Published online Dec 28, 2009. doi: 10.3748/wjg.15.6086
Revised: November 19, 2009
Accepted: November 26, 2009
Published online: December 28, 2009
AIM: To evaluate the usefulness of a balloon overtube to assist colorectal endoscopic submucosal dissection (ESD) using a gastroscope.
METHODS: The results of 45 consecutive patients who underwent colorectal ESD were analyzed in a single tertiary endoscopy center. In preoperative evaluation of access to the lesion, difficulties were experienced in the positioning and stabilization of a gastroscope in 15 patients who were thus assigned to the balloon-guided ESD group. A balloon overtube was placed with a gastroscope to provide an endoscopic channel to the lesion in cases with preoperatively identified difficulties related to accessibility. Colorectal ESD was performed following standard procedures. A submucosal fluid bleb was created with hyaluronic acid solution. A circumferential mucosal incision was made to marginate the lesion. The isolated lesion was finally excised from the deeper layers with repetitive electrosurgical dissections with needle knives. The success of colorectal ESD, procedural feasibility, and procedure-related complications were the main outcomes and measurements.
RESULTS: The overall en bloc excision rate of colorectal ESD during this study at our institution was 95.6%. En bloc excision of the lesion was successfully achieved in 13 of the 15 patients (86.7%) in the balloon overtube-guided colorectal ESD group, which was comparable to the results of the standard ESD group with better accessibility to the lesion (30/30, 100%, not statistically significant).
CONCLUSION: Use of a balloon overtube can improve access to the lesion and facilitate scope manipulation for colorectal ESD.
- Citation: Ohya T, Ohata K, Sumiyama K, Tsuji Y, Koba I, Matsuhashi N, Tajiri H. Balloon overtube-guided colorectal endoscopic submucosal dissection. World J Gastroenterol 2009; 15(48): 6086-6090
- URL: https://www.wjgnet.com/1007-9327/full/v15/i48/6086.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.6086
Endoscopic submucosal dissection (ESD) has evolved to become one of the therapeutic options for the treatment of early stage gastric cancers in Asian countries[1,2]. In Japan, ESD has been increasingly applied to various levels of the gastrointestinal tract and results of initial experiences in a few high volume endoscopy centers have demonstrated the technical feasibility of this unique and aggressive approach, even in the colon. However, the number of institutions allowed to perform this procedure is still restricted because colorectal ESD is technically more challenging, and may carry a higher risk of perforation and the most common sequela, bacterial peritonitis[3-6]. Inherited anatomic variability in the colon such as a long tubal structure, folds, or looping in mobile segments of the colon attached to the mesentery may hinder any endoscopic intervention in the colon. A further challenge arises from the need for a longer colonoscope; this can increase procedural workloads due to the need for careful, intuitive manipulation of the tip of the scope during needle knife dissections of the paper thin submucosal tissue plane[7,8]. At our institution, the gastroscope is therefore often preferentially used even for colorectal ESD, despite the shorter scope length, for deep scope intubation.
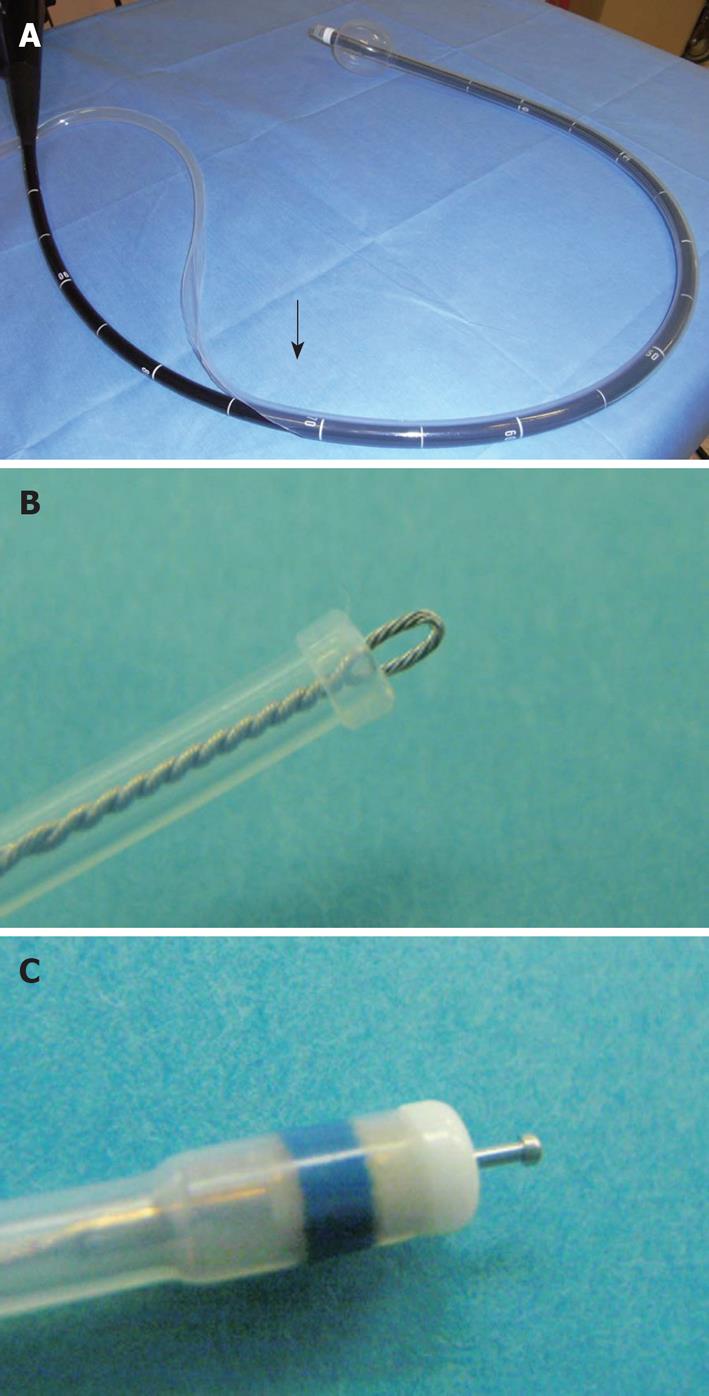
Use of a balloon overtube in enteroscopy provides optimal traction on the intestinal wall, thereby facilitating scope intubation. By inflation and withdrawal of a balloon attached to the tip of the overtube, the intestinal wall can be pleated on the overtube. This balloon overtube-guided technique has enabled a standardized total enteroscopy and has also provided a shorter direct access to the innermost locations in the gastrointestinal tract[9-11]. In addition, this approach has facilitated various interventions in the small intestine including biopsy, hemostasis and most recently polypectomy and endoscopic mucosal resection (EMR). Based on these results in the small intestine, we postulated that the a balloon overtube could form an ideal platform for colorectal ESD[12,13].
We used a balloon overtube as an endoscopic channel and platform for colorectal ESD in cases in which access to the lesion with a gastroscope was difficult. Here we review the results of colorectal ESD in our institution to evaluate whether the balloon overtube-guided technique could improve access and scope manipulation during colorectal ESD.
From October 2008 to March 2009 we performed colorectal ESD in 45 patients. The mean age of the patients was 70.7 years (range, 58 to 83 years). Indications for colorectal ESD were: 1. laterally spreading tumors (LST) over 20 mm in size, 2. lesions evaluated as being difficult to remove en bloc regardless of lesion size, e.g. local residual recurrent tumors after endoscopic removal and flat or depressed mucosal lesions. Histopathology of the lesions was preoperatively confirmed as adenoma or cancer with magnifying endoscopy or biopsy.
In all cases, access to the lesion was preoperatively evaluated using a therapeutic gastroscope (GIF Q260-J, Olympus, Tokyo, Japan). When circumferential access to the lesion with the tip of the endoscope was difficult, the access was considered difficult with a standard gastroscopic approach and the lesion was indicated for balloon overtube-guided ESD.
ESD procedure with a gastroscope: A transparent cap (D201-11802; 2 mm Olympus, Tokyo, Japan) fitted therapeutic gastroscope (GIF-Q260-J, Olympus, Tokyo, Japan) was used for the conventional ESD group. The gastroscope is equipped with a water jet system, which supplies a continuous jet of high-pressure water to wash out blood and mucous during the endoscopic dissection. Two types of needle knives specially designed for ESD with minor modifications to the diathermy wire tip (Flex knife, KD-630L, Olympus, Tokyo Japan or Dual knife, KD-650Q, Olympus, Tokyo Japan) were used for the standard ESD procedure with a VIO300D high-frequency generator (ERBE, Elektromedizin, Tubingen, Germany). Ten percent sodium hyaluronate solution mixed with a small amount of indigo carmine and epinephrine hydrochloride was used as the injection solution to create a submucosal safety bleb[8]. A circumferential mucosal incision was made using one of the needle knives on endocut I (effect 2, interval 2, duration 2) mode. After the horizontal margination of the lesion from the surrounding normal mucosa, the electrosurgical dissection of the submucosal tissue plane was continuously performed with repetitive electrosurgical needle knife dissections. When bleeding and vascular structures were encountered, hemostasis were performed with point cauteries with the diathermy tip of the needle knife with Swift Coag mode 45W (effect 3) or a coagulation forceps (Coagrasper FD411-QR, Olympus, Tokyo, Japan) with Swift Coag mode 45W (effect 3). Patient posture rotations were carried out as needed to improve access to the lesion and deflect the overlying mucosa away from the dissection plane by gravity.
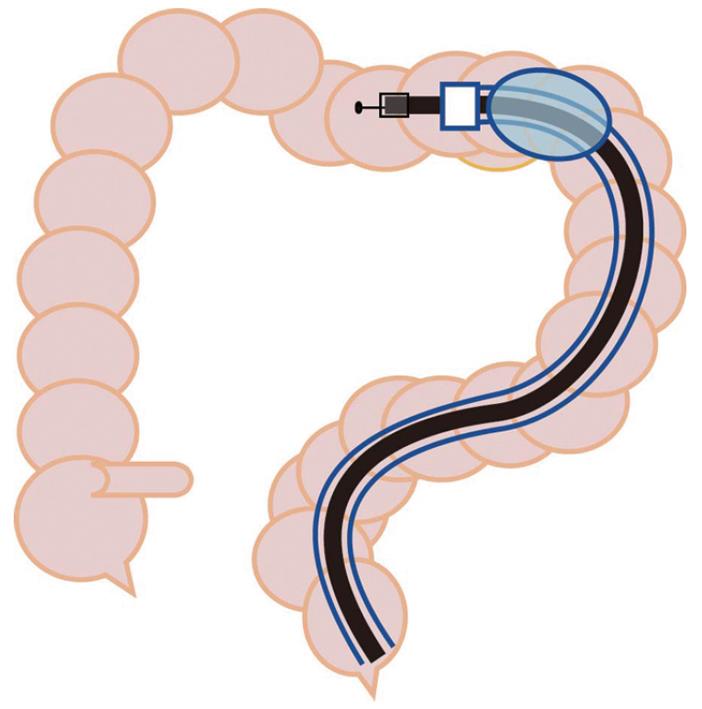
Single balloon overtube-guided ESD: Balloon overtube-guided ESD was performed with a standard diagnostic gastroscope (GIF-Q260, Olympus, Tokyo, Japan) with a 9.2-mm outer diameter. A balloon overtube with a 13.2-mm outer diameter, 11-mm inner diameter over-tube (ST-SB1, total length 1400 mm Olympus, Tokyo, Japan) designed for enteroscopy was shortened to 70 cm in length from the distal end leaving the balloon inflation tube intact. A gastroscope preloaded into the length-adjusted overtube was then inserted into the colon and the lesion was accessed using techniques similar to balloon enteroscopy (Figure 1). A transparent hood (D201-10704; 4 mm, Olympus, Tokyo, Japan) was attached to the tip of the endoscope. The procedural processes for ESD and the tool set used in the balloon overtube-guided procedure were the same for the ESD procedure performed without the overtube.
The study protocol was approved by the Institutional Ethical Committee of Kanto Medical Center NTT. Written informed consent was obtained from each patient before the ESD procedure.
The significance of differences between patient characteristics and clinicopathological features was determined using χ2 test, the Mann-Whitney U test, or Student t test as appropriate. P values < 0.05 were considered statistically significant.
In the preoperative evaluation of accessibility with a gastroscope, fifteen patients were identified as difficult, and were enrolled in the balloon overtube-guided ESD group. Thirty patients, who met the circumferential access criteria, were treated with the standard ESD method using a gastroscope without the overtube.
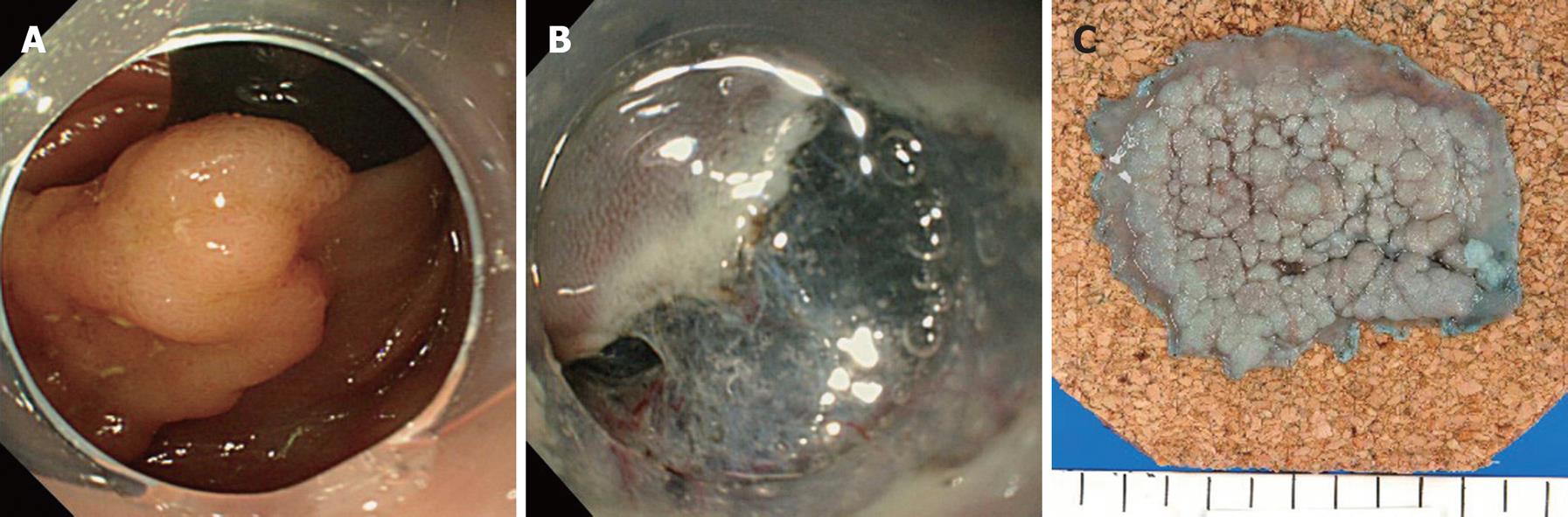
The overall en bloc excision rate of colorectal ESD was 95.6%. In the patients treated with the standard ESD method with a gastroscope, en bloc excision of the lesion was performed successfully in all 30 patients (100%). The lesions were located in the cecum in 2 patients, in the ascending colon in 10 patients, in the transverse colon in 2 patients, in the descending colon in 2 patients, in the sigmoid colon in 6 patients, and in the rectum in 8 patients. The median procedure time was 60 min (12-200 min). The median size of the lesion was 35 mm (SD: 13-98), and the median resected specimen size was 43 mm (17-112 mm). There were no perforations, however, post-ESD bleeding occurred in one case, which required repeated endoscopic hemostasis.
En bloc excision of the lesion was successfully achieved in 13 of the 15 patients (86.7%) in the balloon overtube-guided colorectal ESD group. In 2 cases of failure in the single balloon overtube-guided group, the endoscope did not reach the lesion due to elongation of the sigmoid colon. These two patients were eventually treated by piecemeal snare EMR using a colonoscope (CF-Q240L, Olympus, Japan). One lesion was located in the ascending colon and the other was in the transverse colon. Lesions in the balloon overtube-guided group were located in the transverse colon in 10 patients, the descending colon in 3 patients, the ascending colon in 1 patient and the sigmoid colon in 1 patient. The median procedure time was 80 min (30-160 min). The median size of the tumors was 27 mm (10-46 mm), and the median resected specimen size was 38 mm (18-57 mm). There were no severe complications such as perforation or bleeding.
There were no significant differences in the age (P = 0.352), sex (P = 0.292), lesion size (P = 0.472), or resected specimen size (P = 0.597) between the two groups. Lesions were more frequently located in the transverse colon in the balloon overtube-guided ESD group (10 vs 2, P < 0.001). Operation time was longer in the balloon overtube-guided group (P = 0.050).
On pathology, twenty lesions were diagnosed as tubular adenomas [44.4%, 15 in the ESD without overtube group, 5 in the ESD with overtube group; P = NS (not statistically significant)], and 25 were diagnosed as adenocarcinomas (55.6%, 15 in the ESD without overtube group, 10 in the ESD with overtube group; P = NS). Four patients had submucosal invasion (3 in the ESD without overtube group, 1 in the ESD with overtube group, P = NS) and one patient also had venous involvement. None of the patients had lymphatic involvement.
The development of endoscopic snare polypectomy represents one of the most important achievements in the history of flexible endoscopy. This approach benefits patients enormously by reducing the physical burden associated with colonic polyp removal compared to traditional surgical colectomy. Due to the limited size of the snare, removal of large polyps by snare polypectomy requires a piecemeal resection, which may lead to incomplete tumor removal. Local residual recurrence may occur after piecemeal colonic polyp excision in between 3% to 27% of cases[4]. In general, the majority of recurrent colorectal lesions are not clinically significant and can be managed with repeated endoscopic interventions. However, since some endoscopically removed lesions require additional surgical resection due to invasion into the deeper layers and vasculature, en bloc tumor excision is of interest not only for minimization of local tumor recurrence, but also for ensuring the precise histopathological evaluation of the sampled specimen[14]. In this study, three patients had shallow submucosal invasions and one patient had a solid submucosal invasion with vascular involvement that required additional surgical treatment. If it were possible to overcome the major technical difficulties associated with colorectal ESD, we believe this treatment could be an appropriate therapeutic option for colonic lesions that are difficult to remove en bloc, and this approach may be better accepted in western countries with a higher incidence of colonic polyps and cancers[15].
In this study, preoperative evaluation showed that the majority of lesions in the transverse colon were in a difficult location and were assigned to the balloon overtube-guided group (10 vs 2). The transverse colon is the mobile segment most distant from the anus, and hence could be embarrassing due to the situation of the distal side of the colon. Mid-transverse colon may present as a sharp bend in patients with a redundant and drooping transverse colon. Reformation of sigmoid looping may generate friction for scope passage interfering with scope manipulation. Use of the balloon overtube provided an anchor on the colon wall giving optimal traction to maintain a shorter, straighter and more stabilized access to the lesion during ESD (Figure 2). Lesions that were preoperatively identified as being in a difficult location in the transverse colon could be accessed repeatedly using a diagnostic gastroscope by guidance of the balloon overtube, with the exception of one case with a surprisingly elongated sigmoid and severe adhesion. In addition, stabilized access via the overtube allowed direct and intuitive scope manipulation and en bloc tumor excision could be completed with the standard ESD technique in all attempts (Figure 3). Furthermore, a thin diagnostic gastroscope used in the balloon overtube-guided group could be more smoothly inserted into the submucosal tissue plane following a minimal mucosal isolation of the surgical margin. Once the cap fitted tip of the endoscope was inserted into the submucosal space thus created, electrosurgical dissection of the submucosa, the most error prone procedural process in ESD, could be safely performed with a clear visualization of the working field by deflecting the overlying isolated mucosa from the dissection plane. Although the single balloon-guided technique seems to be a promising approach to reduce the technical challenges of colorectal ESD, some important questions still remain unanswered. Two lesions, one located in the ascending colon and the other in the transverse colon were still difficult to access even with use of the balloon overtube. Both lesions were eventually treated with a colonoscope in a piecemeal fashion. In order to conclusively demonstrate that use of the balloon overtube can reduce technical difficulties in colorectal ESD, this novel approach should be directly compared with the standard ESD techniques using a colonoscope. Additionally, development of an overtube specially designed for the colorectal ESD of larger diameter to enable passage of a colonoscope could potentially reduce procedural difficulties and operation time further.
In conclusion, our preliminary experience suggests that the combined use of the balloon overtube and a thin diagnostic gastroscope is an effective and useful platform for colorectal ESD, especially in cases with difficult to access target lesions. Further studies are needed in which this novel technique is compared to the existing ESD techniques.
Endoscopic submucosal dissection (ESD) is technically more challenging for colorectal lesions than other locations in the gastrointestinal tract due to the anatomic characteristics of the colon and difficulties establishing stabilized manipulation of a long colonoscope.
Use of a balloon overtube in enteroscopy provides optimal traction on the intestinal wall thereby facilitating scope intubation. This balloon overtube-guided approach has facilitated various interventions in the small intestine including biopsy, hemostasis and most recently polypectomy and endoscopic mucosal resection (EMR). Based on these results in the small intestine, the authors postulated that the balloon overtube could form an ideal platform for colorectal ESD. In this study, the authors reviewed the results of the balloon overtube-guided colorectal ESD technique.
Colorectal ESD for the treatment of large superficial colorectal tumors is technically feasible, can improve en bloc resection rates, and is also less invasive compared to surgical treatment. However, colorectal ESD is technically more difficult and carries a higher risk of perforation than ESD at other levels of the gastrointestinal tract. Use of a balloon overtube improved access to the lesion and scope manipulation during colorectal ESD by shortening and straightening the access.
If it were possible to overcome the major technical difficulties associated with colorectal ESD, the authors believe colorectal ESD could be an appropriate therapeutic option for colonic lesions that are difficult to remove en bloc, and this approach may be better accepted in Western countries with a higher incidence of colonic polyps and cancers.
ESD has evolved to become one of the therapeutic options for treatment of early stage gastric cancers in Asian countries. In Japan, ESD has been increasingly applied to various levels of the gastrointestinal tract and results of initial experiences in a few high volume endoscopy centers have demonstrated the technical feasibility of this unique and aggressive approach, even in the colon.
ESD for colorectal tumors is not generally recommended because of the technical difficulties and complications, including perforation. The authors performed ESD in 45 cases using gastroscope with a low perforation rate.
| 1. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. |
| 2. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. |
| 3. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973. |
| 4. | Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol. 2008;43:641-651. |
| 5. | Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592-1597. |
| 6. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. |
| 7. | Toyanaga T, Man-I M, Ivanov D, Sanuki T, Morita Y, Kutsumi H, Inokuchi H, Azuma T. The results and limitations of endoscopic submucosal dissection for colorectal tumors. Acta Chir Iugosl. 2008;55:17-23. |
| 8. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. |
| 9. | Sunada K, Yamamoto H. Double-balloon endoscopy: past, present, and future. J Gastroenterol. 2009;44:1-12. |
| 10. | Kawamura T, Yasuda K, Tanaka K, Uno K, Ueda M, Sanada K, Nakajima M. Clinical evaluation of a newly developed single-balloon enteroscope. Gastrointest Endosc. 2008;68:1112-1116. |
| 11. | Pasha SF, Harrison ME, Das A, Corrado CM, Arnell KN, Leighton JA. Utility of double-balloon colonoscopy for completion of colon examination after incomplete colonoscopy with conventional colonoscope. Gastrointest Endosc. 2007;65:848-853. |
| 12. | Kita H, Yamamoto H. New indications of double balloon endoscopy. Gastrointest Endosc. 2007;66:S57-S59. |
| 13. | Das A. Future perspective of double balloon endoscopy: newer indications. Gastrointest Endosc. 2007;66:S51-S53. |
| 14. | Sumiyama K, Gostout CJ. Novel techniques and instrumentation for EMR, ESD, and full-thickness endoscopic luminal resection. Gastrointest Endosc Clin N Am. 2007;17:471-485, v-vi. |
| 15. | Antillon MR, Bartalos CR, Miller ML, Diaz-Arias AA, Ibdah JA, Marshall JB. En bloc endoscopic submucosal dissection of a 14-cm laterally spreading adenoma of the rectum with involvement to the anal canal: expanding the frontiers of endoscopic surgery (with video). Gastrointest Endosc. 2008;67:332-337. |
Peer reviewer: Dr. Ahmet Tekin, Department of General Surgery, IMC Hospital, Istiklal Cad no: 198, 33100, Mersin, Turkey
S- Editor Wang YR L- Editor Webster JR E- Editor Lin YP