Published online Dec 28, 2009. doi: 10.3748/wjg.15.6061
Revised: October 14, 2009
Accepted: October 21, 2009
Published online: December 28, 2009
AIM: To analyze the impact of the GNAS1 T393C polymorphism on prognosis and histopathology of gastric cancer.
METHODS: Genomic DNA was extracted from paraffin-embedded tissues of 122 patients with primary gastric carcinoma and from the blood of 820 healthy white individuals. Allelic discrimination was performed by quantitative real-time polymerase chain reaction. Genotyping was correlated with histopathologic parameters and with overall survival according to the Kaplan-Meier approach and with multivariate analysis by multiple stepwise regression.
RESULTS: Thirty-nine (32%) patients displayed a CC genotype, 57 (46.7%) a CT genotype and 26 (21.3%) a TT genotype. The frequency of the C allele (fC) in the patient group was 0.55, which was not significantly different from that of healthy blood donors. The distribution was compatible with the Hardy-Weinberg equilibrium. Analysis of clinicopathological parameters did not show any significant correlation of the T393C genotype with gender (P = 0.50), differentiation (P = 0.29), pT-category (P = 0.19), pN-category (P = 0.30), pM-category (P = 0.25), R-category (P = 0.95), the classifications according to WHO (P = 0.34), Laurén (P = 0.16), Goseki (P = 1.00) and Ming (P = 0.74). Dichotomization between C+ (CC+CT) and C-genotypes (TT), however, revealed significantly more advanced tumor stages (P = 0.023) and lower survival rates (P = 0.043) for C allele carriers.
CONCLUSION: The present study provides strong evidence to suggest that the GNAS1 T393C allele carrier status influences tumor progression and survival in gastric cancer with higher tumor stages and a worse outcome for C allele carriers.
-
Citation: Alakus H, Mönig SP, Warnecke-Eberz U, Alakus G, Winde G, Drebber U, Schmitz KJ, Schmid KW, Riemann K, Siffert W, Bollschweiler E, Hölscher AH, Metzger R. Association of the
GNAS1 T393C polymorphism with tumor stage and survival in gastric cancer. World J Gastroenterol 2009; 15(48): 6061-6067 - URL: https://www.wjgnet.com/1007-9327/full/v15/i48/6061.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.6061
Gastric cancer has substantially decreased in incidence over the past decades, but it still remains one of the most common cancers in the world and the second most frequent cause of cancer-related death after lung cancer[1]. Most patients are diagnosed with advanced gastric cancer, and overall survival remains poor[2,3]. The 5-year survival rate for gastric cancer is still only at 40%[4,5].
Of particular interest are prognostic factors, as they give the basis to identify gastric cancer patients with high-risk and poor prognosis. The identification of patients with poor outcome can help to set up novel treatment strategies at the beginning of treatment and may lead to better and more individualized therapy strategies with better survival[2]. Current efforts in research are therefore focused on the detection and validation of biomarkers and genetic markers that give additional information about prognosis to classical prognostic factors such as the TNM classification. The majority of new detected markers are related to properties of the tumor itself, e.g. somatic mutations or differential expression of genes or proteins. However, difficulties in standardization of such markers often prevent their routine application in clinical practice[6].
In recent years, studies have focused on the detection of single nucleotide polymorphisms (SNPs) that have a prognostic impact in cancer. One major advantage of SNPs as prognostic markers is that they can be determined independently from the availability and quality of tumor material as they can be easily evaluated from a blood sample from individual patients.
The T393C polymorphism of the gene GNAS1 is one such polymorphism. This SNP is located in exon 5 of the gene GNAS1, which encodes the ubiquitously expressed Gαs subunit of heterotrimeric G proteins. Previous studies indicate that increased expression of Gαs enhances apoptosis[7,8] and that Gαs mRNA expression is different between T393C genotypes[9]. For various solid tumors, previous studies demonstrated that patient survival and tumor progression depended on T393C genotype[10-17].
Until now, nothing has been published about the impact of the GNAS1 T393C polymorphism on gastric cancer. Thus, the aim of the present study was to determine the influence of this polymorphism on prognosis in gastric cancer. Furthermore, we looked for possible correlations between the GNAS1 T393C polymorphism and clinicopathological parameters.
Of 159 patients, who were treated surgically between May 1996 and January 2005 for primary gastric carcinoma at the Department of General, Visceral and Cancer Surgery of the University of Cologne, 13 (8.2%) patients with a second tumor, a previous operation of the upper digestive tract or missing paraffin-embedded tissue from normal cells, and 24 (15.1%) patients with neoadjuvant treatment received before surgery were excluded. Excluded patients did not differ in age and gender from the remaining patients.
All of the included 122 patients [median age 67.6 years, range 33-87 years; 78 (63.9%) male, 44 (36.1%) female] were initially treated by operation with curative intention. Gastroscopic examination, endoscopic ultrasound and computed tomography (CT) of the chest and abdomen were performed before surgery on all patients for clinical staging.
One hundred and six (86.9%) of the 122 patients underwent a gastrectomy with D2-lymphadenectomy (compartment I and II) and in 16 (13.1%) cases, a subtotal gastrectomy with D2-lymphadenectomy was performed. The median number of resected lymph nodes was 36.0 (range 15-80).
The present study was performed according to the guidelines of the local Research Ethics Commission.
The specimens were removed en bloc and the lymph nodes of the specimens were dissected with the cooperation of surgeons and pathologists according to a standardized protocol. The resected specimens were routinely fixed in 5% phosphate-buffered formalin and embedded in paraffin. Histopathologic examination of all resected specimens consisted of a thorough and standardized evaluation of the tumor stage, residual tumour (R) category, grading and the number of resected and infiltrated lymph nodes. The gastric lymph nodes were documented according to the classification of the Japanese Research Society of Gastric Cancer (JRSGC) with lymph node groups 1 to 13[18]. The tumor localization was defined according to the International Classification of Diseases for Oncology. The lesions were further classified and graded in accordance with WHO recommendations, the Laurén-classification and tumor differentiation. Postoperative staging was performed according to the 6th edition of the TNM-classification of malignant tumors[19].
DNA was extracted from paraffin-embedded tissues from resection boundaries containing exclusively normal cells using a DNA extraction kit (QIAamp, Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Genotyping was performed in 96-well plates by 5’nuclease assay (TaqMan) using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Darmstadt, Germany).
The pre-developed TaqMan assay ID C_9901536_10 (Applied Biosystems, Darmstadt, Germany) was used for genotyping of GNAS1 T393C polymorphism (dbSNP rs7121). Polymerase chain reaction (PCR) reactions contained 10 ng DNA, 200 μmol/L dNTPs and 900 nmol/L primers (Figure 1).
PCR conditions were: 95°C for 10 min followed by 40 cycles of 15 s at 92°C and 60 s at 60°C.
The Caucasian control sample consisted of 820 healthy white individuals who were recruited at the local Department for Transfusion Medicine, University Hospital, Essen. All samples were collected at random from subjects donating blood. The details of this sample have been published previously[12].
Associations between T393C genotype and clinicopathological parameters were evaluated using the χ2 test. Pearson’s χ2 was used for Hardy-Weinberg analysis and to examine differences in allele frequencies between our patient group and the reference group. Relations to overall survival were evaluated with univariate analysis according to the Kaplan-Meier approach using the log-rank test to assess statistical differences between groups. Prognostic factors were determined by multiple stepwise regression analysis using the Cox model. Only potential prognostic factors were included in the multivariate analysis. The level of significance was set at P < 0.05 and P values were for 2-sided testing. All statistical tests were performed using the Software Package SPSS for Windows, Version 17.0 (Chicago, IL, USA).
Thirty-nine (32.0%) patients displayed a CC genotype, 57 (46.7%) a CT genotype and 26 (21.3%) a TT genotype. The frequency of the C allele (fC) in the entire patient group was 0.55, which is not significantly different from that of healthy blood donors (Table 1). The distribution was compatible with the Hardy-Weinberg equilibrium.
| T393C | Patients (n = 122) | Reference group (n = 820) | χ2 | P | Odds ratio | 95% CI | ||||
| Allele | C 135 (55.3) | T 109 (44.7) | C 873 (53.2) | T 767 (46.7) | 0.38 | 0.54 | 0.92 | 0.70-1.20 | ||
| Genotype | CC 39 (32.0) | CT 57 (46.7) | TT 26 (21.3) | CC 235 (28.7) | CT 403 (49.1) | TT 182 (22.2) | 0.37 | 0.54 | 0.92 | |
Clinicopathological characteristics of the whole patient group with genotype distribution are displayed in Table 2. Thirty (24.6%) patients showed an early gastric carcinoma (pT1). In 73 (59.8%) cases, lymph node metastasis (pN+) was detected. An M1 category was found in 23 (18.9%) patients with localized peritoneal carcinosis, distant lymph node metastasis (M1 lymph) or single liver metastasis (M1 Hep). Patients with diffuse peritoneal or multiple liver metastasis had been treated non-surgically and were excluded from the study.
| All | T393C genotypes | P | |||
| CC | CT | TT | |||
| n (%) | 122 (100) | 39 (32) | 57 (46.7) | 26 (21.3) | |
| Gender | |||||
| Male | 78 (63.9) | 25 (32.1) | 34 (43.6) | 19 (24.4) | |
| Female | 44 (36.1) | 14 (31.8) | 23 (52.3) | 7 (15.9) | 0.274 |
| WHO | |||||
| Papillary/Tubular/Mucinous | 76 (62.3) | 23 (30.3) | 34 (44.7) | 19 (25) | |
| Signet-ring cancer | 38 (31.1) | 12 (31.6) | 19 (50) | 7 (18.4) | |
| Other | 8 (6.6) | 4 (50) | 4 (50) | 0 | 0.340 |
| Differentiation | |||||
| Well/Moderate (G1-G2) | 42 (34.4) | 12 (28.6) | 22 (52.4) | 8 (19) | |
| Poor (G3-G4) | 80 (65.6) | 27 (33.8) | 35 (43.8) | 18 (22.5) | 0.805 |
| Laurén | |||||
| Intestinal | 52 (42.6) | 16 (30.8) | 25 (48.1) | 11 (21.2) | |
| Diffuse | 55 (45.1) | 17 (30.9) | 29 (52.7) | 9 (16.4) | |
| Mixed | 15 (12.3) | 6 (40) | 3 (20) | 6 (40) | 0.171 |
| Ming | |||||
| Expanding | 47 (38.5) | 14 (29.8) | 24 (51.1) | 9 (19.1) | |
| Infiltrative | 75 (61.5) | 25 (33.3) | 33 (44) | 17 (22.7) | 0.620 |
| pT-category | |||||
| T1 | 30 (24.6) | 7 (23.3) | 13 (43.3) | 10 (33.3) | |
| T2 | 44 (36.1) | 12 (27.3) | 22 (50) | 10 (22.7) | |
| T3 | 38 (31.1) | 14 (36.8) | 18 (47.4) | 6 (15.8) | |
| T4 | 10 (8.2) | 6 (60) | 4 (40) | 0 | 0.110 |
| pN-category | |||||
| N0 | 49 (40.2) | 11 (22.4) | 24 (49) | 14 (28.6) | |
| N1 | 34 (27.9) | 13 (38.2) | 13 (38.2) | 8 (23.5) | |
| N2 | 14 (11.5) | 6 (42.9) | 6 (42.9) | 2 (14.3) | |
| N3 | 25 (20.5) | 9 (36) | 14 (56) | 2 (8) | 0.196 |
| pM-category | |||||
| M0 | 99 (81.1) | 30 (30.3) | 45 (45.5) | 24 (24.2) | |
| M1 | 23 (18.9) | 9 (39.1) | 12 (52.2) | 2 (8.7) | 0.101 |
| R-category | |||||
| R0 | 118 (96.7) | 38 (32.5) | 54 (46.2) | 25 (21.4) | |
| R1/R2 | 4 (3.3) | 1 (25) | 2 (50) | 1 (25) | 0.950 |
| UICC stage | |||||
| Ia | 26 (21.3) | 5 (19.2) | 11 (42.3) | 10 (38.5) | |
| Ib | 22 (18) | 7 (31.8) | 12 (54.5) | 3 (13.6) | |
| II | 18 (14.8) | 4 (22.2) | 7 (38.9) | 7 (38.9) | |
| IIIa | 11 (9) | 4 (36.4) | 5 (45.5) | 2 (18.2) | |
| IIIb | 4 (3.3) | 2 (50) | 2 (50) | 0 | |
| IV | 41 (33.6) | 17 (41.5) | 20 (48.8) | 4 (9.8) | 0.023 |
Analysis of clinicopathological parameters did not show any significant correlation of the T393C genotype with gender (P = 0.50), differentiation (P = 0.29), pT-category (P = 0.19), pN-category (P = 0.30), pM-category (P = 0.25), R-category (P = 0.95), or the classifications according to WHO (P = 0.34), Laurén (P = 0.16), Goseki (P = 1.0), Ming (P = 0.74) and UICC (P = 0.15).
When genotypes were dichotomized in C+ (CC+CT) and C-genotypes (TT), a significantly higher rate of advanced tumor stages (stage III and IV), according to the UICC classification, was seen for C allele carriers (P = 0.023). Only 6 (23.1%) of 26 patients with TT genotype were diagnosed with a tumor stage of III or IV. In contrast, an advanced tumor stage was detected in 50 (52.1%) of 96 C allele carriers.
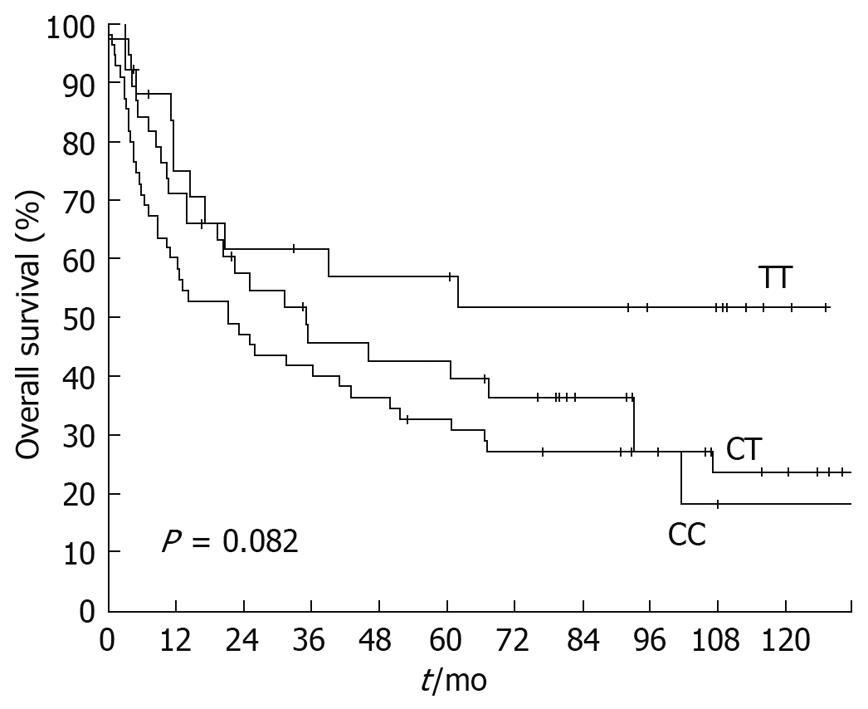
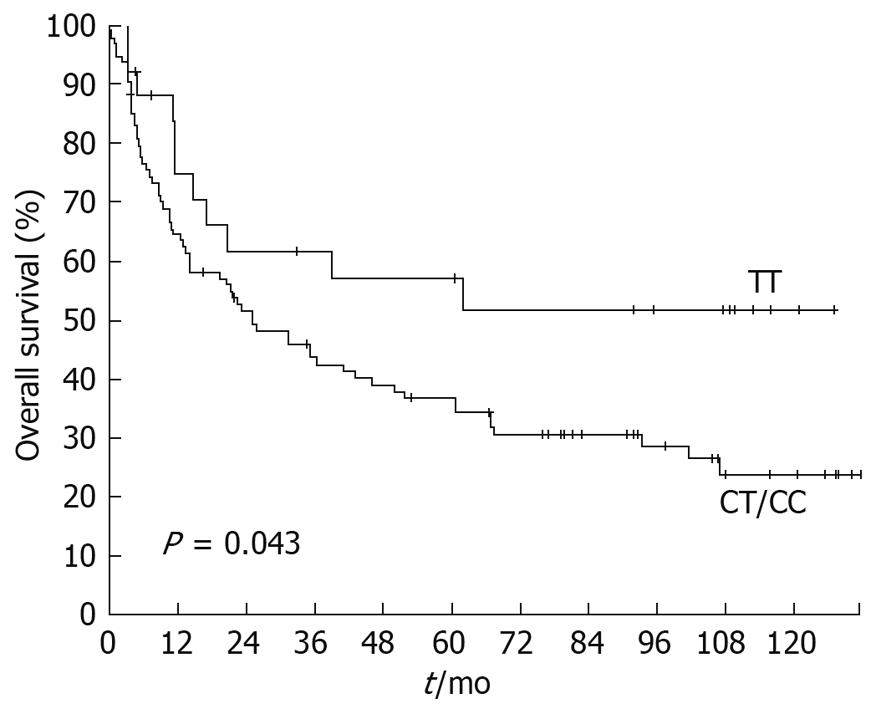
Overall survival dependent on T393C genotypes is displayed in Figure 2. The 5-year survival rate for patients with a TT genotype was 56.9% (SE ± 10.4%), followed by patients with CC genotype with a 5-year survival rate of 42.6% (SE ± 8.3%). Heterozygous CT patients showed a 5-year survival rate of 32.7% (SE ± 6.3%). Survival was not significantly associated with the T393C genotype when the three genotypes were compared (P = 0.082). However, dichotomization between C+ (CC+CT) and TT demonstrated a significantly (P = 0.043) lower survival rate for C allele carriers (Figure 3) with a 5-year survival rate for the C+ group of only 36.7% (SE ± 5.1%) vs 56.9% (SE ± 10.4%) for the TT group.
In the multivariate Cox regression analysis, known prognostic factors for gastric cancer (pT, pN, pM and R-category) and T393C genotype with dichotomization between C+ (CC+CT) and TT were included. pT-category (P < 0.001), R-category (P = 0.022) and pM-category (P = 0.027) maintained their prognostic independence (Table 3). pN-category (P = 0.55), and the T393C genotype (P = 0.33) lost their prognostic independence.
| Covariate | n | Univariate analysis | Multivariate analysis | ||||
| P value | 5-yr-SR (%) | SE (±%) | P value | HR | 95% CI | ||
| pT-category | < 0.001 | < 0.001 | |||||
| pT1 | 30 | 85.4 | 6.8 | 1 | |||
| pT2 | 44 | 44.5 | 7.8 | < 0.001 | 6.212 | 2.31-16.70 | |
| pT3 | 38 | 5.4 | 3.7 | < 0.001 | 13.026 | 4.44-38.23 | |
| pT4 | 10 | 33.3 | 15.7 | 0.001 | 7.838 | 2.24-27.46 | |
| pN-category | < 0.001 | 0.549 | |||||
| pN0 | 49 | 61.6 | 7.4 | 1 | |||
| pN1 | 34 | 47.1 | 8.6 | 0.226 | 0.663 | 0.34-1.29 | |
| pN2 | 14 | 16.9 | 10.9 | 0.986 | 0.993 | 0.43-2.27 | |
| pN3 | 25 | 8.0 | 5.4 | 0.814 | 0.905 | 0.40-2.07 | |
| T393C SNP | 0.043 | 0.333 | |||||
| CC/CT | 96 | 36.7 | 5.1 | 1 | |||
| TT | 26 | 56.9 | 10.4 | 0.712 | 0.36-1.42 | ||
| pM-category | < 0.001 | 0.027 | |||||
| M0 | 99 | 48.1 | 5.2 | 1 | |||
| M1 | 23 | 9.2 | 6.2 | 2.087 | 1.09-4.01 | ||
| R-category | < 0.001 | 0.022 | |||||
| R0 | 118 | 42.3 | 4.7 | 1 | |||
| R+ | 4 | 0 | 0 | 3.128 | 1.18-8.27 | ||
Gastric cancer is the fourth most common cancer with approximately 800 000 new cases per year and the second leading cause of cancer-related death worldwide[20]. Many patients have advanced disease at the time of diagnosis, resulting in poor prognosis and high mortality[2,21,22]. Pretreatment staging of the disease is of high importance as it provides the basis for selecting the most appropriate therapeutic strategy[23]. Based on the preoperative staging, patients with early stage tumors are treated by endoscopic mucosal resection, while patients with advanced tumors are treated by partial or total gastrectomy[5]. Accurate staging is also the basis for selecting patients for neoadjuvant, adjuvant or palliative treatment[24]. By identifying patients with poor outcome, novel treatment strategies could be set up at the beginning of treatment which can lead to better and more individualized therapy strategies with superior survival[2].
The present study demonstrated that besides the known prognostic factors pT, pM, pN and R-category, the T393C polymorphism was also a significant prognostic factor in the univariate analysis with a survival benefit for homozygous TT patients. In addition, it demonstrated that compared to C allele carriers, homozygous TT patients were diagnosed with significantly less advanced tumor stages according to UICC, which is possibly the main reason why the T393C genotype lost its independence in the multivariate analysis.
The gene GNAS1 is mapped to chromosome 20q13 and consists of 13 exons. Somatic activating mutations of GNAS1 have been implicated in the etiology of McCune Albright Syndrome[25] and sporadic, isolated endocrine tumors[26,27] which supports a role of GNAS1 in tumor initiation and progression.
Recent studies have shown that genotypes of the T393C polymorphism are significantly associated with survival of patients suffering from colorectal cancer, bladder cancer, clear cell renal carcinoma, intrahepatic cholangiocarcinoma, invasive breast carcinoma and squamous cell carcinoma of the larynx, oropharynx and hypopharynx (Table 4)[10-14].
| Cancer type | Yr | n | Effect | Benefit (survival) |
| Gastric cancer | 2009 | 122 | The present study demonstrates a significant survival benefit for the TT genotype with a 5-yr-survival rate of 56.9% vs the CC/CT group with a 5-yr-survival rate of only 36.7% (P = 0.043) | TT-genotype |
| Squamous cell cancer of larynx[15] | 2008 | 157 | Survival was significantly dependent on the T393C genotype in advanced American Joint Committee on Cancer (AJCC) stages (III-IV) with higher 5-yr survival rates for TT, followed by TC and CC (P = 0.0437) | TT-genotype |
| Oro- and hypo-pharyngeal squamous cell carcinoma[16] | 2008 | 202 | C homozygous patients displayed a higher risk for disease progression than T homozygous patients (P = 0.019) and a higher risk for death (P = 0.015). In multivariate analysis, besides cancer stage and tumor localization, the T393C polymorphism was an independent prognostic factor for disease progression and death | TT-genotype |
| Clear cell renal cell carcinoma[11] | 2006 | 150 | Tumor progression, development of metastasis and tumor-related death was significantly associated with the T393C polymorphism. In multivariate analysis CC patients were at highest risk for progression or tumor-related death compared with T-allele carriers (P = 0.018) | TT-genotype |
| Chronic lymphocytic leukemia[17] | 2006 | 144 | Median progression-free survival was significantly higher for T-allele carriers (P = 0.007). In multivariate analysis, the T393C polymorphism kept its prognostic independence (P = 0.01) besides of ZAP-70 (P = 0.005) and Binet stage (P < 0.001). Regarding overall survival, CC genotypes were significantly at highest risk for death compared to T-alleles both in univariate (P < 0.001) and multivariate analysis (P = 0.002) | TT-genotype |
| Bladder cancer[10] | 2005 | 254 | Progression-free survival (P = 0.011), metastasis-free survival (P = 0.001) and cancer-specific survival (P = 0.014) were significantly increased in TT genotypes compared with CC genotypes. In multivariate analysis, the T393C polymorphism kept its prognostic independence | TT-genotype |
| Sporadic colorectal cancer[12] | 2005 | 151 | In UICC stages I to II, the 5-yr survival rate was significantly (P = 0.009) higher in TT genotypes (88%) compared with TC (71%) and CC genotypes (50%). In multivariate analysis, the T393C polymorphism was also an independent prognostic factor. No significant effect could be seen for UICC stages III to IV | TT-genotype |
| Cholangio-carcinoma[14] | 2007 | 87 | Disease-specific overall survival was significantly dependent on the T393C genotype (P = 0.02), with TT genotypes showing reduced survival compared to patients carrying at least one C allele. In multivariate analysis (TT/C+) the T393C genotype kept its prognostic independence (P = 0.04) | CC-genotype |
| Breast carcinoma[13] | 2007 | 279 | Overall survival was significantly (P = 0.033) associated with the T393C polymorphism with lowest survival rates for the TT-genotype and highest survival rate for the CC-genotype. In multivariate analysis, the TT-genotype still had a significant survival benefit compared to the CC genotype (P = 0.045) | CC-genotype |
| Esophageal cancer[28] | 2009 | 51 | T393C polymorphism was significantly associated with tumor response to Cisplatin/5-FU-based radiochemotherapy. 63% of the T allele carriers had a minor histopathologic response (MiHR) with more than 10% residual vital tumor cells in resection specimens. For the CC genotype MiHR was seen only in 20%. In binary logistic regression analysis, the T393C genotype kept its independence (P < 0.05) | CC-genotype |
Comparable to previous results in bladder cancer, clear cell renal carcinoma and colorectal cancer, the present study also demonstrated significantly higher survival rates for TT genotypes in gastric cancer (Figure 3). Patients with the TT genotype showed a 5-year survival rate of 57%, whereas the 5-year survival rate for C allele carriers was only at 37%.
In contrast to our findings in gastric cancer and previous findings in the above-mentioned tumor types, an unfavourable clinical course for T allele carriers has been described in studies of invasive breast cancer and intrahepatic cholangiocarcinoma, suggesting that the biological effect of the T393C polymorphism may be different in different tumor types. In a recent study, we demonstrated that this polymorphism is a predictive molecular marker for tumor response to cisplatin/5-FU-based radiochemotherapy in esophageal cancer, with CC genotypes mostly showing a major response[28].
In vitro experiments suggest that expression of Gαs is associated with enhanced apoptosis[7,8]. The second messenger, cyclic AMP, which is generated by activated Gαs, seems to play a major role in this proapoptotic process. An increased concentration of the intracellular second messenger, cyclic AMP promotes apoptosis in several cell types including leukemic cells[29], ovarian cancer cells[30], and lymphoma cells[25]. Increased Gαs expression in tissues of patients with TT genotypes may therefore confer enhanced apoptosis in 393T allele carriers. Hypothetically, this mechanism may contribute to the described more favorable clinical course and the less advanced tumor stages of homozygous TT patients. This hypothesis remains to be supported by additional functional studies which were beyond the scope of the present study. The T393C polymorphism as a risk factor for gastric cancer could not be established in the present study.
In conclusion, this study demonstrated for the first time that in primary gastric cancer, homozygous GNAS1 393T patients have less advanced tumor stages and higher survival rates than C allele carriers. These findings further support the concept of a general role for the GNAS1 T393C polymorphism in tumor progression.
Identification of gastric cancer patients with poor outcome can help to set up novel treatment strategies at the beginning of treatment and may lead to better and more individualized therapy strategies with better survival. In recent years, studies have focused on the detection of single nucleotide polymorphisms (SNPs) as prognostic molecular markers in cancer.
The GNAS1 T393C polymorphism is located in exon 5 of the gene GNAS1. In this study the authors describe, for the first time, the impact of this SNP in gastric cancer. The study demonstrates that the GNAS1 T393C polymorphism affects tumor stage and prognosis in gastric cancer.
For various solid tumors, previous studies have demonstrated that patient survival and tumor progression depend on the GNAS1 T393C genotype. In the present study, the authors have described for the first time that the GNAS1 T393C polymorphism affects tumor stage and prognosis in gastric cancer.
The GNAS1 T393C polymorphism will contribute to identifying high-risk patients with gastric cancer and might help to establish a more individualized treatment strategy for gastric cancer.
A single-nucleotide polymorphism (SNP) is a DNA sequence variation occurring when a single nucleotide - A, T, C, or G - in the genome differs between members of a species. The GNAS1 T393C is located in exon 5 of the gene GNAS1. For several cancer types, studies have demonstrated that patient survival is affected by this SNP.
Overall, this paper provides information on GNAS1 T393C allele carrier status which influences tumor progression and survival in gastric cancer, with higher tumor stages and worse outcome for C allele carriers.
| 1. | Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33-64, 1. |
| 2. | Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449-461. |
| 3. | Sánchez-Bueno F, Garcia-Marcilla JA, Perez-Flores D, Pérez-Abad JM, Vicente R, Aranda F, Ramirez P, Parrilla P. Prognostic factors in a series of 297 patients with gastric adenocarcinoma undergoing surgical resection. Br J Surg. 1998;85:255-260. |
| 4. | Bollschweiler E, Breckheimer S, Mönig SP, Hölscher AH. [The prognostic relevance of age and comorbidity in patients with resected gastric cancer]. Zentralbl Chir. 2009;134:71-76. |
| 5. | Brennan MF. Current status of surgery for gastric cancer: a review. Gastric Cancer. 2005;8:64-70. |
| 6. | Yasui W, Oue N, Aung PP, Matsumura S, Shutoh M, Nakayama H. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86-94. |
| 7. | Krumins AM, Barber R. Examination of the effects of increasing Gs protein on beta2-adrenergic receptor, Gs, and adenylyl cyclase interactions. Biochem Pharmacol. 1997;54:61-72. |
| 8. | Yang X, Lee FY Sr, Wand GS. Increased expression of Gs(alpha) enhances activation of the adenylyl cyclase signal transduction cascade. Mol Endocrinol. 1997;11:1053-1061. |
| 9. | Yan L, Herrmann V, Hofer JK, Insel PA. beta-adrenergic receptor/cAMP-mediated signaling and apoptosis of S49 lymphoma cells. Am J Physiol Cell Physiol. 2000;279:C1665-C1674. |
| 10. | Frey UH, Eisenhardt A, Lümmen G, Rübben H, Jöckel KH, Schmid KW, Siffert W. The T393C polymorphism of the G alpha s gene (GNAS1) is a novel prognostic marker in bladder cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:871-877. |
| 11. | Frey UH, Lümmen G, Jäger T, Jöckel KH, Schmid KW, Rübben H, Müller N, Siffert W, Eisenhardt A. The GNAS1 T393C polymorphism predicts survival in patients with clear cell renal cell carcinoma. Clin Cancer Res. 2006;12:759-763. |
| 12. | Frey UH, Alakus H, Wohlschlaeger J, Schmitz KJ, Winde G, van Calker HG, Jöckel KH, Siffert W, Schmid KW. GNAS1 T393C polymorphism and survival in patients with sporadic colorectal cancer. Clin Cancer Res. 2005;11:5071-2077. |
| 13. | Otterbach F, Callies R, Frey UH, Schmitz KJ, Wreczycki C, Kimmig R, Siffert W, Schmid KW. The T393C polymorphism in the gene GNAS1 of G protein is associated with survival of patients with invasive breast carcinoma. Breast Cancer Res Treat. 2007;105:311-317. |
| 14. | Schmitz KJ, Lang H, Frey UH, Sotiropoulos GC, Wohlschlaeger J, Reis H, Takeda A, Siffert W, Schmid KW, Baba HA. GNAS1 T393C polymorphism is associated with clinical course in patients with intrahepatic cholangiocarcinoma. Neoplasia. 2007;9:159-165. |
| 15. | Lehnerdt GF, Franz P, Winterhoff S, Bankfalvi A, Grehl S, Lang S, Schmid KW, Siffert W, Jahnke K, Frey UH. The GNAS1 T393C polymorphism predicts survival in patients with advanced squamous cell carcinoma of the larynx. Laryngoscope. 2008;118:2172-2176. |
| 16. | Lehnerdt GF, Franz P, Zaqoul A, Schmitz KJ, Grehl S, Lang S, Schmid KW, Siffert W, Jahnke K, Frey UH. Overall and relapse-free survival in oropharyngeal and hypopharyngeal squamous cell carcinoma are associated with genotypes of T393C polymorphism of the GNAS1 gene. Clin Cancer Res. 2008;14:1753-1758. |
| 17. | Frey UH, Nückel H, Sellmann L, Siemer D, Küppers R, Dürig J, Dührsen U, Siffert W. The GNAS1 T393C polymorphism is associated with disease progression and survival in chronic lymphocytic leukemia. Clin Cancer Res. 2006;12:5686-5692. |
| 18. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10-24. |
| 19. | Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94:2511-2516. |
| 20. | Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107-2116. |
| 21. | Hansson LE, Sparén P, Nyrén O. Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230:162-169. |
| 22. | Kim JP, Kwon OJ, Oh ST, Yang HK. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann Surg. 1992;216:269-278; discussion 278-279. |
| 23. | Fleming ID, Cooper JS, Henson DE, Hutter RP, Kennedy BJ, Murphy GP, O'Sullivan B, Sobin LH, Yarbro JW. Manual for staging of cancer. American Joint Committee on Cancer (AJCC). 5th ed. Philadelphia, PA: Lippincott-Raven 1997; 71-76. |
| 24. | Menges M. [Diagnosis, staging and therapy of gastric cancer]. Z Gastroenterol. 2004;42:767-773. |
| 25. | Yan L, Herrmann V, Hofer JK, Insel PA. beta-adrenergic receptor/cAMP-mediated signaling and apoptosis of S49 lymphoma cells. Am J Physiol Cell Physiol. 2000;279:C1665-C1674. |
| 26. | Collins MT, Sarlis NJ, Merino MJ, Monroe J, Crawford SE, Krakoff JA, Guthrie LC, Bonat S, Robey PG, Shenker A. Thyroid carcinoma in the McCune-Albright syndrome: contributory role of activating Gs alpha mutations. J Clin Endocrinol Metab. 2003;88:4413-4417. |
| 27. | Lyons J, Landis CA, Harsh G, Vallar L, Grünewald K, Feichtinger H, Duh QY, Clark OH, Kawasaki E, Bourne HR. Two G protein oncogenes in human endocrine tumors. Science. 1990;249:655-659. |
| 28. | Alakus H, Warnecke-Eberz U, Bollschweiler E, Mönig SP, Vallböhmer D, Brabender J, Drebber U, Baldus SE, Riemann K, Siffert W. GNAS1 T393C polymorphism is associated with histopathological response to neoadjuvant radiochemotherapy in esophageal cancer. Pharmacogenomics J. 2009;9:202-207. |
| 29. | Myklebust JH, Josefsen D, Blomhoff HK, Levy FO, Naderi S, Reed JC, Smeland EB. Activation of the cAMP signaling pathway increases apoptosis in human B-precursor cells and is associated with downregulation of Mcl-1 expression. J Cell Physiol. 1999;180:71-80. |
| 30. | Srivastava RK, Srivastave AR, Cho-Chung YS. Synergistic effects of 8-Cl-cAMP and retinoic acids in the inhibition of growth and induction of apoptosis in ovarian cancer cells: induction of retinoic acid receptor beta. Mol Cell Biochem. 2000;204:1-9. |
Peer reviewer: You-Yong Lu, Professor, Beijing Molecular Oncology Laboratory, Peking University School of Oncology and Beijing Institute for Cancer Reaearch, #52, Fucheng Road, Haidian District, Beijing 100036, China
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP