Published online Feb 14, 2009. doi: 10.3748/wjg.15.675
Revised: September 12, 2008
Accepted: September 19, 2008
Published online: February 14, 2009
The aim of this study was to illustrate the role of non-invasive imaging tools such as ultrasonography, multi-detector row computed tomography, and magnetic resonance imaging in the evaluation of pediatric and adult liver recipients and potential liver donors, and in the detection of potential complications arising from liver transplantation.
- Citation: Caruso S, Miraglia R, Maruzzelli L, Gruttadauria S, Luca A, Gridelli B. Imaging in liver transplantation. World J Gastroenterol 2009; 15(6): 675-683
- URL: https://www.wjgnet.com/1007-9327/full/v15/i6/675.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.675
In liver transplantation (LT) candidates, the goal of imaging is to evaluate the intra- and extra-hepatic anatomy, identify conditions that can complicate LT, and stage the neoplastic disease.
Preoperative assessment of potential living liver donors requires the evaluation of liver parenchyma to identify steatosis or lesions; the accurate evaluation of intra and extrahepatic biliary and vascular anatomy to identify congenital variants and, overall, to detect the dominant arterial branch to segment 4 to prevent accidental removal at surgery; and an accurate estimation of the volume of both liver lobes to exclude complications related to graft volume [small-for-size grafts or large-for-size grafts, characterized by a graft-to-recipient weight ratio (GRWR) less than 1%, and more than 3%, respectively].
In the post-transplant period, the goal of imaging is to identify vascular and biliary complications. The long-term follow-up also allows clinicians to identify recurrence of the primary disease and/or detect disease related to long-term immunosuppression.
In the pediatric recipient a wide spectrum of diffuse and focal diseases are indications for LT. Biliary atresia represents at least 50% of all pediatric transplantation, while the most common hepatic malignancy leading to LT is hepatoblastoma[1–5]. Other indications for LT in pediatric patients are Alagille syndrome, cystic fibrosis, tyrosinemia type 1 (associated with a high risk of hepatocellular carcinoma (HCC) development), Wilson’s disease, Langerhans’ cell histiocytosis, HCC, infantile hepatic hemangioendothelioma type II (IHE), and hemangiomatosis[5–10].
In the adult recipient the most common indication for LT is still hepatitis-related liver cirrhosis with or without HCC. HCC is the fifth most common neoplasm in the world, and the third most common cause of cancer-related death. In adults it typically occurs within the cirrhotic liver. Non-alcoholic post-hepatitic cirrhosis is the most common association, but any condition that causes cirrhosis may potentially lead to HCC, including conditions such as inborn errors of metabolism. Exposure to chemical carcinogens can also cause the development of HCC. LT based on the Milan criteria is considered the optimal treatment for HCC, especially in patients with underlying chronic liver disease, because it offers a potential cure for both HCC and the underlying liver disease. The prognosis of cirrhotic patients depends on the occurrence and progression of HCC. &agr;-feto protein (AFP) alone shows low predictivity and sensitivity for the screening of HCC, and so imaging plays a key role in the surveillance of the cirrhotic patient[11–14].
Other focal diseases that require LT in adult patients are metastasis of neuroendocrine tumors, adenomatosis, giant angiomas, hepatic epitheloid hemangioendothelioma, and cholangiocarcinoma in selected cases, while the most common diffuse liver diseases in adults that require LT are primary biliary cirrhosis, primary sclerosing cholangitis, polycystic liver disease, Caroli’s disease and Budd-Chiari Syndrome[15]. Fulminant hepatic failure can occur in both pediatric and adult patients[16].
US is usually the first imaging modality in the evaluation of a transplant candidate, independent of the disease, because it is easy to perform, widely available, relatively inexpensive, and is cost effective. US can detect morphological changes in the liver, hepatic focal lesions (cystic or solid) or abdominal masses, and signs of portal hypertension such as hypersplenism, perihepatic or perisplenic varices, and ascites.
US plays a specific role in the diagnosis of biliary atresia and in the screening of HCC in cirrhotic patients[17–20].
In biliary atresia, US may show the absence, or reduction in size, of the gallbladder and the presence of a triangular cord (TC) sign (thickness of 4 mm or more of the anterior wall of the right portal vein, seen near the portal bifurcation). The identification of a TC sign results in a sensitivity of 80%, a specificity of 98%, a positive predictive value of 94%, a negative predictive value of 94%, and an accuracy of 94% for diagnosing biliary atresia[17].
US is the most common examination for the screening of HCC in cirrhotic patients, usually performed at either 3-, 6- or 12-mo intervals, although the sensitivity and specificity reported in the literature show a wide heterogeneity, ranging from 58% to 89%, and from 75% to 94%, respectively[18–20].
On gray-scale US, HCC is predominantly hypoechoic and sometimes isoechoic, with a thin hypoechoic halo corresponding to the tumor capsule. In diffuse HCC, there is subtle disruption of the normal echo pattern, with anechoic areas due to necrosis. Color Doppler and power Doppler modes permit a real-time evaluation of the hemodynamics in liver tumors. There are, however, many limitations that can affect the assessment of tumor hemodynamics[21].
For the diagnosis of HCC, contrast-enhanced US (CEUS) is recommended by the European Association for the Study of the Liver (EASL) as the modality for evaluation of the vascularity of hepatic nodules in cirrhotic patients. Two dynamic imaging studies that show arterial hypervascularity and washout in the portal venous phase for diagnosis of HCC ranging from 1 cm to 2 cm in diameter are required. For a mass greater than 2 cm, the coincident findings of characteristic arterial vascularization that is seen on at least two imaging techniques, or hypervascularity in one imaging technique associated with washout in the portal venous and/or delayed phase, may be used to confidently establish the diagnosis without biopsy[22–26].
However, there is currently no indication for the use of microbubble contrast agents to increase the detection rate of HCC in patients undergoing US surveillance. In fact, with CEUS one can only observe the perfusion in selected lesions identified with other imaging modalities, and not in the whole liver[23–25].
US also shows a high sensitivity and specificity for excluding portal vein thrombosis (PVT). In patients with hypervascular tumors such as HCC, it is important to establish the nature of the thrombus because tumoral vascular invasion worsens prognosis and may result in exclusion from the LT program. The presence of pulsatile arterial signals inside the thrombus at color Doppler ultrasound (CDUS) is reported to be a highly sensitive and specific sign of malignant PVT[27]. CEUS, using sulfur hexafluoride microbubbles (SonoVue, Bracco SpA), seems to increase sensitivity (88%) and accuracy (92.5%) when distinguishing between benign and malignant PVT[28].
In liver recipients, MDCT provides important information about liver morphology (normal or cirrhotic), intrahepatic and extrahepatic malignancy, venous benign and/or malignant thrombosis, patency of main portal vein, portosystemic collateral due to portal hypertension (spleno-renal spontaneous shunt, gastroesophageal and/or paracaval varices, and paraumbilical and caput medusae), celiac stenosis, splenic artery aneurysm, congenital arterial variants, patency, and anomalies of the inferior vena cava[29]. These findings may influence the decision to transplant, or the surgical planning of arterial and venous reconstruction. In addition, combined arterial, portal venous, and delayed-phase imaging improves the sensitivity of MDCT in detecting hypervascular neoplasms such as HCC or neuroendocrine metastases, and can also detect other tumors that enhance in a delayed phase, such as cholangi-ocarcinoma[30–33].
In pediatric candidates, the studies are usually performed with and without isosmolar or lower osmolar contrast media intravenous (c.m.i.v.) injection (Iodixanol 320 mgI/mL, Optiray 320, respectively) at a dose of 1.5 mL/kg, and at a rate that depends on the age of the patient (0.5-4 mL/s). When needed, the patient is anesthetized with intravenous propofol (0.5-1 mg/kg), without intubation. Images of the liver are acquired in the cranium-caudal direction, with slice thickness 1.25 mm or 0.625 mm, collimation 2.5 mm and table speed 7.5 mm per gantry rotation. Usually only three phases are acquired: unenhanced phase, arterial phase, and portal venous phase. Postprocessing of the dataset offers a variety of advanced three-dimensional models of the hepatic artery and vein using multi-planar reconstruction (MPR), maximum intensity projection (MIP), and volume rendering (VR) reconstructions. The volume of the liver is usually calculated, using dedicated software, in pediatric recipients as a guide in the donor-to-dimensional matching[3435].
In adult candidates, MDCT studies are usually performed with and without iodinated c.m.i.v. with a dose ranging from 1.5 mL/kg to 1.8 mL/kg of body weight, at a rate of 4-5 mL/s. Images of the liver are acquired in the cranium-caudal direction, during a single breath-hold acquisition, with slice thickness 1.25 mm or 0.625 mm, collimation 2.5 mm and table speed 7.5 mm per gantry rotation. A triple or quadruple-phase protocol is used: unenhanced phase, arterial phase, and portal venous phase, without and with late phase, respectively. Before the study, patients receive 500 mL of water as an oral contrast agent. Bolus tracking or test bolus technique (10 mL of contrast material at 5 mL/s) is used to calculate the correct time of the arterial phase. The portal venous phase and late phase acquisitions are generally obtained after 60 s and 180 s from the beginning of contrast injection, respectively. MIP and MPR reconstructions are usually made[30–33].
MRI is a non-invasive and sensitive technique that is devoid of ionizing radiation. For this reason, MRI is the preferred modality in the assessment of pediatric recipients. MRI examinations are usually performed using a head coil for small infants, or body coils for larger children. All images are acquired in the axial plane in breath-hold, or with suspended respiration if under general anesthesia. If necessary, contrast medium is injected. Using Gadobenato dimeglumina 0.1 mL/kg (Bracco, SpA) it is possible to obtain information about perfusion of the liver, changes in the parenchyma and the vasculature related to cirrhosis and portal hypertension (PVT, varices, ascites), and to detect vascular congenital anomalies. In addition, the multiphasic contrast enhancement study can detect malignancy in the liver, and locoregional involvement.
Mangafodipir trisodium (MnDPDP, Teslascan, GE) is a contrast agent composed of a water-soluble chelate complex salt that is between a paramagnetic manganese (Mn2+ ) ion and the ligand dipyridoxyl diphosphate, a vitamin B6 analogue; 50%-60% of the contrast administered is excreted through the gastrointestinal tract. For this reason, Teslascan has recently been used for the early diagnosis of biliary atresia, based on the absence of the bowel excretion of contrast material[36].
In adult recipients, especially in cirrhotic patients, MRI plays a role in detecting and differentiating HCC from other regenerative or dysplastic nodules, because it is more sensitive than multiphasic contrast-enhanced MDCT. However, it is still unclear whether MRI is more sensitive than MDCT in detecting HCC[192026].
MR cholangio-pancreatography (MRCP) using single shot fast spin echo (SSFSE) single and multisection, parallel and radial acquisition can well depict disease (such as sclerosing cholangitis, Caroli’s disease) of the biliary tree in adults, while in pediatric recipients, it is limited by the small caliber of the duct, thus rarely visible in neonates. In biliary atresia, it can help to demonstrate the absence of the gallbladder.
US is usually the first imaging modality for the evaluation of potential donors because it can identify hepatic lesions, obtain important information on the anatomy of the great vessels, such as hepatic veins and portal system, and evaluate the presence of steatosis. Due to a lack of accepted methods for quantification of steatosis on imaging, in many hospitals a biopsy is incorporated in the work-up, while in other centers, a biopsy is performed only in cases of suspicion based on clinical or imaging grounds[37].
MDCT is the most important tool in the assessment of potential donors. MDCT can precisely depict congenital variants, if present, that can influence the surgical technique, identify focal lesions (hemangiomas, focal nodular hyperplasia, adenomas) or diffuse liver diseases (steatosis, hemocromatosis), and calculate the volume of the two liver lobes.
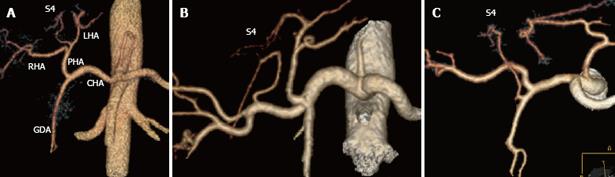
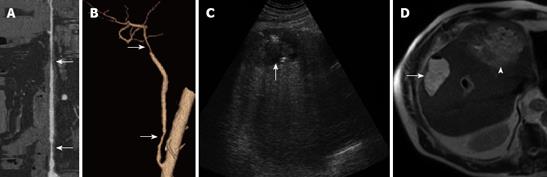
Congenital arterial variants are frequent, and are found in approximately 45% of donors. The identification of the dominant arterial branch to segment 4 is very important because its integrity is indispensable for the regeneration of the residual left hemiliver. This artery usually arises from the left hepatic artery (LHA), while in 25% of cases it arises from the right hepatic artery (RHA) or from both the LHA and RHA (Figure 1).
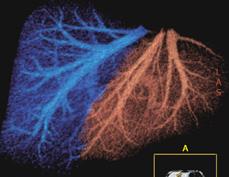
Anatomical variants of the portal system occur in 20% of the donor population; although the anomalies are not a contraindication to surgery, they must be known because they may require multiple portal anastomoses during the implantation of the right lobe into the recipient (Figure 2).
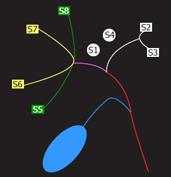
Identifying the hepatic venous anatomy is a fundamental step because it determines the hepatectomy plane that runs 1 cm to the right of the middle hepatic vein (MHV). Both accessory hepatic veins of the right inferior lobe (68% of the donor population), and large branching veins (> 5 mm) draining into the MHV from the right lobe require separate anastomosis to prevent venous congestion in the graft[37–41] (Figure 3).
Accurate volume of both liver lobes needs to be estimated to ensure that the hepatic mass is adequate for both liver donor and recipient.
MDCT scan studies are performed with and without iodinated c.m.i.v. at a dose ranging from 1.5 mL/kg to 1.8 mL/kg of body weight, and at a rate of 4-5 mL/s. Images of the liver are acquired in the cranium-caudal direction, during a single breath-hold acquisition, with slice thickness 1.25 mm or 0.625 mm, collimation 2.5 mm and table speed 7.5 mm per gantry rotation. Before the study, patients receive 500 mL of water as an oral contrast agent. Usually, a triple-phase protocol is used: unenhanced phase, arterial phase, and portal venous phase. Bolus tracking or test bolus technique (10 mL of contrast material at 4/5 mL per second) is used to calculate the correct time of the arterial phase. The peak enhancement plus 2 s is deemed as the start of the arterial acquisition to depict the arterial system. The portal venous phase is generally taken 70 s after the contrast agent has been injected to determine the exact delineation of the portal and hepatic veins. MIP and VR image reconstruction of the artery and portal venous system are usually created in the post-processing stage. The portal-venous acquisition is used for the volumetric evaluation, using dedicated software, in the postprocessing of the right and left lobe[37–39].
Some authors have proposed an all-in-one protocol to depict the biliary system, using a biliary contrast agent (Biliscopin; Schering, Berlin, Germany). However, a high incidence of adverse reactions to the biliary contrast agent, ranging from mild and self-resolving to severe systemic adverse reactions (shock-syndrome and death), has been observed[38].
MRCP is currently considered the primary imaging tool for biliary anatomy evaluation in potential living liver donors. In fact, it is performed with new generation units equipped with high performance gradient and phased-array coils, allowing for high quality heavily T2-weighted images with increased spatial resolution in a few seconds or with respiratory triggering, eliminating most motion-related artifacts.
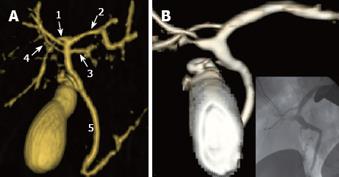
Only 57% of donors have a conventional biliary anatomy (Figure 4). Although the congenital variants of biliary anatomy do not represent a contraindication to liver donation, they must be identified before surgery to prevent ligation of major branches of the right lobe in the recipient and/or of the liver lobe in the donor. Multiple biliary anastomoses during the implantation of the right lobe into the recipient can be required to avoid atrophy due to biliary obstruction.
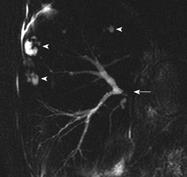
Improvements in hepatocyte-specific contrast agents with biliary excretion (mangafodipir trisodium and gadobenate dimeglumine) seem to have increased the accuracy of MRI in depicting the biliary system[4142] (Figure 5).
Some studies propose MRI as a single imaging modality for the preoperative assessment of potential donors to depict the arterial, portal and venous anatomy using MR-angiography with 3D sequence after the administration of extracellular c.m.i.v. However, MR-angiography can rarely depict the artery supplying segment 4[4043].
US and CDUS are the most important tools in the follow-up of LT patients because they show high sensitivity and specificity in detecting vascular complications.
During transplantation, CDUS is usually performed to detect the intraparenchymal flows (arterial, portal and venous), and to evaluate the velocity of flow and waveform to detect very early complications such as hyperacute hepatic artery or PVT[44].
After LT, CDUS is usually performed once a day during the first week, and once a week in the following 2 mo, and is key in the suspicion or identification of vascular or biliary complications.
Hepatic artery thrombosis (HAT) occurs more frequently in pediatric recipients (9%-42%) than in adult recipients (4%-12%). It frequently leads to graft failure, due to biliary wall necrosis with bilomas, biliary leakage, and hepatic infarction[45].
CDUS is able to identify up to 92% of cases of HAT, demonstrating the absence of flow in the common hepatic artery and in the intrahepatic branches. In younger subjects, in the event of complete hepatic artery thrombosis, intrahepatic flow can be sustained by small collateral neoformed vessels from the superior mesenteric artery. In these cases, the flow has a tardus parvus waveform. In adults, the formation of collateral vessels is almost never sufficient to prevent ischemic biliary complications. Ultrasound findings can be false positive if the hepatic artery is small or stenotic, if the flow is very slow, or if there is coexistent systemic hypotension. If US does not show an arterial flow, administration of contrast media (microbubble) can help to improve the flow visualization in the HA, differentiating between thrombosis and a patent artery in patients without HA flow on conventional Doppler US[46–49].
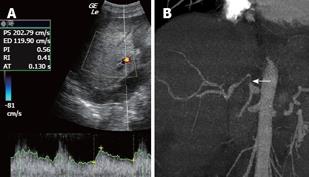
Hepatic artery stenosis is reported in 5%-10% of transplant recipients and can be anastomotic (in 70% of cases), perianastomotic or intrahepatic. It is most frequently caused by an error in surgical technique or by arterial damage during explantation. Near the stenosis, the Doppler ultrasound shows a focal velocity greater than 2 mL/s, and turbulence; more distally, it detects a tardus parvus arterial waveform with a resistance index lower than 0.5 and a systolic acceleration time (between the end of the diastole and the first systolic peak) greater than 0.08 s[46–49] (Figures 6 and 7).
Post-transplant PVT is extremely rare in adults. In children, particularly with biliary atresia, post-transplant PVT, although not usual, is not rare. The underlying problems in small children are not only due to smaller caliber vessels but also due to hypoplastic and sclerotic vessels brought about by pre-transplant recurrent cholangitis[50]. Color Doppler ultrasound shows the absence of flow in the portal vein, and whether it is anechogenic (recent thrombosis) or echogenic (old thrombosis). If the thrombosis is recent, it can be treated with local thrombolysis and mechanical thrombectomy.
Portal vein stenosis is more frequent in partial liver transplants than in whole liver transplants. The suspicion of stenosis arises if color Doppler shows a turbulent flow with focal aliasing in the stenotic tract, and a velocity gradient of > 4-fold[46–49].
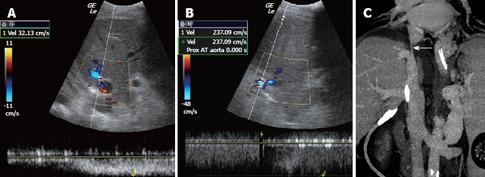
Post-liver transplant stenosis of the inferior vena cava is rare and generally secondary to technical issues. CDUS shows an increased trans-anastomotic velocity gradient (> 4 times) and the loss of the tracing’s normal phasicity[444649] (Figure 8).
In partial liver transplants, hepatic vein stenosis is a frequent complication. CDUS of the hepatic veins reveals a slow (< 10 cm/s) and monophasic flow[444649].
All the vascular complications described, when suspected on US and CDUS, need confirmation with contrast-enhanced MDCT, contrast-enhanced MR or with angiography.
US shows low diagnostic accuracy in identifying biliary complications, particularly in early anastomotic stenosis without a significant intrahepatic biliary duct dilatation. US is, however, an accurate tool for evaluating necrosis, bilomas, or abscess of the graft.
MDCT angiography is the best option for confirming the ultrasonographic suspicion of early and late vascular complications (HAT, main portal vein or inferior vena cava (IVC) stenosis or thrombosis)[51]. In addition, it permits a good assessment of liver parenchyma and other abdominal organs, and the evaluation of bilomas (Figure 9), bleeding, abdominal or hepatic abscesses (Figure 7), adrenal infarction, and intestinal perforation or obstruction. MDCT can identify biliary duct dilatation, even if the anastomosis is not easy to depict.
MDCT also plays a key role in detecting late complications, such as recurrence of the primary disease, post-transplant lymphoproliferative disease (PTLD), Kaposi’s sarcoma or other malignancies related to long-term immunosuppression.
MRCP after LT is the modality of choice for the diagnosis and management of biliary complications, and shows a sensitivity ranging from 87.5% to 95.3%, a positive predictive value ranging from 92.3% to 97.6%, and an accuracy ranging from 90.4% to 95.2%[52–54]. The T2W images easily identify fluid collection (bilomas, bile leakage, biliary duct dilatation) that appear strongly hyperintense, while MRCP using SSFSE single and multisection, parallel and radial acquisition can finely depict filling defects, anastomotic or non-anastomotic stenosis, or irregularities of the biliary duct.
Bile duct complications in the various series vary from 7% to 50%. In partial liver transplants, biliary complications are more frequent because the diameters of the bile ducts to be anastomosed are smaller, and multiple biliary anastomoses are often necessary. Most of these complications occur within the first 3 mo, even though biliary stenoses and gallstones can occur months and years after transplantation.
Bile extravasation has an incidence ranging from 5% to 19%, and may occur in the T-tube insertion site, in the region of the anastomosis, or intrahepatically.
Intrahepatic bile leakage (biloma) entails the suspicion of bile duct necrosis secondary to HAT. In these cases, a new transplant is almost always necessary. However, the percutaneous drainage of a biloma can prevent sepsis and increase the likelihood of graft survival. In partial liver transplants, bile can also leak from a large bile duct damaged at the time of liver “splitting,” or from the surface of the resection margin, which exposes thousands of small bile ducts (Figure 9).
Stenoses can be classified as anastomotic or non-anastomotic. Anastomotic stenoses are the result of postoperative fibroses or of errors associated with surgical technique (Figure 9). Non-anastomotic stenoses can be intra- or extrahepatic, single or multiple, and are often due to ischemic damage. In these cases, it is necessary to assess the patency of the hepatic artery. Rarely are they due to prolonged graft ischemia time, chronic rejection or cytomegalovirus infections. In transplants related to sclerosing cholangitis, intrahepatic stenosis can indicate disease recurrence.
Rare causes of bile duct obstruction are the dislocation/obstruction of the T-tube, biliary sludge, gallstones or an excessive choledochus length after the choledochocholedochostomy. The mucocele of the residual cystic duct can cause bile duct stenosis resulting from extrinsic compression.
Enhancement with mangafodipir trisodium improves the performance of MRCP for the detection and exclusion of biliary abnormalities after orthotopic LT[55].
Imaging plays a primary role in LT. It is used in the assessment of the recipient, the assessment of the potential living liver donor, and the detection of early and late complications. US, MDCT and MRI have different roles, depending on accuracy, in depicting the different goal in each period of the orthotopic LT.
| 1. | Hasegawa T, Kimura T, Sasaki T, Okada A. Living-related liver transplantation for biliary atresia associated with polysplenia syndrome. Pediatr Transplant. 2002;6:78-81. |
| 2. | Meyers RL. Tumors of the liver in children. Surg Oncol. 2007;16:195-203. |
| 3. | Avila LF, Luis AL, Hernandez F, Garcia Miguel P, Jara P, Andres AM, Lopez Santamaria M, Tovar JA. Liver transplantation for malignant tumours in children. Eur J Pediatr Surg. 2006;16:411-414. |
| 4. | Emre S, McKenna GJ. Liver tumors in children. Pediatr Transplant. 2004;8:632-638. |
| 5. | Sevmis S, Karakayali H, Ozcay F, Canan O, Bilezikci B, Torgay A, Haberal M. Liver transplantation for hepatocellular carcinoma in children. Pediatr Transplant. 2008;12:52-56. |
| 6. | Colombo C, Battezzati PM, Crosignani A, Morabito A, Costantini D, Padoan R, Giunta A. Liver disease in cystic fibrosis: A prospective study on incidence, risk factors, and outcome. Hepatology. 2002;36:1374-1382. |
| 7. | Kvittingen EA. Tyrosinaemia--treatment and outcome. J Inherit Metab Dis. 1995;18:375-379. |
| 8. | Schilsky ML. Diagnosis and treatment of Wilson’s disease. Pediatr Transplant. 2002;6:15-19. |
| 9. | Zandi P, Panis Y, Debray D, Bernard O, Houssin D. Pediatric liver transplantation for Langerhans’ cell histiocytosis. Hepatology. 1995;21:129-133. |
| 10. | Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, Schirmacher P, Weitz J, Friess H, Buchler MW. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108-2121. |
| 11. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. |
| 12. | Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, Bhoori S, Lee SG. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001-1007. |
| 13. | Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Yamashita Y, Maehara Y. Liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:124-130. |
| 14. | Ishizaki Y, Kawasaki S. The evolution of liver transplantation for hepatocellular carcinoma (past, present, and future). J Gastroenterol. 2008;43:18-26. |
| 15. | Seaberg EC, Belle SH, Beringer KC, Schivins JL, Detre KM. Liver transplantation in the United States from 1987-1998: updated results from the Pitt-UNOS Liver Transplant Registry. Clin Transpl. 1998;43:17-37. |
| 16. | Mondragon R, Mieli-Vergani G, Heaton ND, Mowat AP, Vougas V, Williams R, Tan KC. Liver transplantation for fulminant liver failure in children. Transpl Int. 1992;5 Suppl 1:S206-S208. |
| 17. | Lee HJ, Lee SM, Park WH, Choi SO. Objective criteria of triangular cord sign in biliary atresia on US scans. Radiology. 2003;229:395-400. |
| 18. | Chalasani N, Horlander JC Sr, Said A, Hoen H, Kopecky KK, Stockberger SM Jr, Manam R, Kwo PY, Lumeng L. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94:2988-2993. |
| 19. | Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000;95:1535-1538. |
| 20. | Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, Brown JJ, McFarland EG, Middleton WD, Balfe DM. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226:533-542. |
| 21. | Gaiani S, Volpe L, Piscaglia F, Bolondi L. Vascularity of liver tumours and recent advances in doppler ultrasound. J Hepatol. 2001;34:474-482. |
| 22. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. |
| 23. | Maruyama H, Yoshikawa M, Yokosuka O. Current role of ultrasound for the management of hepatocellular carcinoma. World J Gastroenterol. 2008;14:1710-1719. |
| 24. | Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol. 2008;48:848-857. |
| 25. | Dai Y, Chen MH, Fan ZH, Yan K, Yin SS, Zhang XP. Diagnosis of small hepatic nodules detected by surveillance ultrasound in patients with cirrhosis: Comparison between contrast-enhanced ultrasound and contrast-enhanced helical computed tomography. Hepatol Res. 2008;38:281-290. |
| 26. | Taouli B, Krinsky GA. Diagnostic imaging of hepatocellular carcinoma in patients with cirrhosis before liver transplantation. Liver Transpl. 2006;12:S1-S7. |
| 27. | Dodd GD 3rd, Memel DS, Baron RL, Eichner L, Santiguida LA. Portal vein thrombosis in patients with cirrhosis: does sonographic detection of intrathrombus flow allow differentiation of benign and malignant thrombus? AJR Am J Roentgenol. 1995;165:573-577. |
| 28. | Tarantino L, Francica G, Sordelli I, Esposito F, Giorgio A, Sorrentino P, de Stefano G, Di Sarno A, Ferraioli G, Sperlongano P. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging. 2006;31:537-544. |
| 29. | Pannu HK, Maley WR, Fishman EK. Liver transplantation: preoperative CT evaluation. Radiographics. 2001;21:S133-S146. |
| 30. | Kanematsu M, Oliver JH 3rd, Carr B, Baron RL. Hepatocellular carcinoma: the role of helical biphasic contrast-enhanced CT versus CT during arterial portography. Radiology. 1997;205:75-80. |
| 31. | Iannaccone R, Laghi A, Catalano C, Rossi P, Mangiapane F, Murakami T, Hori M, Piacentini F, Nofroni I, Passariello R. Hepatocellular carcinoma: role of unenhanced and delayed phase multi-detector row helical CT in patients with cirrhosis. Radiology. 2005;234:460-467. |
| 32. | Ronzoni A, Artioli D, Scardina R, Battistig L, Minola E, Sironi S, Vanzulli A. Role of MDCT in the diagnosis of hepatocellular carcinoma in patients with cirrhosis undergoing orthotopic liver transplantation. AJR Am J Roentgenol. 2007;189:792-798. |
| 33. | Zhao H, Zhou KR, Yan FH. Role of multiphase scans by multirow-detector helical CT in detecting small hepatocellular carcinoma. World J Gastroenterol. 2003;9:2198-2201. |
| 34. | Cheng YF, Chen CL, Jawan B, Huang TL, Chen TY, Chen YS, Wang CC, de Villa V, Wang SH, Wah CK. Multislice computed tomography angiography in pediatric liver transplantation. Transplantation. 2003;76:353-357. |
| 35. | Ravindra KV, Guthrie JA, Woodley H, Davison S, McClean P, Prasad KR, Stringer MD. Preoperative vascular imaging in pediatric liver transplantation. J Pediatr Surg. 2005;40:643-647. |
| 36. | Ryeom HK, Choe BH, Kim JY, Kwon S, Ko CW, Kim HM, Lee SB, Kang DS. Biliary atresia: feasibility of mangafodipir trisodium-enhanced MR cholangiography for evaluation. Radiology. 2005;235:250-258. |
| 37. | Low G, Wiebe E, Walji AH, Bigam DL. Imaging evaluation of potential donors in living-donor liver transplantation. Clin Radiol. 2008;63:136-145. |
| 38. | Schroeder T, Radtke A, Kuehl H, Debatin JF, Malago M, Ruehm SG. Evaluation of living liver donors with an all-inclusive 3D multi-detector row CT protocol. Radiology. 2006;238:900-910. |
| 39. | Chen WH, Xin W, Wang J, Huang QJ, Sun YF, Xu Q, Yu SN. Multi-slice spiral CT angiography in evaluating donors of living-related liver transplantation. Hepatobiliary Pancreat Dis Int. 2007;6:364-369. |
| 40. | Makuuchi M, Sugawara Y. Technical progress in living donor liver transplantation for adults. HPB (Oxford). 2004;6:95-98. |
| 41. | Ayuso JR, Ayuso C, Bombuy E, De Juan C, Llovet JM, De Caralt TM, Sanchez M, Pages M, Bruix J, Garcia-Valdecasas JC. Preoperative evaluation of biliary anatomy in adult live liver donors with volumetric mangafodipir trisodium enhanced magnetic resonance cholangiography. Liver Transpl. 2004;10:1391-1397. |
| 42. | Sirvanci M, Duran C, Ozturk E, Balci D, Dayangac M, Onat L, Yuzer Y, Tokat Y, Killi R. The value of magnetic resonance cholangiography in the preoperative assessment of living liver donors. Clin Imaging. 2007;31:401-405. |
| 43. | Cheng YF, Chen CL, Huang TL, Chen TY, Lee TY, Chen YS, Wang CC, de Villa V, Goto S, Chiang YC. Single imaging modality evaluation of living donors in liver transplantation: magnetic resonance imaging. Transplantation. 2001;72:1527-1533. |
| 44. | Choi JY, Lee JY, Lee JM, Kim SH, Lee MW, Han JK, Choi BI. Routine intraoperative Doppler sonography in the evaluation of complications after living-related donor liver transplantation. J Clin Ultrasound. 2007;35:483-490. |
| 45. | Ametani F, Itoh K, Shibata T, Maetani Y, Tanaka K, Konishi J. Spectrum of CT findings in pediatric patients after partial liver transplantation. Radiographics. 2001;21:53-63. |
| 46. | Hom BK, Shrestha R, Palmer SL, Katz MD, Selby RR, Asatryan Z, Wells JK, Grant EG. Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology. 2006;241:267-274. |
| 47. | Dodd GD 3rd, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology. 1994;192:657-661. |
| 48. | Vit A, De Candia A, Como G, Del Frate C, Marzio A, Bazzocchi M. Doppler evaluation of arterial complications of adult orthotopic liver transplantation. J Clin Ultrasound. 2003;31:339-345. |
| 49. | Tamsel S, Demirpolat G, Killi R, Aydin U, Kilic M, Zeytunlu M, Parildar M, Oran I, Ucar H. Vascular complications after liver transplantation: evaluation with Doppler US. Abdom Imaging. 2007;32:339-347. |
| 50. | Chen CL, Concejero A, Wang CC, Wang SH, Lin CC, Liu YW, Yong CC, Yang CH, Lin TS, Chiang YC. Living donor liver transplantation for biliary atresia: a single-center experience with first 100 cases. Am J Transplant. 2006;6:2672-2679. |
| 51. | Kayahan Ulu EM, Coskun M, Ozbek O, Tutar NU, Ozturk A, Aytekin C, Haberal M. Accuracy of multidetector computed tomographic angiography for detecting hepatic artery complications after liver transplantation. Transplant Proc. 2007;39:3239-3244. |
| 52. | Linhares MM, Gonzalez AM, Goldman SM, Coelho RD, Sato NY, Moura RM, Silva MH, Lanzoni VP, Salzedas A, Serra CB. Magnetic resonance cholangiography in the diagnosis of biliary complications after orthotopic liver transplantation. Transplant Proc. 2004;36:947-948. |
| 53. | Aufort S, Molina E, Assenat E, Rigole H, Bauret P, Calvet C, Navarro F, Fabre JM, Blanc P, Taourel P. [Value of MRCP for diagnosis of biliary complications after liver transplantation]. J Radiol. 2008;89:221-227. |
| 54. | Valls C, Alba E, Cruz M, Figueras J, Andia E, Sanchez A, Llado L, Serrano T. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. AJR Am J Roentgenol. 2005;184:812-820. |
| 55. | Bridges MD, May GR, Harnois DM. Diagnosing biliary complications of orthotopic liver transplantation with mangafodipir trisodium-enhanced MR cholangiography: comparison with conventional MR cholangiography. AJR Am J Roentgenol. 2004;182:1497-1504. |