Published online Dec 21, 2009. doi: 10.3748/wjg.15.5983
Revised: October 27, 2009
Accepted: November 3, 2009
Published online: December 21, 2009
AIM: To compare neoadjuvant chemoradiotherapy and surgery with surgery alone for resectable esophageal carcinoma.
METHODS: We used MEDLINE and EMBASE databases to identify eligible studies and manual searches were done to ensure no studies were missed. Trial validity assessment was performed and a trial quality score was assigned.
RESULTS: Eleven randomized controlled trials (RCTs) including 1308 patients were selected. Neoadjuvant chemoradiotherapy significantly improved the overall survival compared with surgery alone. Odds ratio (OR) [95% confidence interval (CI), P value], expressed as neoadjuvant chemoradiotherapy and surgery vs surgery alone, was 1.28 (1.01-1.64, P = 0.05) for 1-year survival, 1.78 (1.20-2.66, P = 0.004) for 3-year survival, and 1.46 (1.07-1.99, P = 0.02) for 5-year survival. Postoperative mortality increased in patients treated by neoadjuvant chemoradiotherapy (OR: 1.68, 95% CI: 1.03-2.73, P = 0.04), but incidence of postoperative complications was similar in two groups (OR: 1.14, 95% CI: 0.88-1.49, P = 0.32). Neoadjuvant chemoradiotherapy lowered the local-regional cancer recurrence (OR: 0.64, 95% CI: 0.41-0.99, P = 0.04), but incidence of distant cancer recurrence was similar (OR: 0.94, 95% CI: 0.68-1.31, P = 0.73). Histological subgroup analysis indicated that esophageal squamous cell carcinoma did not benefit from neoadjuvant chemoradiotherapy, OR (95% CI, P value) was 1.16 (0.85-1.57, P = 0.34) for 1-year survival, 1.34 (0.98-1.82, P = 0.07) for 3-year survival and 1.41 (0.98-2.02, P = 0.06) for 5-year survival.
CONCLUSION: Neoadjuvant chemoradiotherapy can raise the survival rate of patients with esophageal adenocarcinoma.
- Citation: Jin HL, Zhu H, Ling TS, Zhang HJ, Shi RH. Neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: A meta-analysis. World J Gastroenterol 2009; 15(47): 5983-5991
- URL: https://www.wjgnet.com/1007-9327/full/v15/i47/5983.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5983
Esophageal carcinoma (EC) is the sixth commonest cause of tumor-related death around the world[1]. It is endemic in Asia, southern and eastern Africa, and northern France[2,3]. North America and many western European countries are low-incidence regions, but the nearly 6-fold increase in the incidence of esophageal adenocarcinoma (EAC) in the past three decades and the corresponding 7-fold increase in mortality are remarkable. Surgery has always been considered as the standard treatment for patients with resectable esophageal cancer, but the effectiveness of surgery alone was unsatisfactory and the median survival of patients treated by surgery alone rarely exceeded 18 mo[4]. So clinicians always make efforts to seek for new treatment strategies to prolong the survival time of patients with EC. Recently neoadjuvant chemoradiotherapy plus surgery has been studied widely, but opinions vary among clinicians as to the therapeutic effect of the new method, and the outcomes of randomized controlled trials (RCTs) were not consistent. Published meta-analyses did not reach a consensus, some of which was short of enough RCTs or adopted unpublished data. The current study aims to perform a meta-analysis to compare neoadjuvant chemoradiotherapy plus surgery with surgery alone for resectable EC by enough eligible published RCTs to date.
Computerized bibliographic and manual searches were done to identify all eligible published literature between 1980 and 2008. MEDLINE and EMBASE were the primary source of RCTs, with the following key words: esophageal cancer, surgery, radiotherapy, chemotherapy, neoadjuvant chemoradiotherapy and RCT. Manual searches were performed by reviewing articles and abstracts cited in the published meta-analysis and RCTs.
The eligible studies must meet the following inclusion criteria: (1) It must be a prospective RCT which compares neoadjuvant chemoradiotherapy plus surgery with surgery alone in the initial management of resectable EC; (2) Outcomes must include survival data; (3) There was no statistical significance in factors such as sex, age, type of pathology, tumour stage between the two groups; and (4) Studies were analyzed by intention-to-treat patients. Trials were not excluded because of cancer histology (squamous cell carcinoma or adenocarcinoma) or language of publication. Unpublished reports, abstracts and theses were excluded. This meta-analysis was performed according to the QUOROM statement[5].
All data were abstracted by three independent researchers and the methodological qualities of all RCTs were assessed by three aspects: blinding, randomization and handling withdrawals and dropouts[6]. If researchers had discrepancies in assessing RCTs, a consensus was reached by discussion.
Outcomes including 1-year survival, 3-year survival, 5-year survival, postoperative mortality, incidence of postoperative complication, incidence of local-regional cancer recurrence and incidence of distant cancer recurrence were analyzed. In two trials[7,8] we used the Kaplan-Meier estimate of the 1-year survival and 3-year survival in the two groups and the data for the 5-year survival was obtained from another trial[9] in the same way. The remaining data were directly available in the corresponding RCTs. Evaluation of therapeutic effectiveness, including survival rate and incidence of recurrence, was performed in all patients who were enrolled in these trials, but for postoperative events, data were calculated only based on the number of patients who underwent surgery as the denominator. Sensitivity analyses were performed to identify the effect of histological subtype (squamous cell carcinoma or adenocarcinoma) and scheduling of neoadjuvant chemoradiotherapy (concurrent or sequential) on survival.
Data were analyzed by RevMan 4.2.10. χ2 tests were used to assess heterogeneity of study results and a planned cut-off for significance of P≤ 0.05. If P > 0.05, we used a fixed effect model, otherwise we used a random effect model. The odds ratios (OR) among the frequency of events in both neoadjuvant chemoradiotherapy plus surgery group (CRT group) and surgery alone group (S group) was calculated and these OR are presented as a point estimate with 95% confidence intervals (CI) and P values in parentheses. The significance level was set at 5%. Funnel plot analysis did not suggest publication bias against negative trials.
Eleven randomized studies were identified from 1980 to 2008 and the main features of the trials included in the meta-analysis are shown in Table 1[7-17]. All studies were published literature. Nine countries including Australia, United States of America, China, France, Ireland, Japan, Korea, Norway and Thailand were involved in the RCTs.
| Country | Year of RCT published | SCC or AC | Schedule of radiotherapy | Schedule of chemotherapy | Concurrent or sequential | Time of surgery |
| Norway | 1992 | SCC | 35 Gy | Cisplatin: 20 mg/m2 D1-5, D15-19 | Sequential | Not report |
| 1.75 Gy/d | Bleomycin: 10 mg/m2 D1-5, D15-19 | |||||
| 5 d/wk for 4 wk | ||||||
| Thailand | 1994 | SCC | 40 Gy | Cisplatin: 100 mg/m2 D1, 29 | Concurrent | 4 wk after completion of chemotherapy |
| 2 Gy/d | FU: 1000 mg/m2 D1, D29-32 | |||||
| 5 d/wk for 4 wk | ||||||
| France | 1994 | SCC | 20 Gy | Cisplatin: 100 mg/m2 D1,21 | Sequential | D42 |
| 2 Gy/d | FU: 600 mg/m2 D2-5, D22-25 | |||||
| D8-19 | ||||||
| Ireland | 1996 | AC | 40 Gy | Cisplatin: 75 mg/m2 D7 | Concurrent | 8 wk after CRT |
| 2.67 Gy/ d | FU: 15 mg/kg D1-5 | |||||
| D1-5, 8-12, 15-19 | Week 1 and week 6 | |||||
| France | 1997 | SCC | 37 Gy | Cisplatin: 80 mg/m2 D0-2 | Sequential | 2-4 wk after CRT |
| 3.7 Gy/d | ||||||
| 5 d/wk for 2 wk | ||||||
| USA | 2001 | SCC and AC | 45 Gy | Cisplatin: 20 mg/m2 D1-5, 17-21 | Concurrent | D42 |
| 1.5 Gy bid | FU: 300 mg/m2 D1-21 | |||||
| D1-5, 8-12, 15-19 | Vinblastine: 1 mg/m2 D1-4, 17-20 | |||||
| China | 2003 | SCC | 36 Gy | Cisplatin: 25 mg/m2 D2-5, D22-25 | Sequential | 3 wk after CRT |
| 3 Gy/d | FU: 1000 mg/m2 D1-5 | |||||
| D21-24, 28-31, 35-38 | 500 mg/m2 D21-25 | |||||
| Korea | 2004 | SCC | 45.6 Gy | Cisplatin: 60 mg/m2 D1, 21 | Concurrent | 3-4 wk after completion of radiotherapy |
| 1.2 Gy bid | FU: 1000 mg/m2 D2-5 | |||||
| D1-28 | ||||||
| Australia | 2005 | SCC and AC | 35 Gy | Cisplatin: 80 mg/m2 D1 | Concurrent | 3-6 wk after completion of radiotherapy |
| 2.33 Gy/d | FU: 800 mg/m2 D2-5 | |||||
| 5 d/wk for 3 wk | ||||||
| Japan | 2006 | SCC | 40 Gy | Cisplatin: 7 mg/m2 | Concurrent | 35-40 d after CRT |
| 2 Gy/d | FU: 350 mg/m2 | |||||
| 5 d/wk for 4 wk | 5 d/wk for 4-6 wk | |||||
| USA | 2008 | SCC and AC | 50.4 Gy | Cisplatin: 100 mg/m2 D1,29 | Concurrent | 3-8 wk after CRT |
| 1.8 Gy/d | FU: 1000 mg/m2 D1-4, D29-32 | |||||
| 5 d/wk for 5.5 wk |
The studies were carried out from 1983 to 2002 and the literatures were published between 1992 and 2008. Because double blinding can not be performed due to the inherent difficulty of the design of the trial (e.g. chemotherapy and radiotherapy) and the method of randomization was not reported in most trials, the RCT quality scores ranged from 1 to 3 (5-point scale) and the average was 2.3[5,6]. Of these 11 studies, seven were restricted to patients with esophageal squamous cell carcinoma (ESCC) only, one was restricted to patients with EAC only, and the remaining three trials enrolled patients with either ESCC or EAC. The 11 RCTs included 1308 patients, 659 of whom received neoadjuvant chemoradiotherapy before surgery, and the remaining 649 patients received surgery alone. Nearly all the patients in the S group underwent surgery, yet there were more patients in the CRT group who had not completed the planned treatment regimen for various causes such as side effects of chemotherapy or metastasis of cancer before surgery. The tumor stage of the most patients in the 11 studies ranged from I-III (1987 UICC), but more advanced tumor stage (IVa) was also seen in two RCTs[9,11]. In addition, tumor stages were classified in the RCT by Le Prise et al[15] according to the 1978 American Joint Committee on Cancer, which was not a TNM staging. Finally tumor stage was not reported in the RCT by Walsh et al[16].
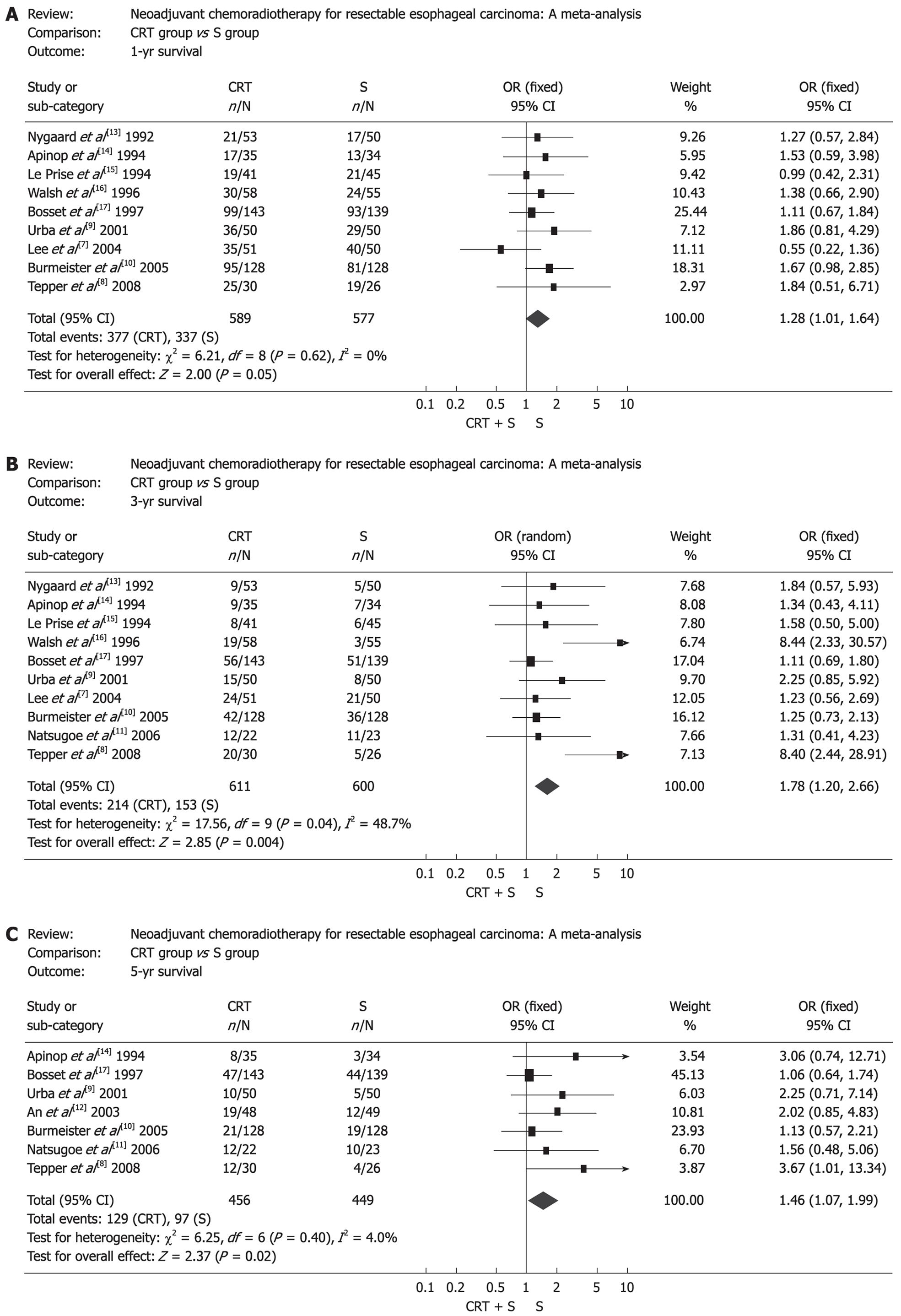
The effect of neoadjuvant chemoradiotherapy on survival rate is shown in Figure 1. Obviously, there was statistical significance in survival rate between the two groups. OR (95% CI, P value), expressed as neoadjuvant chemoradiotherapy plus surgery vs surgery alone, was 1.28 (1.01-1.64, P = 0.05) for 1-year survival, 1.78 (1.20-2.66, P = 0.004) for 3-year survival and 1.46 (1.07-1.99, P = 0.02) for 5-year survival. Subgroup analysis showed that there was no survival benefit from neoadjuvant chemoradiotherapy in EC patients when chemotherapy and radiotherapy were given sequentially. On the contrary, EC patients benefited from concurrent chemoradiotherapy. The corresponding OR (95% CI, P value) is shown in Table 2. Moreover, patients with ESCC did not get any survival benefit from neoadjuvant chemoradiotherapy and corresponding OR (95% CI, P value) is shown in Table 3. In addition, another subgroup analysis indicated that the 3-year survival in CRT group was significantly higher than that of S group in patients of the USA and Europe, but in patients of Asia, it was a pessimistic result (Table 4).
| Schedule of CRT | Overall survival | No. of studies | No. of patients | OR (95% CI) | P | |
| CRT + S | S | |||||
| Sequential | 1 yr | 3 | 237 | 234 | 1.12 (0.77, 1.64) | 0.56 |
| 3 yr | 3 | 237 | 234 | 1.24 (0.82, 1.88) | 0.31 | |
| 5 yr | 2 | 191 | 188 | 1.24 (0.81, 1.91) | 0.32 | |
| Concurrent | 1 yr | 6 | 352 | 343 | 1.41 (1.03, 1.94) | 0.03 |
| 3 yr | 7 | 374 | 366 | 2.12 (1.20, 3.76) | 0.011 | |
| 5 yr | 5 | 265 | 261 | 1.72 (1.10, 2.71) | 0.02 | |
| Overall survival | No. of studies | No. of patients | OR (95% CI) | P | |
| CRT + S | S | ||||
| 1 yr | 6 | 368 | 368 | 1.16 (0.85, 1.57) | 0.34 |
| 3 yr | 7 | 390 | 391 | 1.34 (0.98, 1.82) | 0.07 |
| 5 yr | 5 | 293 | 295 | 1.41 (0.98, 2.02) | 0.06 |
| Population | No. of studies | No. of patients | OR (95% CI) | P | |
| CRT + S | S | ||||
| USA | 2 | 80 | 76 | 3.74 (1.77, 7.88) | 0.0005 |
| Europe | 4 | 295 | 289 | 1.59 (1.09, 2.33) | 0.02 |
| Asia | 3 | 108 | 107 | 1.27 (0.72, 2.24) | 0.40 |
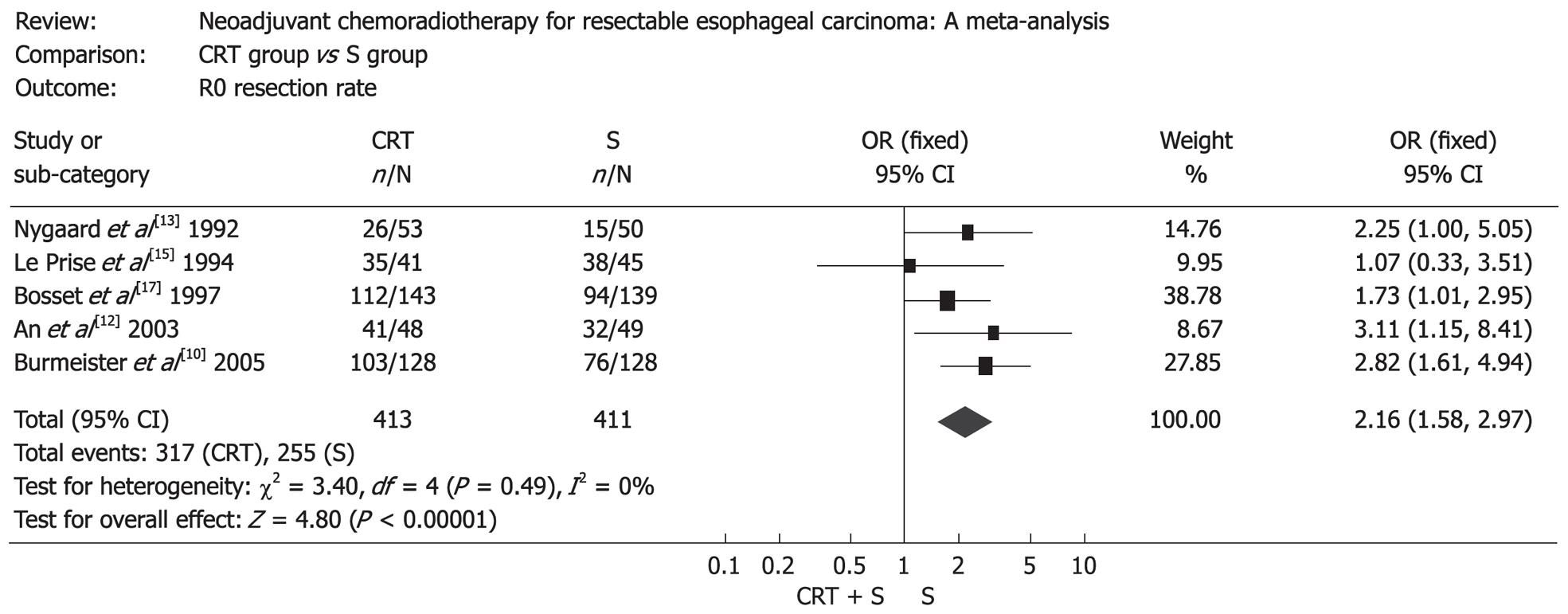
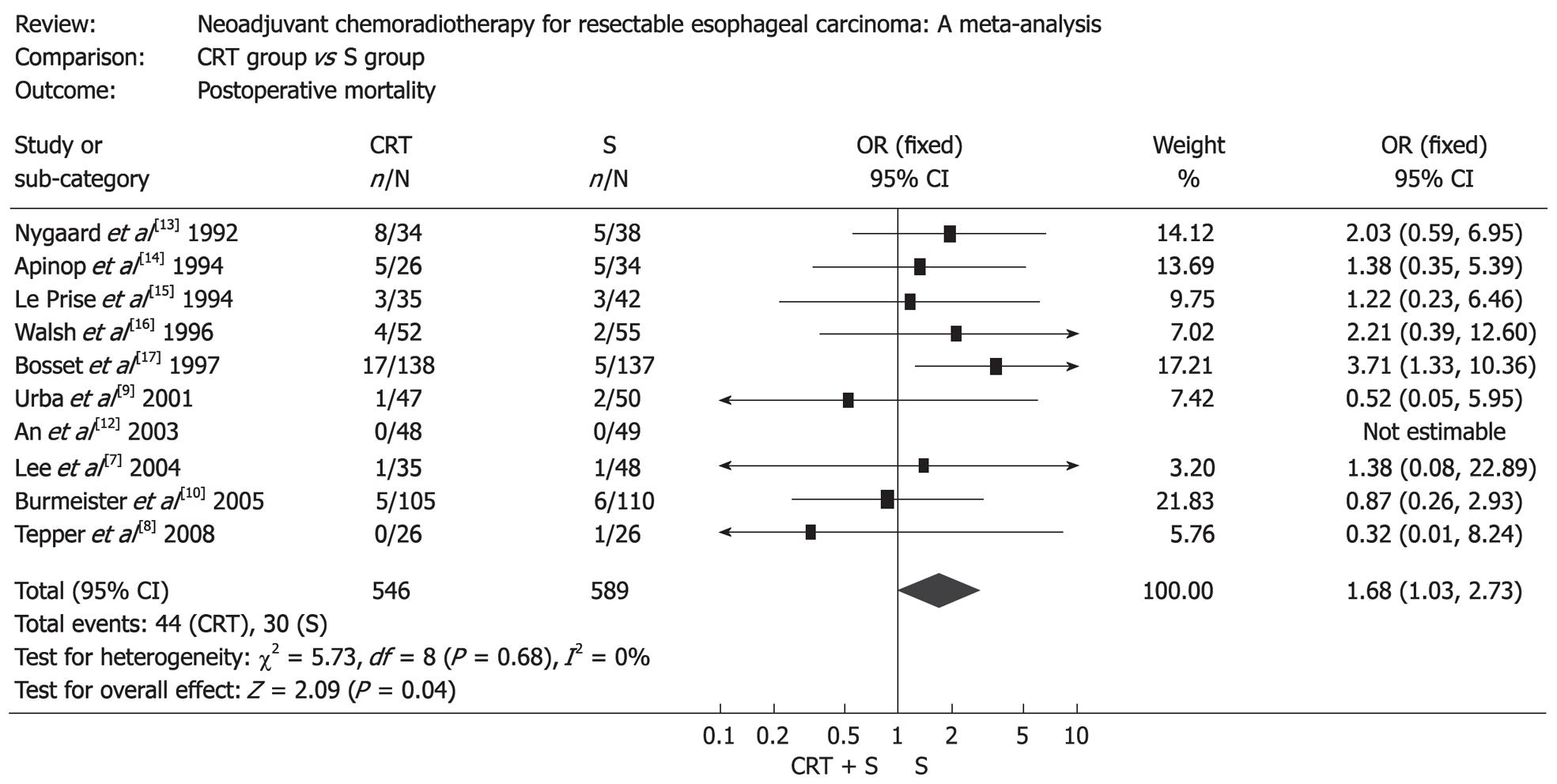
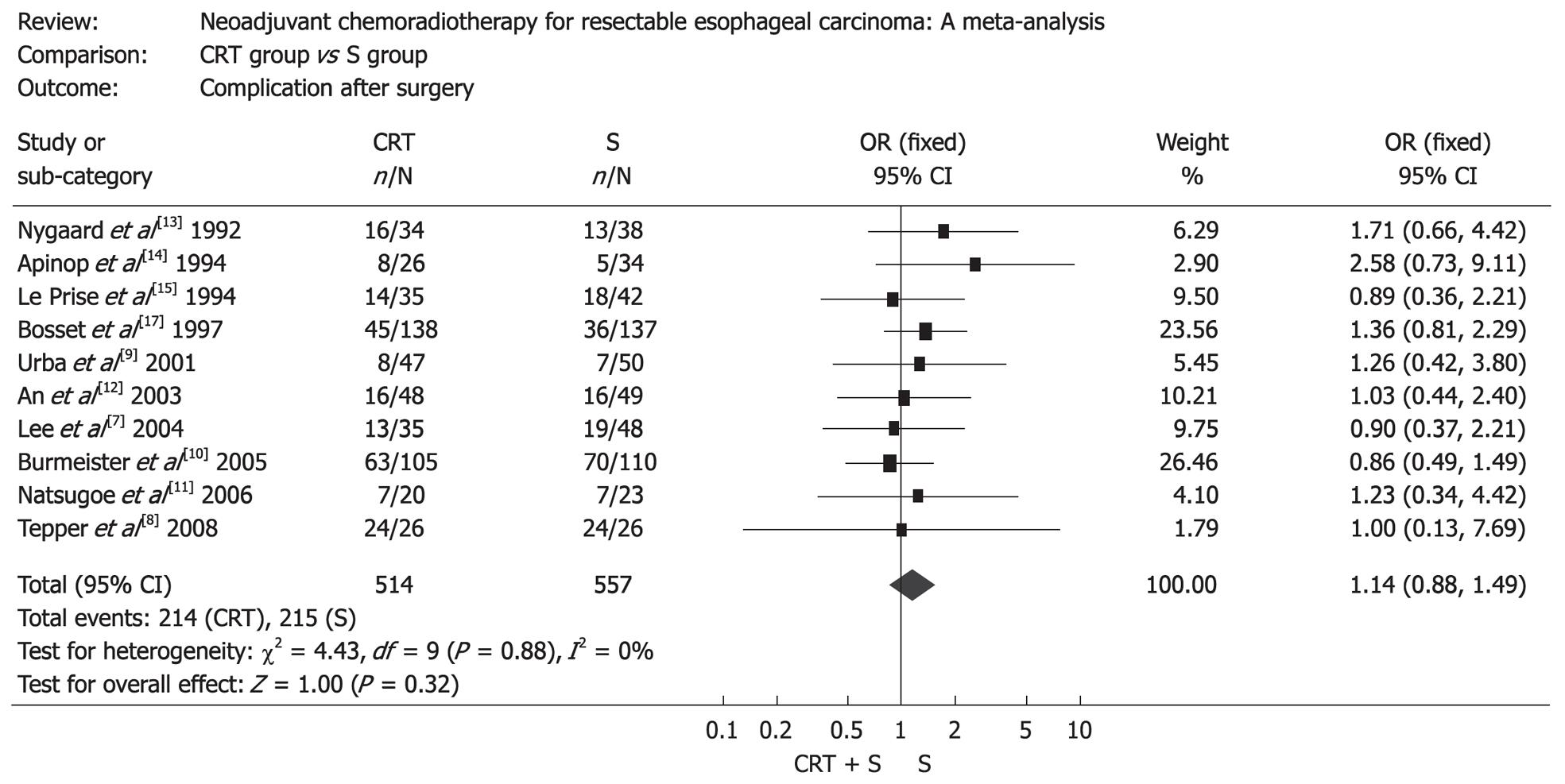
The resection rate in patients treated with surgery alone was markedly higher than that in patients treated with preoperative chemoradiotherapy (OR: 0.36, 95% CI: 0.24-0.54, P < 0.00001), but patients treated with preoperative chemoradiotherapy were more likely to obtain a complete resection (R0 resection), which was defined as gross disease removed with negative margins (OR: 2.16, 95% CI: 1.58-2.97, P < 0.00001) (Figure 2). Mortality after surgery varied from 0% to 23.5% in CRT group (the highest mortality was in the RCT by Nygaard et al[13]) and from 0% to 14.7% in S group (the highest mortality was in the RCT by Apinop et al[14]). Mortality after surgery in CRT group was higher than that in S group (OR: 1.68, 95% CI: 1.03-2.73, P = 0.04) (Figure 3). But if the RCT by Nygaard et al[13] or the RCT by Bosset et al[17] were excluded, there was no significant difference between the two groups and corresponding OR (95% CI, P value) was 1.66 (0.99-2.79, P = 0.06) and 1.30 (0.75-2.28, P = 0.35). The postoperative complications included nonfatal and fatal complications. There was no significant difference between the two groups (OR: 1.14, 95% CI: 0.88-1.49, P = 0.32) (Figure 4).
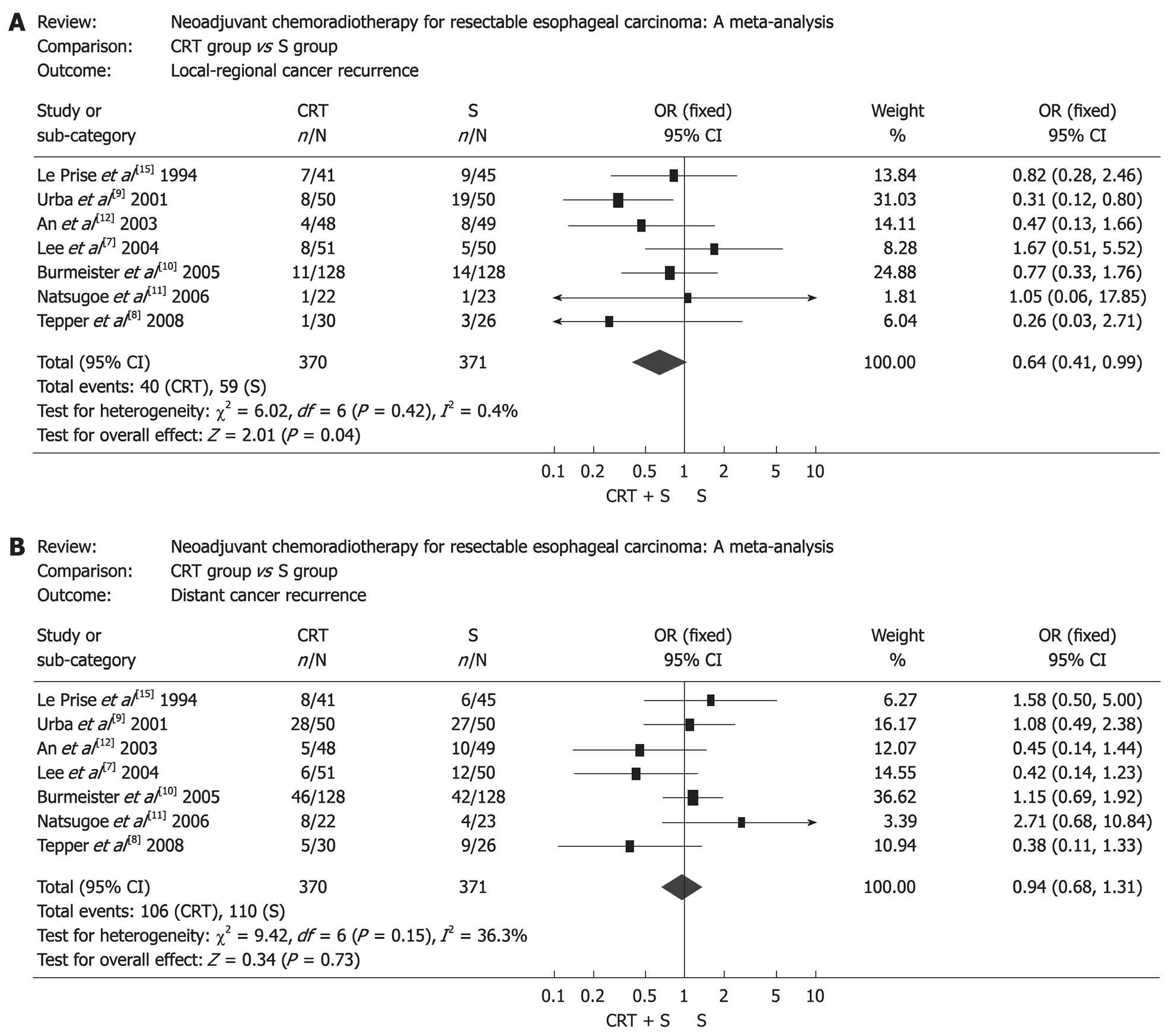
First treatment failure was defined as unequivocal histological or radiological evidence of tumor recurrence for the first time after surgery wherever the tumor relapsed. Seven RCTs provided related data on tumor recurrence. The patients treated by preoperative chemoradiotherapy had lower incidence of local recurrence (OR: 0.64, 95% CI: 0.41-0.99, P = 0.04) (Figure 5A), but the two groups had no significant difference in distant recurrence (OR: 0.94, 95% CI: 0.68-1.31, P = 0.73) (Figure 5B).
Our meta-analysis indicated that patients treated by neoadjuvant chemoradiotherapy had more survival benefit compared with patients treated by surgery alone, including 1-year survival, 3-year survival and 5-year survival. But subgroup analysis demonstrated patients with ESCC could not benefit from neoadjuvant chemoradiotherapy. The meta-analysis performed by Fiorica et al[18] suggested that neoadjuvant chemoradiotherapy plus surgery significantly lowered the 3-year mortality compared with surgery alone, but there was no statistical significance between the two groups if all RCTs including patients with EAC were excluded. Another meta-analysis performed by Gebski et al[19] demonstrated that both patients with ESCC and patients with EAC benefited from neoadjuvant chemoradiotherapy and corresponding OR (95% CI, P value) were 0.84 (0.71-0.99, P = 0.04) and 0.75 (0.59-0.95, P = 0.02). Since the former P value approached 0.05, our conclusion should be cautiously done. Thus we presume that only patients with EAC could benefit from neoadjuvant chemoradiotherapy. Another subgroup indicated patients in Europe and the USA benefited from neoadjuvant chemoradiotherapy; however, patients in Asia did not. This result indicated that different population had different response to neoadjuvant chemoradiotherapy and this may be associated with ethnic difference.
Though patients receiving neoadjuvant chemoradiotherapy had higher survival than patients treated by surgery alone, our meta-analysis showed that the incidence of surgery-related death was higher in the CRT group. Moreover, some patients lost the chance of surgery for the metastasis of tumor or some patients died before surgery for the aggravation of disease. Neoadjuvant chemoradiotherapy made local tissue harder and easier to bleed and as a result fatal postoperative complications such as anastomotic leakage and respiratory insufficiency increased. This may account for the higher postoperative mortality of patients in CRT group. However, sensitivity analysis showed no significant difference between the two groups after excluding the RCT by Nygaard et al[13], performed between 1983 and 1988, which was the earliest one among all the RCTs included in this meta-analysis. Probably there was no effective treatment for severe postoperative complications due to the relative undeveloped medical conditions at that time. In fact, the postoperative mortality in the RCTs published after 2000 was significantly lower than those published before 2000. In a word, it is possible that the postoperative mortality of patients receiving neoadjuvant chemoradiotherapy is not significantly higher than that of patients treated by surgery alone under present medical conditions.
Outcomes of our meta-analysis revealed that patients treated by surgery alone had more possibility to undergo the scheduled surgery, however, the rate of complete resection in CRT group was higher than that in S group. This may account for the lower incidence of local recurrence in CRT group, which was another result of this meta-analysis. In the 11 RCTs, only part of patients in CRT group can obtain clinical relief or pathological response. Two RCTs[12,14] compared the survival time between the patients who obtained clinical relief (including complete response and partial response) and the patients who failed to obtain clinical relief (including stable disease and disease progression), and found that the former was markedly greater than the latter. So, if some biological molecules can predict the response of EC patients to neoadjuvant chemoradiotherapy, EC patients will suffer from less physical miseries. Furthermore, this could avoid waste and enhance targeted treatment. Some studies[20-23] have indicated that Hsp27, DNA-PKcs, ERCC1 and c-erbB-2 were potential biological molecules, which were related to the response of EC patients to neoadjuvant chemoradiotherapy. Studies in this field are meaningful and promising.
One study reported that the effect of sequential chemoradiotherapy was superior to that of concurrent chemoradiotherapy in treating lung cancer[24]. In EC, however, outcome was exactly opposite to those obtained from lung cancer. This meta-analysis showed that EC patients only benefited from preoperative concurrent chemoradiotherapy. Concurrent chemoradiotherapy may work by inhibiting the growth of local tumor and micrometastasis, moreover concurrent chemotherapy can increase the sensitivity of tumor to radiotherapy.
Some studies[25-27] indicated that 40%-75% of patients with resectable EC (T1-3N0-1M0) judged according to clinical examination or surgery had subclinical metastasis or tumor had already invaded the adjacent organs or tissues. Accurate tumor staging is crucial to the prognosis of EC patients receiving surgery. Therefore, further measures should be taken to improve the accuracy of tumor staging. Currently, endoscopic ultrasound (EUS) is the most accurate method for staging EC for T and N stage[28]. Although helical computed tomography still appears insensitive for the identification of T4 or metastatic involvement of celiac lymph node disease in esophageal cancer, EUS with fine needle aspiration and FDG-PET [fluorine 18-labeled fluorodeoxyglucose (FDG) positron emission tomography (PET)] can make up for this shortcoming[29]. Part of patients in RCTs[9,11] included in this meta-analysis had metastasis of non-regional lymph nodes. Investigators considered those lymph nodes could be included in the radiation port and should be resected at surgery[9]. In fact, this condition belongs to IVa according to TNM staging. We suggest that EC patients (IVa) should not give up the chance of surgery, and they will benefit from neoadjuvant chemoradiotherapy plus surgery too.
In conclusion, this meta-analysis showed that patients with ESCC did not benefit from preoperative concurrent chemoradiotherapy and patients with EAC may be the real beneficiaries of the treatment protocol. Compared with patients treated by surgery alone, patients receiving neoadjuvant chemoradiotherapy more likely obtained complete resection and had lower local cancer recurrence. Neoadjuvant chemoradiotherapy was connected with a little higher mortality after surgery. But it did not increase the incidence of postoperative complications. In addition, patients in Europe and the USA more likely benefited from neoadjuvant chemoradiotherapy than those in Asia, and this is worth of further studies.
Esophageal carcinoma (EC) is one of the major malignant diseases worldwide. Surgery alone cannot obtain satisfactory effects in patients with EC.
Neoadjuvant chemoradiotherapy has been a hotspot for EC treatment research. Several related randomized controlled trials (RCTs) have been published, but opinions vary among clinicians as to the therapeutic effect of the new method. It remains uncertain whether patients with resectable EC can benefit from neoadjuvant chemoradiotherapy.
Several meta-analyses on the neoadjuvant chemoradiotherapy for EC have been published so far, some of which lacked adequate RCTs or used unpublished data. In this study, the authors collected relatively comprehensive data and all the data were from the published literature. It was found that patients with esophageal squamous cell carcinoma did not benefit from neoadjuvant chemoradiotherapy, while patients with esophageal adenocarcinoma (EAC) were the real beneficiaries. In addition, the authors analyzed the impact of geographical differences on the efficacy of the treatment protocol and found that patients in Europe and the USA more likely benefited from neoadjuvant chemoradiotherapy than those in Asia.
Results of this study indicate that neoadjuvant chemoradiotherapy is an effective treatment protocol, which is beneficial to patients with EAC in Europe and the USA.
Neoadjuvant chemoradiotherapy: Chemotherapy and radiotherapy are given to patients with cancer before surgery.
This work is a meta-analysis including 11 randomized prospective studies that analyze the advantages of the use of the neoadjuvant chemoradiotherapy vs surgery alone in the treatment of the EC. The results are interesting and suggest that neoadjuvant chemoradiotherapy is beneficial to patients with EAC.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 2. | Parkin DM, Muir CS. Cancer Incidence in Five Continents. Comparability and quality of data. IARC Sci Publ. 1992;45-173. |
| 3. | Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450-464. |
| 4. | Khushalani N. Cancer of the esophagus and stomach. Mayo Clin Proc. 2008;83:712-722. |
| 5. | Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896-1900. |
| 6. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. |
| 7. | Lee JL, Park SI, Kim SB, Jung HY, Lee GH, Kim JH, Song HY, Cho KJ, Kim WK, Lee JS. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15:947-954. |
| 8. | Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-1092. |
| 9. | Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305-313. |
| 10. | Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D, Millar J. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659-668. |
| 11. | Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Yokomakura N, Ishigami S, Owaki T, Aikou T. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis Esophagus. 2006;19:468-472. |
| 12. | An FS, Huang JQ, Xie YT, Chen SH, Rong TH. [A prospective study of combined chemoradiotherapy followed by surgery in the treatment of esophageal carcinoma]. Zhonghua Zhongliu Zazhi. 2003;25:376-379. |
| 13. | Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, Mäntyla M, Modig H, Munck-Wikland E, Rosengren B. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104-1109; discussion 1110. |
| 14. | Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology. 1994;41:391-393. |
| 15. | Le Prise E, Etienne PL, Meunier B, Maddern G, Ben Hassel M, Gedouin D, Boutin D, Campion JP, Launois B. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779-1784. |
| 16. | Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462-467. |
| 17. | Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, Lozach P, Ollier JC, Pavy JJ, Mercier M. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161-167. |
| 18. | Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, Falchi AM, Craxì A, Cammà C. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925-930. |
| 19. | Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226-234. |
| 20. | Miyazaki T, Kato H, Faried A, Sohda M, Nakajima M, Fukai Y, Masuda N, Manda R, Fukuchi M, Ojima H. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005;25:2749-2755. |
| 21. | Noguchi T, Shibata T, Fumoto S, Uchida Y, Mueller W, Takeno S. DNA-PKcs expression in esophageal cancer as a predictor for chemoradiation therapeutic sensitivity. Ann Surg Oncol. 2002;9:1017-1022. |
| 22. | Warnecke-Eberz U, Metzger R, Miyazono F, Baldus SE, Neiss S, Brabender J, Schaefer H, Doerfler W, Bollschweiler E, Dienes HP. High specificity of quantitative excision repair cross-complementing 1 messenger RNA expression for prediction of minor histopathological response to neoadjuvant radiochemotherapy in esophageal cancer. Clin Cancer Res. 2004;10:3794-3799. |
| 23. | Miyazono F, Metzger R, Warnecke-Eberz U, Baldus SE, Brabender J, Bollschweiler E, Doerfler W, Mueller RP, Dienes HP, Aikou T. Quantitative c-erbB-2 but not c-erbB-1 mRNA expression is a promising marker to predict minor histopathologic response to neoadjuvant radiochemotherapy in oesophageal cancer. Br J Cancer. 2004;91:666-672. |
| 24. | Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88:1210-1215. |
| 25. | Diehl LF. Radiation and chemotherapy in the treatment of esophageal cancer. Gastroenterol Clin North Am. 1991;20:765-774. |
| 26. | Katlic MR, Wilkins EW Jr, Grillo HC. Three decades of treatment of esophageal squamous carcinoma at the Massachusetts General Hospital. J Thorac Cardiovasc Surg. 1990;99:929-938. |
| 27. | Kelsen D. Multimodality therapy for adenocarcinoma of the esophagus. Gastroenterol Clin North Am. 1997;26:635-645. |
| 28. | Meyenberger C, Fantin AC. Esophageal carcinoma: current staging strategies. Recent Results Cancer Res. 2000;155:63-72. |
| 29. | Romagnuolo J, Scott J, Hawes RH, Hoffman BJ, Reed CE, Aithal GP, Breslin NP, Chen RY, Gumustop B, Hennessey W. Helical CT versus EUS with fine needle aspiration for celiac nodal assessment in patients with esophageal cancer. Gastrointest Endosc. 2002;55:648-654. |
Peer reviewers: Luis Grande, Professor, Department of Surgery, Hospital del Mar, Passeig Marítim 25-29, Barcelona 08003, Spain; Marco G Patti, MD, Professor, Director, Center for Esophageal Diseases, University of Chicago Pritzker School of Medicine, 5841 S. Maryland Avenue, MC 5095, Room G 201, Chicago, IL 60637, United States; Marcelo A Beltran, MD, Chairman, Department of Surgery, Hospital de La Serena, PO Box 912, IV Region, La Serena, Chile
S- Editor Wang JL L- Editor Ma JY E- Editor Zheng XM