Published online Feb 7, 2009. doi: 10.3748/wjg.15.513
Revised: December 10, 2008
Accepted: December 17, 2008
Published online: February 7, 2009
Hepatocellular carcinoma (HCC) is a major health problem, being the sixth most common cancer world-wide. Dysregulation of the balance between proliferation and cell death represents a pro-tumorigenic principle in human hepatocarcinogenesis. This review updates the recent relevant contributions reporting molecular alterations for HCC that induce an imbalance in the regulation of apoptosis. Alterations in the expression and/or activation of p53 are frequent in HCC cells, which confer on them resistance to chemotherapeutic drugs. Many HCCs are also insensitive to apoptosis induced either by death receptor ligands, such as FasL or TRAIL, or by transforming growth factor-beta (TGF-β). Although the expression of some pro-apoptotic genes is decreased, the balance between death and survival is dysregulated in HCC mainly due to overactivation of anti-apoptotic pathways. Indeed, some molecules involved in counteracting apoptosis, such as Bcl-XL, Mcl-1, c-IAP1, XIAP or survivin are over-expressed in HCC cells. Furthermore, some growth factors that mediate cell survival are up-regulated in HCC, as well as the molecules involved in the machinery responsible for cleavage of their pro-forms to an active peptide. The expression and/or activation of the JAK/STAT, PI3K/AKT and RAS/ERKs pathways are enhanced in many HCC cells, conferring on them resistance to apoptotic stimuli. Finally, recent evidence indicates that inflammatory processes, as well as the epithelial-mesenchymal transitions that occur in HCC cells to facilitate their dissemination, are related to cell survival. Therefore, therapeutic strategies to selectively inhibit anti-apoptotic signals in liver tumor cells have the potential to provide powerful tools to treat HCC.
- Citation: Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol 2009; 15(5): 513-520
- URL: https://www.wjgnet.com/1007-9327/full/v15/i5/513.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.513
Apoptosis represents a physiological way to eliminate excess cells during both liver development and regeneration[1]. Indeed, insufficient apoptosis has been associated with development and progression of tumors of the liver and the biliary tree[12]. Hepatocellular carcinoma (HCC) is a major health problem, being the sixth most common cancer world-wide[3]. It is a heterogeneous tumor commonly associated with chronic liver diseases which frequently culminate in cirrhosis, such as alcoholic cirrhosis and chronic hepatitis B and C infections. During recent years, major advancements in the knowledge of this complex disease have been reported[3]. This review is an effort to update the recent relevant contributions reporting molecular alterations for HCC that induce an imbalance in the regulation of apoptosis.
Among the most common alterations observed in HCC are mutations in the p53 tumor suppressor gene (TP53)[4]. Different chemotherapeutic agents require p53 to induce apoptosis. Indeed, tumors with a disruption in the p53 pathway are generally resistant to chemotherapy. The presence of specific mutational hotspots in TP53 in different types of human cancer implicates environmental carcinogens and endogenous processes. In this sense, somatic mutations at the third base in codon 249 of TP53 in HCC have been related to exposure to aflatoxin B1 (AFB1), in association with HBV infection[4]. Chronic infection with HBV and HCV viruses and exposure to oxidative stress, including hemochromatosis or inflammation, induce damage in the DNA and mutations in cancer-related genes, including TP53. Thus, it would seem plausible that p53 mutation might operate in either HCC initiation or progression, depending on the context. However, adenoviral delivery of p53 recombinant DNA into mice models bearing hepatocellular carcinomas did not apparently suppress tumor growth[5]. DePinho et al in a recent work[6] have helped to clarify this point. They have demonstrated that the effect of p53 loss in hepatocellular carcinoma that is associated with chronic liver disease is dependent on cellular context, in particular intact or dysfunctional telomeres, and they have hypothesized that a decreased p53 function might contribute to hepatocyte survival in the presence of telomere-induced chromosomal instability.
The transforming growth factor-beta (TGF-β) family of cytokines plays a physiological role during embryonic development and its misregulation can result in tumorigenesis[7]. TGF-β-1 is an important regulatory suppressor factor in hepatocytes, inhibiting proliferation[8] and inducing cell death[9]. Paradoxically, TGF-β may also modulate other pro-tumorigenic processes, such as cell invasion, immune regulation or microenvironment modification[7]. Blocking TGF-β up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells[10]. Furthermore, liver tumors expressing late TGF-β-responsive genes (anti-apoptotic and metastatic) display a higher invasive phenotype and increased tumor recurrence when compared to those that show an early TGF-β signature (suppressor genes)[11]. Indeed, the escape from the antiproliferative and pro-apoptotic actions of TGF-β might be a prerequisite for hepatocarcinoma progression[12].
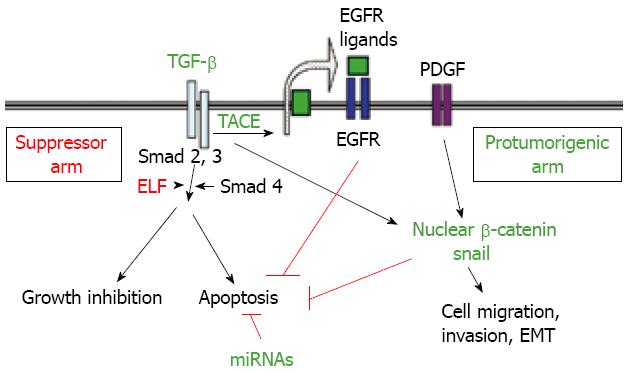
Disruption of the TGF-β pathway occurs in HCC[13] and might cause dysregulation of apoptosis. In favour of this hypothesis, recent studies have demonstrated that overexpression of SMAD3 reduces susceptibility to develop hepatocarcinoma, by sensitizing hepatocytes to apoptosis through down-regulation of Bcl-2[12]. However, perturbations at receptor or SMAD levels do not appear to be as frequent as they are in colon or pancreatic cancer[13] and expression of TGF-β is up-regulated in a great percentage of HCC patients[1113]. Thus, other possible ways to disrupt TGF-β signalling might exist and they remain to be explored. Interestingly, Mishra et al have recently demonstrated that HCC might arise from loss of TGF-β signalling adaptor protein embryonic liver foldrin (ELF), a crucial Smad3/4 adaptor[1415]. HCC cells might also overexpress a specific set of microRNAs (miRNAs) that would allow the escape from TGF-β-induced apoptosis[1617]. Furthermore, recent results have indicated that TGF-β might play a dual role in controlling apoptosis in hepatocytes and hepatoma cells. On one hand, it induces cell death, but on the other it could activate anti-apoptotic signals, the epidermal growth factor receptor (EGFR) being required for this effect[18–20]. Indeed, EGF is an important survival signal for TGF-β-induced apoptosis in hepatocytes[21]. The enzyme phosphatidylinositol 3-kinase (PI3K) mediates the effect of EGF on TGF-β-induced death by acting upstream from the mitochondrial changes, probably counteracting TGF-β-induced oxidative stress[22]. The autocrine loop of EGFR activated by TGF-β in hepatoma cells would require a high activity of TACE/ADAM17[20], the metalloprotease responsible for shedding of the pro-tumor necrosis factor (proTNF-α) that it is also necessary for shedding of the EGF family of growth factors[23]. Although the possible role of an increased expression of TACE/ADAM17 in the development of human hepatocellular carcinoma (HCC) has been barely studied, a recent report indicates that the quantities of ADAM17 mRNA vary among different pathological types of HCC, but are significantly higher in poorly differentiated HCC than in well or moderately differentiated HCC[24]. Overexpression of TACE/ADAM17 might confer an advantage on HCC cells by impairing TGF-β-induced apoptosis through transactivation of the EGFR. Concluding, HCC cells might impair the suppressor arm in TGF-β-signalling, with enhancement of the response to this factor in terms of tumor progression and invasion (Figure 1).
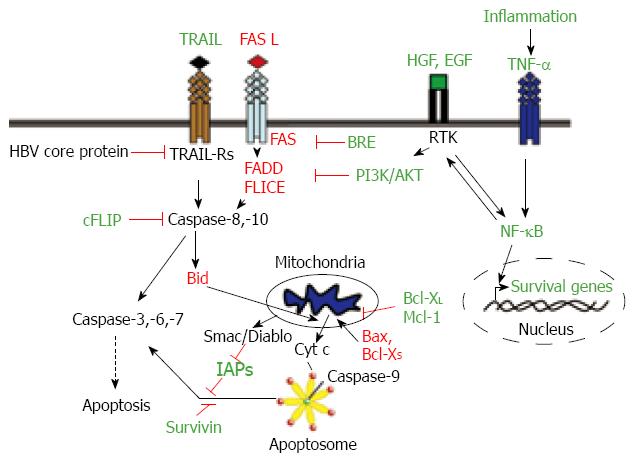
HCCs show resistance to apoptosis mediated by several death receptors. The majority of the HCCs show one or more alterations in the Fas pathway molecules, which inhibit Fas-mediated apoptosis[25]. The status of Fas and Fas ligand (FasL) expression can predict HCC recurrence[26]. Loss of response to Fas in HCC cells may be produced either by down-regulation of Fas expression[2527], concomitant with decreased expression of downstream molecules, such as FADD or FLICE[27], or by up-regulation or over-activation of molecules that counteract its pro-apoptotic effect, including nuclear factor-kappaB (NF-κB), Bcl-2 or Bcl-XL[28–30]. The cellular FLICE/caspase-8-inhibitory protein (cFLIP), an intracellular inhibitor of caspase-8 activation, is constitutively expressed in human HCC cell lines and displays higher levels in HCC tissues than in nontumor liver tissues[31]. It has also been described that HCC tissues show overexpression of BRE, an antiapoptotic protein that binds to the cytoplasmic domains of tumour necrosis factor (TNF) receptor-1 and Fas, attenuating death-receptor initiated apoptosis[32]. Furthermore, it has been suggested that extracellular factors might counteract Fas-induced apoptosis in HCC cells. Indeed, hepatocyte growth factor (HGF), through activation of the PI3K/AKT pathway, suppresses Fas-mediated cell death in human HCC cell lines, by inhibiting Fas-death-inducing signalling complex (DISC) formation, especially FADD and caspase 8 interaction[33] (Figure 2).
TNF-related apoptosis-inducing ligand (TRAIL) selectively induces apoptosis in various transformed cell lines but not in almost normal tissues[34]. HCC cells constitutively express TRAIL mRNA and protein, but there are contradictory and confusing data about the expression of the different TRAIL receptors in HCC cells and tissues[35–37]. Certain evidence indicates that most HCC cells are insensitive towards TRAIL-mediated apoptosis, suggesting that the presence of mediators can inhibit the TRAIL cell-death-inducing pathway in HCC[3637]. It has been reported that hepatitis B virus core protein inhibits TRAIL-induced apoptosis by blocking the expression of the TRAIL receptor 2 (TRAIL-R2/DR5)[38]. Overactivation of NF-κB and Bcl-XL in HCC cells might also restrain the TRAIL-mediated apoptosis[39]. After an initial debate about the potential liver toxicity of TRAIL in freshly isolated human hepatocytes[37], there is a recent interest in the development of new therapeutic approaches that can sensitize HCC cells to TRAIL-induced apoptosis. Indeed, it has been proposed that TRAIL, in combination with chemotherapeutic agents, may have potential in the treatment of HCC[40]. Of clinical relevance, proteasome inhibitors and histone deacetylase (HDAC) inhibitors might sensitize HCC cells but not primary human hepatocytes for TRAIL-induced apoptosis[4142].
It is worthy of note that many of the genetic alterations observed in HCC lead to an imbalance in the pro- and anti-apoptotic members of the Bcl-2 family[43]. Bcl-XL is overexpressed in a great percentage of HCCs[44], and so is Mcl-1[45]. In contrast, pro-apoptotic members of the family, such as Bax or Bcl-XS are down-regulated in HCC with dysfunction in the p53 pathway[46]. Furthermore, recent results have indicated that some pro-apoptotic members of the BH-3-only family, such as Bid, show decreased expression in HCC related to hepatitis B or C infection[47].
Recent investigations have revealed that nearly 90% of clinical tumors from advanced HCC patients express high levels of X-linked inhibitor-of-apoptosis protein (XIAP), a well known inhibitor of caspases. Studies in established HCC cell lines with different metastatic capabilities indicated a correlation of metastasis with resistance to apoptosis and increased expression of XIAP[48]. Interestingly, it had previously been suggested that XIAP might also function as a cofactor in TGF-β signalling[49]. Thus, overexpression of XIAP might confer resistance to the apoptotic effects of TGF-β, allowing HCC cells to respond to this cytokine in terms of migration and invasion. Genome-wide analyses of tumors in a mouse model of liver cancer and in HCC tissue have recently revealed a recurrent amplification in a region of human chromosome 11q22, delineating cIAP1, the known inhibitor of apoptosis, as one of the candidate oncogenes in the amplicon[50]. Survivin, another member of the family of inhibitor of apoptosis proteins, is also overexpressed in HCC cell lines and tissues[5152] and it has been suggested that it might play a pivotal role in metastasis[53]. Survivin might play an important role in progression of HCC not only by inhibiting apoptosis[54], but also by promoting cell proliferation[51] and may be positively correlated with high risk of disease recurrence and poor prognosis[55]. Concluding, HCC cells show an imbalance in the expression of pro- and anti-apoptotic proteins, which favours cell survival (Figure 2).
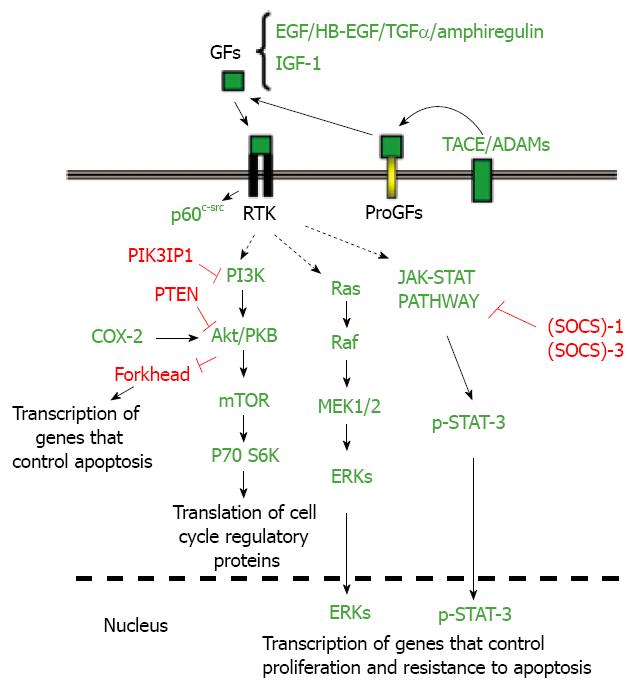
Some autocrine signal activators, such as EGF receptor (EGFR) ligands, might protect liver tumor cells from apoptosis induced by stress, physiological factors or pro-apoptotic drugs[56]. Dysregulation of growth factor signalling, including EGF and IGF-1 pathways, has been well established in human HCCs[5758]. Viral hepatitis infections might contribute to the enhancement of the expression of EGFR ligands[59]. The tyrosine kinase p60c-src is also overactivated in hepatoma cells[5660] that protect themselves from death stimuli[61], and it accounts in a large part for the desensitization of liver tumor cells to TRAIL and CD95. Interestingly, blockade of EGFR or c-Src in primary hepatocytes only marginally increases cell death[5661], which indicates that both tyrosine kinases are critical effectors that specifically protect liver cancer cells from death stimuli, providing a weak point in cancer cells for a potential therapeutic approach.
Signal transducer and activator of transcription (STAT) proteins become activated by tyrosine kinases in response to cytokines and growth factors. It has been reported that suppressor of cytokine signalling (SOCS)-1 and (SOCS)-3, negative regulators of the JAK2-STAT signalling pathway, are silenced by methylation in human hepatoma cell lines and HCC tissues, which leads to constitutive activation of STAT3 in these cells[6263]. Deletion of the (SOCS)-3 gene in hepatocytes promotes the activation of STAT3, resistance to apoptosis and accelerated proliferation, resulting in enhanced hepatitis-induced hepatocarcinogenesis[64]. In addition, hepatitis C virus (HCV) core protein exerts an inhibitory effect on (SOCS)-1 gene expression[65]. Hepatitis viruses also activate STAT-3 via oxidative stress[66–68], which might contribute to cellular transformation[69]. Abrogation of constitutive STAT3 activity sensitizes human hepatoma cells to apoptosis induced by TRAIL or drugs[7071].
The PI3K/Akt pathway is also altered in HCC. The expression of the PTEN gene product is reduced or absent in almost half of HCCs and hepatocyte-specific abrogation of PTEN expression in mice results in the development of HCCs[72]. Recent results have indicated that the expression of a negative regulator of PI3K (phosphatidylinositol-3-kinase interacting protein I: PIK3IP1) is reduced in most cases of human HCC, pointing to a tumor suppressor-like function for this protein[73]. Interestingly, hepatic overexpression of PIK3IP1 negatively regulates PI3K activity in the tissue and suppresses the development of HCC[73].
Overexpression of Ras proteins is frequently observed in HCC[74], at least in part due to epigenetic silencing of inhibitors of the Ras pathway[75]. Furthermore, it has been reported that the expression of different ERK inhibitors, such as the Spred family of Ras/ERK inhibitors or the dual-specificity phosphatase-1 (DUSP1), is dysregulated in HCC[7677]. Activated RAS oncogene collaborates with the hepatitis B virus HBx protein to transform cells by suppressing HBx-mediated apoptosis[78]. Thus, dysregulation of the Ras pathway might also be playing a role in balancing pre-neoplastic hepatocytes towards survival in HBV- or HCV-mediated HCC.
In summary, different molecular alterations may contribute to an enhancement of anti-apoptotic signals in HCC cells that allow them to survive pro-apoptotic stimuli (Figure 3).
A link between inflammation and liver cancer was suspected some years ago, but the precise mechanisms are just beginning to be understood[79]. Recent experimental data support the hypothesis that inflammation promotes carcinogenesis and that NF-κB signalling is at the heart of such inflammation[79]. Different studies have implicated members of the NF-κB/Rel family in both HBV- and HCV-induced neoplastic development of the liver[80]. Several mechanisms have been proposed for activation of NF-κB by the hepatitis virus. Overall, inflammatory hepatitis might activate NF-κB by the concerted action of cytokines, such as TNF-α, chemokines or interleukins, and viral proteins, which likely will promote cell survival of pre-cancerous hepatocytes[80]. Furthermore, a correlation between EGFR ligands and NF-κB activity has been provided by studies in transforming growth factor-alpha (TGF-α)/c-Myc mice. Indeed, an important role for NF-κB in inhibiting c-Myc-induced apoptosis was found essential for hepatocarcinogenesis[81]. Two pro-survival NF-κB targets are an antiapoptotic member of the Bcl-2 family, Bcl-XL, and a member of the caspase inhibitors, XIAP, which are frequently overexpressed in human HCCs, as commented above[4448]. Interestingly, the NF-κB/Bcl-XL/XIAP axis potently counteracts the TGF-β-induced apoptosis[82] and exerts a general cytoprotective role on preneoplastic hepatocytes[83]. Recent results also link NF-κB to the increase in the autocrine expression of EGF receptor ligands, such as TGF-α, in hepatocytes and hepatoma cells[8485]. In summary, overactivation of the NF-κB pathway might generate resistance to apoptosis, through different mechanisms, in HCC cells (Figure 2).
Many epidemiological studies demonstrate that treatment with non-steroidal anti-inflammatory drugs (NSAIDs) reduces the incidence and mortality of certain malignancies, especially gastrointestinal cancer[86]. The cyclooxygenase (COX) enzymes are well known targets of NSAIDs. Overexpression of COX-2 in HCC cells increases proliferation and survival through Akt activation[87]. Accordingly, recent evidence indicates that selective inhibition of COX-2 in HCC cells leads to a marked induction of apoptosis and inhibition of proliferation and, thus, may offer therapeutic and preventive potential in human hepatocarcinogenesis[88]. COX-2 inhibitors might induce apoptosis signalling in HCC cells via death receptors and mitochondria[89]. Recent data have demonstrated that simultaneous inhibition of PI3K/Akt/mTOR and COX-2 activity in in vitro models causes massive apoptosis of neoplastic hepatocytes[90].
During later stages in the development of liver tumors, a loss in cell-cell contacts and the acquisition of fibroblastic-like phenotype is observed. This phenomenon, known as epithelial-to-mesenchymal transition (EMT), might contribute to increasing the migratory and metastatic capabilities of the cells[91]. Cytokines, such as TGF-β and extracellular matrix molecules are thought to fundamentally contribute to the microenvironmental interaction between stromal and malignant cells, and provide the basis for a broad repertoire of epithelial transdifferentiation. Interestingly, EMT of liver cells also results in enhanced resistance to apoptosis[9293], probably due to up-regulation of SNAI1, the gene that codifies for Snail, a repressor of E-cadherin expression that also has effects on cell homeostasis, inhibiting the cell cycle and preventing cell death[94] (Figure 1).
A high percentage of human HCCs show high levels of β-catenin[9596], either through stabilizing mutations of the β-catenin or overexpression of FZD, therefore favouring the intracellular accumulation of the protein[95]. Furthermore, certain evidence indicates that TGF-β might induce nuclear β-catenin accumulation, through induction of PDGF signalling[97] (Figure 1). β-catenin expression leads to elevated EGFR levels in hepatocytes and inmunohistological analysis shows high correlation between the expression of nuclear/cytoplasmic β-catenin and EGFR in most hepatoblastomas[57]. β-catenin also participates in homotypic cell-cell interactions through its association with E-cadherin. Thus, β-catenin accumulation in HCC cells might contribute to impairing E-cadherin expression, mediating the EMT process, migration and survival. Indeed, there is evidence suggesting that up-regulation of CTNNB1, the gene encoding for β-catenin, also contributes to the enhancement of hepatocellular carcinoma cell survival[98].
In summary, a significant number of relevant molecular mechanisms altered in HCC initiation and progression are compromising the balance between survival and apoptotic signals in the pre-neoplastic hepatocytes. Some physiological pro-apoptotic molecules are down-regulated or inactivated in HCC, but the balance between death and survival is mainly disrupted due to overactivation of anti-apoptotic signals. Therefore, liver cancer cells might show stronger requirements of these intracellular pathways to survive. The absence of standard systemic therapy for advanced cases of HCC has changed with the recent positive randomized trial testing the multikinase sorafenib, which represents a breakthrough in the management of this neoplasm[358]. Interestingly, sorafenib induces tumor cell apoptosis in HCC cells, through, at the least, inhibiting the RAF/MEK/ERK pathway[99]. Similar situations might be found with other multikinase inhibitor drugs that are on the way towards approval for HCC therapy[58100]. Of relevance here is certain evidence indicating that erlotinib-induced growth inhibition in HCC cells correlates with overexpression of pro-apoptotic factors like caspase and gadds, as well as down-regulation of anti-apoptotic factors, such as Bcl-XL[101]. Another receptor tyrosine kinase inhibitor, sunitinib, which has also shown intriguing outcomes in advanced HCC[100], is a strong apoptosis inducer in different tumor cells, an effect that is enhanced in the presence of inhibitors of the PI3-K/Akt/mTOR pathway[102]. Bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, has been proven to be efficient in inhibiting the growth of nonmetastatic HCC[103]. Interestingly, recent evidence indicates that VEGF signalling inhibitors might be effective in inhibiting tumorigenesis more through their pro-apoptotic than their anti-angiogenic properties[104]. Therefore, therapeutic strategies to selectively inhibit anti-apoptotic signals in HCC cells might have the potential to provide powerful tools in the future to treat liver cancer.
| 1. | Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024-1033. |
| 2. | Fabregat I, Roncero C, Fernandez M. Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int. 2007;27:155-162. |
| 3. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. |
| 4. | Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166-2176. |
| 5. | Bao JJ, Zhang WW, Kuo MT. Adenoviral delivery of recombinant DNA into transgenic mice bearing hepatocellular carcinomas. Hum Gene Ther. 1996;7:355-365. |
| 6. | Farazi PA, Glickman J, Horner J, Depinho RA. Cooperative interactions of p53 mutation, telomere dysfunction, and chronic liver damage in hepatocellular carcinoma progression. Cancer Res. 2006;66:4766-4773. |
| 8. | Carr BI, Hayashi I, Branum EL, Moses HL. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986;46:2330-2334. |
| 9. | Oberhammer FA, Pavelka M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W, Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci USA. 1992;89:5408-5412. |
| 10. | Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557-1566. |
| 11. | Coulouarn C, Factor VM, Thorgeirsson SS. Transforming growth factor-beta gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology. 2008;47:2059-2067. |
| 12. | Yang YA, Zhang GM, Feigenbaum L, Zhang YE. Smad3 reduces susceptibility to hepatocarcinoma by sensitizing hepatocytes to apoptosis through downregulation of Bcl-2. Cancer Cell. 2006;9:445-457. |
| 13. | Ito N, Kawata S, Tamura S, Takaishi K, Shirai Y, Kiso S, Yabuuchi I, Matsuda Y, Nishioka M, Tarui S. Elevated levels of transforming growth factor beta messenger RNA and its polypeptide in human hepatocellular carcinoma. Cancer Res. 1991;51:4080-4083. |
| 14. | Kitisin K, Ganesan N, Tang Y, Jogunoori W, Volpe EA, Kim SS, Katuri V, Kallakury B, Pishvaian M, Albanese C. Disruption of transforming growth factor-beta signaling through beta-spectrin ELF leads to hepatocellular cancer through cyclin D1 activation. Oncogene. 2007;26:7103-7110. |
| 15. | Baek HJ, Lim SC, Kitisin K, Jogunoori W, Tang Y, Marshall MB, Mishra B, Kim TH, Cho KH, Kim SS. Hepatocellular cancer arises from loss of transforming growth factor beta signaling adaptor protein embryonic liver fodrin through abnormal angiogenesis. Hepatology. 2008;48:1128-1137. |
| 16. | Huang S, He X, Ding J, Liang L, Zhao Y, Zhang Z, Yao X, Pan Z, Zhang P, Li J. Upregulation of miR-23a approximately 27a approximately 24 decreases transforming growth factor-beta-induced tumor-suppressive activities in human hepatocellular carcinoma cells. Int J Cancer. 2008;123:972-978. |
| 17. | Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 2008;68:8191-8194. |
| 18. | Valdes F, Murillo MM, Valverde AM, Herrera B, Sanchez A, Benito M, Fernandez M, Fabregat I. Transforming growth factor-beta activates both pro-apoptotic and survival signals in fetal rat hepatocytes. Exp Cell Res. 2004;292:209-218. |
| 19. | Murillo MM, del Castillo G, Sanchez A, Fernandez M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene. 2005;24:4580-4587. |
| 20. | Caja L, Ortiz C, Bertran E, Murillo MM, Miro-Obradors MJ, Palacios E, Fabregat I. Differential intracellular signalling induced by TGF-beta in rat adult hepatocytes and hepatoma cells: implications in liver carcinogenesis. Cell Signal. 2007;19:683-694. |
| 21. | Fabregat I, Herrera B, Fernandez M, Alvarez AM, Sanchez A, Roncero C, Ventura JJ, Valverde AM, Benito M. Epidermal growth factor impairs the cytochrome C/caspase-3 apoptotic pathway induced by transforming growth factor beta in rat fetal hepatocytes via a phosphoinositide 3-kinase-dependent pathway. Hepatology. 2000;32:528-535. |
| 22. | Carmona-Cuenca I, Herrera B, Ventura JJ, Roncero C, Fernandez M, Fabregat I. EGF blocks NADPH oxidase activation by TGF-beta in fetal rat hepatocytes, impairing oxidative stress, and cell death. J Cell Physiol. 2006;207:322-330. |
| 23. | Borrell-Pages M, Rojo F, Albanell J, Baselga J, Arribas J. TACE is required for the activation of the EGFR by TGF-alpha in tumors. EMBO J. 2003;22:1114-1124. |
| 24. | Ding X, Yang LY, Huang GW, Wang W, Lu WQ. ADAM17 mRNA expression and pathological features of hepatocellular carcinoma. World J Gastroenterol. 2004;10:2735-2739. |
| 25. | Lee SH, Shin MS, Lee HS, Bae JH, Lee HK, Kim HS, Kim SY, Jang JJ, Joo M, Kang YK. Expression of Fas and Fas-related molecules in human hepatocellular carcinoma. Hum Pathol. 2001;32:250-256. |
| 26. | Ito Y, Monden M, Takeda T, Eguchi H, Umeshita K, Nagano H, Nakamori S, Dono K, Sakon M, Nakamura M. The status of Fas and Fas ligand expression can predict recurrence of hepatocellular carcinoma. Br J Cancer. 2000;82:1211-1217. |
| 27. | Shin EC, Shin JS, Park JH, Kim JJ, Kim H, Kim SJ. Expression of Fas-related genes in human hepatocellular carcinomas. Cancer Lett. 1998;134:155-162. |
| 28. | Takahashi M, Saito H, Okuyama T, Miyashita T, Kosuga M, Sumisa F, Yamada M, Ebinuma H, Ishii H. Overexpression of Bcl-2 protects human hepatoma cells from Fas-antibody-mediated apoptosis. J Hepatol. 1999;31:315-322. |
| 29. | Lian Z, Liu J, Pan J, Satiroglu Tufan NL, Zhu M, Arbuthnot P, Kew M, Clayton MM, Feitelson MA. A cellular gene up-regulated by hepatitis B virus-encoded X antigen promotes hepatocellular growth and survival. Hepatology. 2001;34:146-157. |
| 30. | Otsuka M, Kato N, Taniguchi H, Yoshida H, Goto T, Shiratori Y, Omata M. Hepatitis C virus core protein inhibits apoptosis via enhanced Bcl-xL expression. Virology. 2002;296:84-93. |
| 31. | Okano H, Shiraki K, Inoue H, Kawakita T, Yamanaka T, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Fujikawa K. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab Invest. 2003;83:1033-1043. |
| 32. | Chan BC, Ching AK, To KF, Leung JC, Chen S, Li Q, Lai PB, Tang NL, Shaw PC, Chan JY. BRE is an antiapoptotic protein in vivo and overexpressed in human hepatocellular carcinoma. Oncogene. 2008;27:1208-1217. |
| 33. | Suzuki A, Hayashida M, Kawano H, Sugimoto K, Nakano T, Shiraki K. Hepatocyte growth factor promotes cell survival from fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppression. Hepatology. 2000;32:796-802. |
| 34. | Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782-798. |
| 35. | Shiraki K, Yamanaka T, Inoue H, Kawakita T, Enokimura N, Okano H, Sugimoto K, Murata K, Nakano T. Expression of TNF-related apoptosis-inducing ligand in human hepatocellular carcinoma. Int J Oncol. 2005;26:1273-1281. |
| 36. | Chen XP, He SQ, Wang HP, Zhao YZ, Zhang WG. Expression of TNF-related apoptosis-inducing Ligand receptors and antitumor tumor effects of TNF-related apoptosis-inducing Ligand in human hepatocellular carcinoma. World J Gastroenterol. 2003;9:2433-2440. |
| 37. | Herr I, Schemmer P, Buchler MW. On the TRAIL to therapeutic intervention in liver disease. Hepatology. 2007;46:266-274. |
| 38. | Du J, Liang X, Liu Y, Qu Z, Gao L, Han L, Liu S, Cui M, Shi Y, Zhang Z. Hepatitis B virus core protein inhibits TRAIL-induced apoptosis of hepatocytes by blocking DR5 expression. Cell Death Differ. 2009;16:219-229. |
| 39. | Zender L, Hutker S, Mundt B, Waltemathe M, Klein C, Trautwein C, Malek NP, Manns MP, Kuhnel F, Kubicka S. NFkappaB-mediated upregulation of bcl-xl restrains TRAIL-mediated apoptosis in murine viral hepatitis. Hepatology. 2005;41:280-288. |
| 40. | Yamanaka T, Shiraki K, Sugimoto K, Ito T, Fujikawa K, Ito M, Takase K, Moriyama M, Nakano T, Suzuki A. Chemotherapeutic agents augment TRAIL-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology. 2000;32:482-490. |
| 41. | Ganten TM, Koschny R, Haas TL, Sykora J, Li-Weber M, Herzer K, Walczak H. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology. 2005;42:588-597. |
| 42. | Pathil A, Armeanu S, Venturelli S, Mascagni P, Weiss TS, Gregor M, Lauer UM, Bitzer M. HDAC inhibitor treatment of hepatoma cells induces both TRAIL-independent apoptosis and restoration of sensitivity to TRAIL. Hepatology. 2006;43:425-434. |
| 43. | Mott JL, Gores GJ. Piercing the armor of hepatobiliary cancer: Bcl-2 homology domain 3 (BH3) mimetics and cell death. Hepatology. 2007;46:906-911. |
| 44. | Takehara T, Liu X, Fujimoto J, Friedman SL, Takahashi H. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology. 2001;34:55-61. |
| 45. | Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, Bodingbauer M, Crevenna R, Monia BP, Peck-Radosavljevic M. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44:151-157. |
| 46. | Beerheide W, Tan YJ, Teng E, Ting AE, Jedpiyawongse A, Srivatanakul P. Downregulation of proapoptotic proteins Bax and Bcl-X(S) in p53 overexpressing hepatocellular carcinomas. Biochem Biophys Res Commun. 2000;273:54-61. |
| 47. | Chen GG, Lai PB, Chan PK, Chak EC, Yip JH, Ho RL, Leung BC, Lau WY. Decreased expression of Bid in human hepatocellular carcinoma is related to hepatitis B virus X protein. Eur J Cancer. 2001;37:1695-1702. |
| 48. | Shi YH, Ding WX, Zhou J, He JY, Xu Y, Gambotto AA, Rabinowich H, Fan J, Yin XM. Expression of X-linked inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes metastasis and tumor recurrence. Hepatology. 2008;48:497-507. |
| 49. | Birkey Reffey S, Wurthner JU, Parks WT, Roberts AB, Duckett CS. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-beta signaling. J Biol Chem. 2001;276:26542-26549. |
| 50. | Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253-1267. |
| 51. | Ito T, Shiraki K, Sugimoto K, Yamanaka T, Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080-1085. |
| 52. | Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758-1767. |
| 53. | Zhu H, Chen XP, Zhang WG, Luo SF, Zhang BX. Expression and significance of new inhibitor of apoptosis protein survivin in hepatocellular carcinoma. World J Gastroenterol. 2005;11:3855-3859. |
| 54. | Duan XX, Ou JS, Li Y, Su JJ, Ou C, Yang C, Yue HF, Ban KC. Dynamic expression of apoptosis-related genes during development of laboratory hepatocellular carcinoma and its relation to apoptosis. World J Gastroenterol. 2005;11:4740-4744. |
| 55. | Ye CP, Qiu CZ, Huang ZX, Su QC, Zhuang W, Wu RL, Li XF. Relationship between survivin expression and recurrence, and prognosis in hepatocellular carcinoma. World J Gastroenterol. 2007;13:6264-6268. |
| 56. | Ortiz C, Caja L, Sancho P, Bertran E, Fabregat I. Inhibition of the EGF receptor blocks autocrine growth and increases the cytotoxic effects of doxorubicin in rat hepatoma cells: role of reactive oxygen species production and glutathione depletion. Biochem Pharmacol. 2008;75:1935-1945. |
| 57. | Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787-3800. |
| 58. | Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312-1327. |
| 59. | Sato Y, Kato J, Takimoto R, Takada K, Kawano Y, Miyanishi K, Kobune M, Sato Y, Takayama T, Matunaga T. Hepatitis C virus core protein promotes proliferation of human hepatoma cells through enhancement of transforming growth factor alpha expression via activation of nuclear factor-kappaB. Gut. 2006;55:1801-1808. |
| 60. | Masaki T, Okada M, Shiratori Y, Rengifo W, Matsumoto K, Maeda S, Kato N, Kanai F, Komatsu Y, Nishioka M. pp60c-src activation in hepatocellular carcinoma of humans and LEC rats. Hepatology. 1998;27:1257-1264. |
| 61. | De Toni EN, Kuntzen C, Gerbes AL, Thasler WE, Sonuc N, Mucha SR, Camaj P, Bruns C, Goke B, Eichhorst ST. P60-c-src suppresses apoptosis through inhibition of caspase 8 activation in hepatoma cells, but not in primary hepatocytes. J Hepatol. 2007;46:682-691. |
| 62. | Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, Harris CC, Herman JG. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet. 2001;28:29-35. |
| 63. | Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406-6417. |
| 64. | Ogata H, Kobayashi T, Chinen T, Takaki H, Sanada T, Minoda Y, Koga K, Takaesu G, Maehara Y, Iida M. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology. 2006;131:179-193. |
| 65. | Miyoshi H, Fujie H, Shintani Y, Tsutsumi T, Shinzawa S, Makuuchi M, Kokudo N, Matsuura Y, Suzuki T, Miyamura T. Hepatitis C virus core protein exerts an inhibitory effect on suppressor of cytokine signaling (SOCS)-1 gene expression. J Hepatol. 2005;43:757-763. |
| 66. | Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599-9604. |
| 67. | Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721-7730. |
| 68. | Waris G, Turkson J, Hassanein T, Siddiqui A. Hepatitis C virus (HCV) constitutively activates STAT-3 via oxidative stress: role of STAT-3 in HCV replication. J Virol. 2005;79:1569-1580. |
| 69. | Yoshida T, Hanada T, Tokuhisa T, Kosai K, Sata M, Kohara M, Yoshimura A. Activation of STAT3 by the hepatitis C virus core protein leads to cellular transformation. J Exp Med. 2002;196:641-653. |
| 70. | Choudhari SR, Khan MA, Harris G, Picker D, Jacob GS, Block T, Shailubhai K. Deactivation of Akt and STAT3 signaling promotes apoptosis, inhibits proliferation, and enhances the sensitivity of hepatocellular carcinoma cells to an anticancer agent, Atiprimod. Mol Cancer Ther. 2007;6:112-121. |
| 71. | Kusaba M, Nakao K, Goto T, Nishimura D, Kawashimo H, Shibata H, Motoyoshi Y, Taura N, Ichikawa T, Hamasaki K. Abrogation of constitutive STAT3 activity sensitizes human hepatoma cells to TRAIL-mediated apoptosis. J Hepatol. 2007;47:546-555. |
| 72. | Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774-1783. |
| 73. | He X, Zhu Z, Johnson C, Stoops J, Eaker AE, Bowen W, DeFrances MC. PIK3IP1, a negative regulator of PI3K, suppresses the development of hepatocellular carcinoma. Cancer Res. 2008;68:5591-5598. |
| 74. | Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117-1128. |
| 75. | Calvisi DF, Ladu S, Gorden A, Farina M, Lee JS, Conner EA, Schroeder I, Factor VM, Thorgeirsson SS. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713-2722. |
| 76. | Yoshida T, Hisamoto T, Akiba J, Koga H, Nakamura K, Tokunaga Y, Hanada S, Kumemura H, Maeyama M, Harada M. Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene. 2006;25:6056-6066. |
| 77. | Calvisi DF, Pinna F, Meloni F, Ladu S, Pellegrino R, Sini M, Daino L, Simile MM, De Miglio MR, Virdis P. Dual-specificity phosphatase 1 ubiquitination in extracellular signal-regulated kinase-mediated control of growth in human hepatocellular carcinoma. Cancer Res. 2008;68:4192-4200. |
| 78. | Kim YC, Song KS, Yoon G, Nam MJ, Ryu WS. Activated ras oncogene collaborates with HBx gene of hepatitis B virus to transform cells by suppressing HBx-mediated apoptosis. Oncogene. 2001;20:16-23. |
| 79. | Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19-26. |
| 80. | Arsura M, Cavin LG. Nuclear factor-kappaB and liver carcinogenesis. Cancer Lett. 2005;229:157-169. |
| 81. | Qiao L, Zhang H, Yu J, Francisco R, Dent P, Ebert MP, Rocken C, Farrell G. Constitutive activation of NF-kappaB in human hepatocellular carcinoma: evidence of a cytoprotective role. Hum Gene Ther. 2006;17:280-290. |
| 82. | Kaur S, Wang F, Venkatraman M, Arsura M. X-linked inhibitor of apoptosis (XIAP) inhibits c-Jun N-terminal kinase 1 (JNK1) activation by transforming growth factor beta1 (TGF-beta1) through ubiquitin-mediated proteosomal degradation of the TGF-beta1-activated kinase 1 (TAK1). J Biol Chem. 2005;280:38599-38608. |
| 83. | Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228-6244. |
| 84. | Murillo MM, Carmona-Cuenca I, Del Castillo G, Ortiz C, Roncero C, Sanchez A, Fernandez M, Fabregat I. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem J. 2007;405:251-259. |
| 85. | Sato Y, Kato J, Takimoto R, Takada K, Kawano Y, Miyanishi K, Kobune M, Sato Y, Takayama T, Matunaga T. Hepatitis C virus core protein promotes proliferation of human hepatoma cells through enhancement of transforming growth factor alpha expression via activation of nuclear factor-kappaB. Gut. 2006;55:1801-1808. |
| 86. | Cervello M, Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J Gastroenterol. 2006;12:5113-5121. |
| 87. | Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38:756-768. |
| 88. | Kern MA, Schubert D, Sahi D, Schoneweiss MM, Moll I, Haugg AM, Dienes HP, Breuhahn K, Schirmacher P. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885-894. |
| 89. | Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H, Fleischer B, Trautwein C, Michalski C, Schulze-Bergkamen H. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006;66:7059-7066. |
| 90. | Ladu S, Calvisi DF, Conner EA, Farina M, Factor VM, Thorgeirsson SS. E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer. Gastroenterology. 2008;135:1322-1332. |
| 91. | Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition--does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5:280-290. |
| 92. | Valdes F, Alvarez AM, Locascio A, Vega S, Herrera B, Fernandez M, Benito M, Nieto MA, Fabregat I. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol Cancer Res. 2002;1:68-78. |
| 93. | Nitta T, Kim JS, Mohuczy D, Behrns KE. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology. 2008;48:909-919. |
| 94. | Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131-1143. |
| 95. | Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901-1915. |
| 96. | Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298-1305. |
| 97. | Fischer AN, Fuchs E, Mikula M, Huber H, Beug H, Mikulits W. PDGF essentially links TGF-beta signaling to nuclear beta-catenin accumulation in hepatocellular carcinoma progression. Oncogene. 2007;26:3395-3405. |
| 98. | Lian Z, Liu J, Li L, Li X, Clayton M, Wu MC, Wang HY, Arbuthnot P, Kew M, Fan D. Enhanced cell survival of Hep3B cells by the hepatitis B x antigen effector, URG11, is associated with upregulation of beta-catenin. Hepatology. 2006;43:415-424. |
| 99. | Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851-11858. |
| 100. | Abou-Alfa GK, Venook AP. The impact of new data in the treatment of advanced hepatocellular carcinoma. Curr Oncol Rep. 2008;10:199-205. |
| 101. | Huether A, Hopfner M, Sutter AP, Baradari V, Schuppan D, Scherubl H. Signaling pathways involved in the inhibition of epidermal growth factor receptor by erlotinib in hepatocellular cancer. World J Gastroenterol. 2006;12:5160-5167. |
| 102. | Ikezoe T, Nishioka C, Tasaka T, Yang Y, Komatsu N, Togitani K, Koeffler HP, Taguchi H. The antitumor effects of sunitinib (formerly SU11248) against a variety of human hematologic malignancies: enhancement of growth inhibition via inhibition of mammalian target of rapamycin signaling. Mol Cancer Ther. 2006;5:2522-2530. |
| 103. | Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992-2998. |
| 104. | Epstein RJ. VEGF signaling inhibitors: more pro-apoptotic than anti-angiogenic. Cancer Metastasis Rev. 2007;26:443-452. |