. Jpn J Clin Oncol. 2007;37:829-835. |
| 22. | Evans D, Miner T, Iannitti D, Akerman P, Cruff D, Maia-Acuna C, Harrington D, Habr F, Chauhan B, Berkenblit A. Docetaxel, capecitabine and carboplatin in metastatic esophagogastric cancer: a phase II study. Cancer Invest. 2007;25:445-448. |