Published online Dec 21, 2009. doi: 10.3748/wjg.15.5972
Revised: October 28, 2009
Accepted: November 4, 2009
Published online: December 21, 2009
AIM: To assess the safety, yield and clinical utility of percutaneous transgastric computed tomography (CT)-guided biopsy of pancreatic tumor using large needles, in selected patients.
METHODS: We reviewed 34 CT-guided biopsies in patients with pancreas mass, of whom 24 (71%) had a direct path to the mass without passing through a major organ. The needle passed through the liver in one case (3%). Nine passes (26%) were made through the stomach. These nine transgastric biopsies which used a coaxial technique (i.e. a 17-gauge coaxial introducer needle and an 18-gauge biopsy needle) were the basis of this study. Immediate and late follow-up CT images to detect complications were obtained.
RESULTS: Tumor tissues were obtained in nine pancreatic biopsies, and histologic specimens for diagnosis were obtained in all cases. One patient, who had a rare sarcomatoid carcinoma, received a second biopsy. One patient had a complication of transient pneumoperitoneum but no subjective complaints. An immediate imaging study and clinical follow-up detected neither hemorrhage nor peritonitis. No delayed procedure-related complication was seen during the survival period of our patients.
CONCLUSION: Pancreatic biopsy can be obtained by a transgastric route using a large needle as an alternative method, without complications of peritonitis or bleeding.
- Citation: Tseng HS, Chen CY, Chan WP, Chiang JH. Percutaneous transgastric computed tomography-guided biopsy of the pancreas using large needles. World J Gastroenterol 2009; 15(47): 5972-5975
- URL: https://www.wjgnet.com/1007-9327/full/v15/i47/5972.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5972
The diagnosis of a pancreatic mass detected by abdominal imaging can be difficult, and therapeutic decisions are based on the ability to diagnose or exclude malignancy[1]. Although most neoplasms are ductal adenocarcinomas, imaging modalities can not reliably be used to diagnose other malignant or benign conditions which may have different treatment options and prognoses[2]. Therefore, tissue diagnosis is often needed before surgery.
Computed tomography (CT)-guided biopsy for tissue diagnosis is well established[3-5]. In a prospective analysis of 125 procedures, CT-directed biopsy for pancreatic lesions had an accuracy of 95.2%[3]. An occasional limitation of the axial CT guidance of such interventional procedures is the presence of intervening vital structures which cannot be avoided even by using a gantry tilt technique[4]. Brandt et al[5] stated that there were no complications with fine, 21-gauge needle passage through the gastrointestinal tract. Fine needle biopsy for pancreatic lesions had an accuracy of 85%, whereas a large needle (16-19 gauge) had an accuracy of 92%[5]. Therefore, in daily practice, large needle biopsy could reduce the repeat biopsy rate. One experimental study in rabbits reported that the transgastric route with an 18-gauge cutting needle could be used for pancreas biopsy, without apparent peritonitis or bleeding[6]. However, only a few studies have reported on the technique of large needle, transgastric-route biopsy of pancreatic lesions in humans[7,8].
Since the initial description in 1992, tissue diagnosis under endoscopic ultrasonography (EUS) guidance has emerged as an important modality for evaluating patients with pancreatic lesions[9-11]. EUS-guided trucut needle biopsy using a 19-gauge needle, performed through the patient’s stomach, is safe and accurate[11]. This procedure, however, is limited to selected patients who are willing to undergo endoscopy. Therefore, a transgastric approach using a large needle for CT-guided biopsy of the pancreatic lesion can be an alternative method of choice.
The aim of the present study was to assess the safety, yield, and clinical utility of percutaneous transgastric CT-guided biopsy in patients with pancreatic masses.
We reviewed the medical records of 34 consecutive CT-guided biopsies of a solid pancreatic mass from one institution (Taipei Veterans General Hospital, Taipei, Taiwan) over a 4-year period. All the patients were unwilling or failed to undergo EUS-guided biopsy. The indication for biopsy of these pancreatic masses was to obtain a diagnosis of malignancy from tissue before the patients received adjuvant chemotherapy or radiotherapy. Those patients with a resectable mass and scheduled for surgery were not included.
We analyzed the records of these biopsy procedures, and 24 had a direct path to the mass which did not pass through a major organ (71%). In one case (3%), the needle passed through the liver. Nine passes (26%) in eight patients were made through the stomach. These nine CT-guided biopsies performed with a transgastric approach were the basis of this study. The patients were one woman and seven men, with an average age of 65 years (range, 35-78 years).
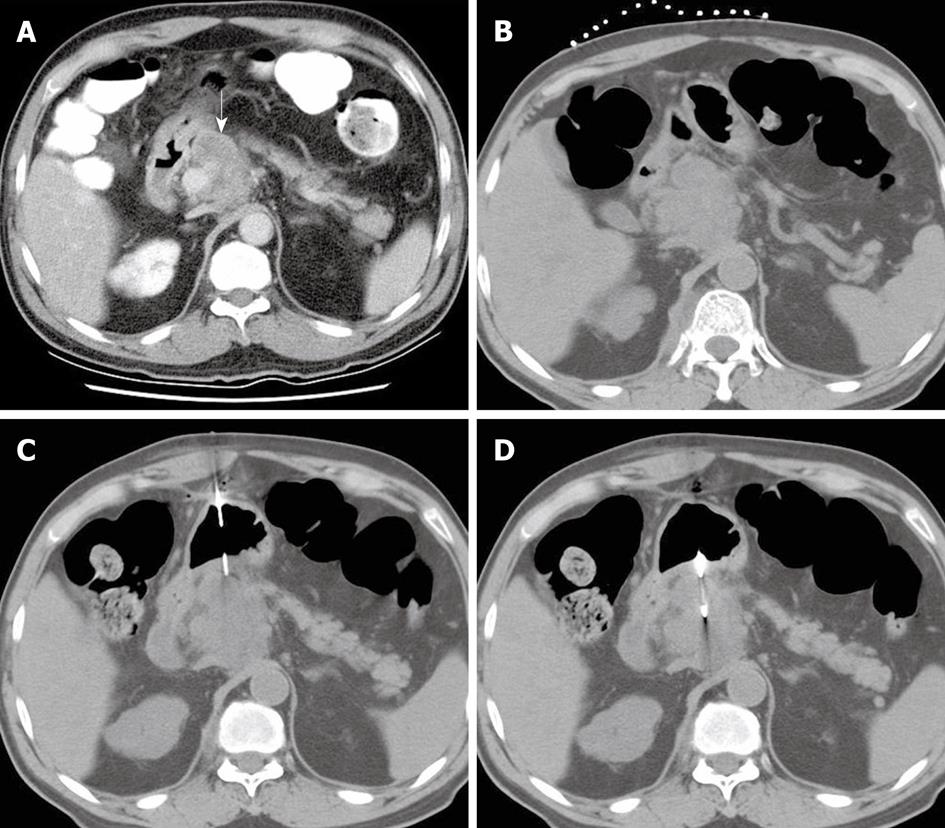
Magnetic resonance imaging (MRI) or CT images before biopsy were reviewed by two experienced radiologists in a joint meeting, and the interpretation reached consensus. The proper pass route and the biopsy site were evaluated (Figure 1A). All patients were hospitalized and fasted overnight before the procedure. Biopsies were monitored with the patient in the supine position and performed on a CT scanner (Siemens, Germany). The reference scan was obtained first, and an opaque marker placed on the patient’s abdomen (Figure 1B). The opaque marker consisted of several parallel segments of angiographic catheter. On the reference image, the accessible pass route and the distance were measured. The coaxial technique was applied with a 17-gauge coaxial introducer needle (Allegiance Health Corporation, McGaw Park, IL, USA) and an 18-gauge Temno biopsy needle (Allegiance Health Corporation, McGaw Park, IL, USA). The coaxial introducer needle penetrated the stomach wall as perpendicularly as possible, with its tip stopped on the edge of the target lesion (Figure 1C). Two to four strips of tissue were obtained in each procedure (Figure 1D).
An immediate follow-up CT image was obtained in all patients to detect possible complications. After each procedure, fasting was not necessary if there were no abnormal findings in follow-up CT images or complaints by the patients. All patients were observed in hospital until their condition was stable. Any delayed procedure-related complication was recorded on the patient’s chart and by follow-up images.
Table 1 shows basic data on our patients who underwent CT-guided transgastric biopsy. Nine biopsies of eight pancreas masses were successfully performed. The tumor sizes ranged from 20 to 75 mm (mean 48.5 mm). The tumor locations were the pancreatic body (62%) and pancreatic head (38%). Histologic diagnoses were obtained in all nine biopsies. There were five adenocarcinomas, one squamous cell carcinoma, one poorly differentiated carcinoma, and one sarcomatoid carcinoma. Because sarcomatoid carcinoma in the pancreas is very rare, our initial biopsy specimen was classified as atypical cells. A second biopsy of the same mass was then obtained and the specimen showed similar histologic findings. Our pathologist then revised the report as a sarcomatoid carcinoma.
| Patient | Age/Gender | Target site (Pancreas) | Size1 (mm) | Histology | Complication | Follow-up |
| 1 | 74/M | Body | 20 | Adenocarcinoma | No | Expired, 8 mo later |
| 2 | 67/M | Body | 25 | Adenocarcinoma | No, TP | Expired, 2 mo later |
| 3 | 78/M | Body | 30 | Adenocarcinoma | No | Expired, 2 mo later |
| 4 | 76/M | Head | 75 | Squamous cell carcinoma | No | Expired, 6 mo later |
| 5 | 77/M | Head | 48 | Adenocarcinoma | No | Expired, 1 mo later |
| 6 | 35/M | Body | 50 | Poorly differentiated carcinoma | No | Expired, 6 mo later |
| 7 | 48/F | Body | 40 | Adenocarcinoma | No | Expired, 19 mo later |
| 8 | 67/M | Head | 20 | Sarcomatoid carcinoma2 | No | Expired, 6 mo later |
One patient had transient, minimal pneumoperitoneum, but no abdominal pain or peritoneal signs were noted. The condition resolved after the patient fasted for 1 d, and was confirmed by an erect chest film. No patient had internal hemorrhage or peritonitis, confirmed by imaging study or during clinical follow-up. There were no late complications, such as tumor spread, in the follow-up images in our patients who had a survival of 1 to 19 mo.
In this study, percutaneous puncture of a pancreatic mass was restricted to patients having advanced disease and who were not candidates for laparotomy. Histologic diagnosis was required in all patients scheduled for chemotherapy, radiotherapy, or both. Ihse et al[12] suggested that biopsy is not mandatory if the clinical suspicion of cancer is high and the surgical team has documented low postoperative mortality and morbidity rates.
Although percutaneous fine needle aspiration biopsy is well established in evaluating pancreatic masses[6], this technique requires experienced cytopathologists for tissue diagnosis. The amount of aspirated material is often suboptimal for multiple histopathologic examination or certain analyses required to detect endocrine tumors of the pancreas[13]. Recently, Li et al[8] reported a successful diagnosis in 69 of 80 patients (86%) suspected of having pancreatic lesions using an 18-20-gauge cutting needle automated biopsy gun, with no serious complications. In our study, pancreatic biopsies were performed with an 18-gauge biopsy gun. A final histological diagnosis from pancreatic masses can be obtained with this method. In contrast, a correct diagnosis from percutaneous fine needle aspiration with limited tissue specimen may be difficult.
In our study, we found that 29% of consecutive biopsies had no direct route for approaching the pancreatic mass without passing through a major organ. Nine passes were made through the stomach and one through the liver. The incidence of an indirect biopsy route was lower than that of previous CT-guided biopsies (40%) and higher than that of ultrasound-guided biopsies (24%)[5]. It is generally accepted and has been clinically proved that, for pancreas biopsy, a fine needle crossing the gastrointestinal tract, rather than through the liver[5], is safe and will not result in complications[5]. One experimental study in rabbits reported that the transgastric route with an 18-gauge cutting needle could be used without apparent peritonitis and bleeding[6]. The EUS-guided trucut needle (19-gauge) biopsy of the pancreas, all performed transgastrically, has been claimed to be safe and accurate[11] in selected patients who agreed to undergo endoscopy.
The biopsy-related complication rate using fine needle is low (0.5% to 3%) and acute pancreatitis is the most frequent complication[14,15]. Zech et al[7]reported only one complication in 57 patients who developed acute pancreatitis after a large core-needle biopsy of the pancreas. In the present study, all nine transgastric biopsies which penetrated both the anterior and posterior stomach walls were performed with 17-gauge coaxial transducer needles and then an 18-gauge biopsy gun. There were no immediate complications such as peritonitis or bleeding. One patient had transient pneumoperitoneum, which resolved after overnight fasting. Late complications, including tumor spread, were not found in follow-up images. However, our study was limited by a relatively short period of follow-up during the survival period of our patients.
The hole made by a gastrostomy catheter is larger than that caused by a biopsy needle. Some authors have reported no untoward effects after the removal of a 10 or 14 French catheter used for percutaneous gastrostomy[16]. The muscular layers run in three directions- oblique, circular and longitudinal- from the inner to the outer stomach wall. This arrangement is considered to be the reason why the stomach wall can be punctured without peritoneal leakage[17].
In our study, the stomach was usually empty and partly collapsed after overnight fasting. It is much more difficult to penetrate the stomach wall when it is “flaccid”. There are two tricks to piercing the stomach wall. The first is to keep the biopsy needle as perpendicular as possible to the gastric wall. If the biopsy needle was tangential, it would slide over rather than penetrate the gastric wall. The second is to advance the needle forcibly and quickly when penetrating the gastric wall. If the needle was advanced slowly, it would tent the gastric wall rather than piercing it. If the stomach wall is tented and can not be punctured, the needle should be withdrawn a little and then advanced again.
We conclude that percutaneous transgastric biopsy of the pancreas in selected patients with a combination of a 17-gauge introducer needle and an 18-gauge biopsy gun can be safe and has a high successful rate.
It is reasonable to obtain a histological diagnosis before treating patients who have pancreatic masses and are unsuitable or unwilling to undergo surgery. As the pancreas is a deep seated organ surrounded by other vital structures, it is a challenge for the physician to obtain an adequate specimen for histological examination. Endoscopic ultrasound-guided biopsy of pancreatic masses has been proved to be a safe and effective method. However, if the hospital has no such facilities or patients are unwilling or intolerant of the procedure, computed tomography (CT)-guided biopsy is an alternative method. In some cases, penetration of other vital organs is unavoidable when approaching the pancreatic mass. In this article, the authors clarified the safety and efficacy of percutaneous transgastric biopsy of pancreatic masses.
The stomach has three muscle layers and can be punctured without evidence of leakage. Percutaneous gastrostomy, either by endoscopy or fluoroscopy guidance, has been widely performed for a long time. Although only the anterior wall is punctured during gastrostomy and a catheter is put in place to block the hole, the authors think it would be safe to pierce both the anterior and posterior wall of the stomach using a large biopsy needle. An experimental study in rabbits also showed that it was safe to perform transgastric biopsy.
Eight patients received 9 CT-guided transgastric biopsies of pancreatic masses located at the pancreas head or body without passing through vital organs. All procedures went smoothly. Only one patient had transient pneumoperitoneum which completely resolved the next day. Although this is a small series, their study shows that it is feasible to perform transgastric biopsy of a pancreatic lesion using a large needle.
Besides pancreatic lesions, there are other types of pathology in the upper abdomen, such as enlarged lymph nodes or loculated fluid. Transgastric biopsy of lymph nodes or aspiration or drainage of fluid could be performed safely.
CT-guided biopsy uses CT scanning to perform a biopsy. When facing a deep seated lesion, or lesion blocked by gas, such as a lung nodule, CT scanning can provide better resolution and clearly shows the biopsy needle reaching the target.
As the authors stated in the introduction of the manuscript, endoscopic ultrasonography guided biopsy of the pancreas is the gold standard to obtain sample tissue for histological diagnosis of pancreatic mass. Percutaneous transgastric CT-guided biopsy for patients with pancreatic mass should be considered an alternative.
| 1. | Tamm E, Charnsangavej C. Pancreatic cancer: current concepts in imaging for diagnosis and staging. Cancer J. 2001;7:298-311. |
| 2. | Cohen SJ, Pinover WH, Watson JC, Meropol NJ. Pancreatic cancer. Curr Treat Options Oncol. 2000;1:375-386. |
| 3. | Welch TJ, Sheedy PF 2nd, Johnson CD, Johnson CM, Stephens DH. CT-guided biopsy: prospective analysis of 1,000 procedures. Radiology. 1989;171:493-496. |
| 4. | Yueh N, Halvorsen RA Jr, Letourneau JG, Crass JR. Gantry tilt technique for CT-guided biopsy and drainage. J Comput Assist Tomogr. 1989;13:182-184. |
| 5. | Brandt KR, Charboneau JW, Stephens DH, Welch TJ, Goellner JR. CT- and US-guided biopsy of the pancreas. Radiology. 1993;187:99-104. |
| 6. | Akan H, Ozen N, Incesu L, Gümüş S, Güneş M. Are percutaneous transgastric biopsies using 14-, 16- and 18-G Tru-Cut needles safe? An experimental study in the rabbit. Australas Radiol. 1998;42:99-101. |
| 7. | Zech CJ, Helmberger T, Wichmann MW, Holzknecht N, Diebold J, Reiser MF. Large core biopsy of the pancreas under CT fluoroscopy control: results and complications. J Comput Assist Tomogr. 2002;26:743-749. |
| 8. | Li L, Liu LZ, Wu QL, Mo YX, Liu XW, Cui CY, Wan DS. CT-guided core needle biopsy in the diagnosis of pancreatic diseases with an automated biopsy gun. J Vasc Interv Radiol. 2008;19:89-94. |
| 9. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. |
| 10. | Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the M. D. Anderson Cancer Center experience. Cancer. 2002;96:174-180. |
| 11. | Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc. 2004;59:185-190. |
| 12. | Ihse I, Axelson J, Dawiskiba S, Hansson L. Pancreatic biopsy: why? When? How? World J Surg. 1999;23:896-900. |
| 13. | Elvin A, Andersson T, Scheibenpflug L, Lindgren PG. Biopsy of the pancreas with a biopsy gun. Radiology. 1990;176:677-679. |
| 14. | Aideyan OA, Schmidt AJ, Trenkner SW, Hakim NS, Gruessner RW, Walsh JW. CT-guided percutaneous biopsy of pancreas transplants. Radiology. 1996;201:825-828. |
| 15. | Jennings PE, Donald JJ, Coral A, Rode J, Lees WR. Ultrasound-guided core biopsy. Lancet. 1989;1:1369-1371. |
| 16. | Ho CS, Yeung EY. Percutaneous gastrostomy and transgastric jejunostomy. AJR Am J Roentgenol. 1992;158:251-257. |
| 17. | Hicks ME, Surratt RS, Picus D, Marx MV, Lang EV. Fluoroscopically guided percutaneous gastrostomy and gastroenterostomy: analysis of 158 consecutive cases. AJR Am J Roentgenol. 1990;154:725-728. |
Peer reviewers: Dr. Aydin Karabacakoglu, Assistant Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey; Alberto Biondi, MD, Department of Surgery, 1st Surgical Division, Catholic University of Rome, Largo A. Gemelli 8, Rome 00168, Italy
S- Editor Wang JL L- Editor Webster JR E- Editor Tian L