Published online Dec 21, 2009. doi: 10.3748/wjg.15.5960
Revised: August 7, 2009
Accepted: August 14, 2009
Published online: December 21, 2009
AIM: To establish the frequency of hyperphosphatemia following the administration of sodium phosphate laxatives in low-risk patients.
METHODS: One hundred consecutive ASA I-II individuals aged 35-74 years, who were undergoing colonic cleansing with oral sodium phosphate (OSP) before colonoscopy were recruited for this prospective study. Exclusion criteria: congestive heart failure, chronic kidney disease, diabetes, liver cirrhosis, intestinal obstruction, decreased bowel motility, increased bowel permeability, and hyperparathyroidism. The day before colonoscopy, all the participants entered a 24-h period of diet that consisted of 4 L of clear fluids with sugar or honey and 90 mL (60 g) of OSP in two 45-mL doses, 5 h apart. Serum phosphate was measured before and after the administration of the laxative.
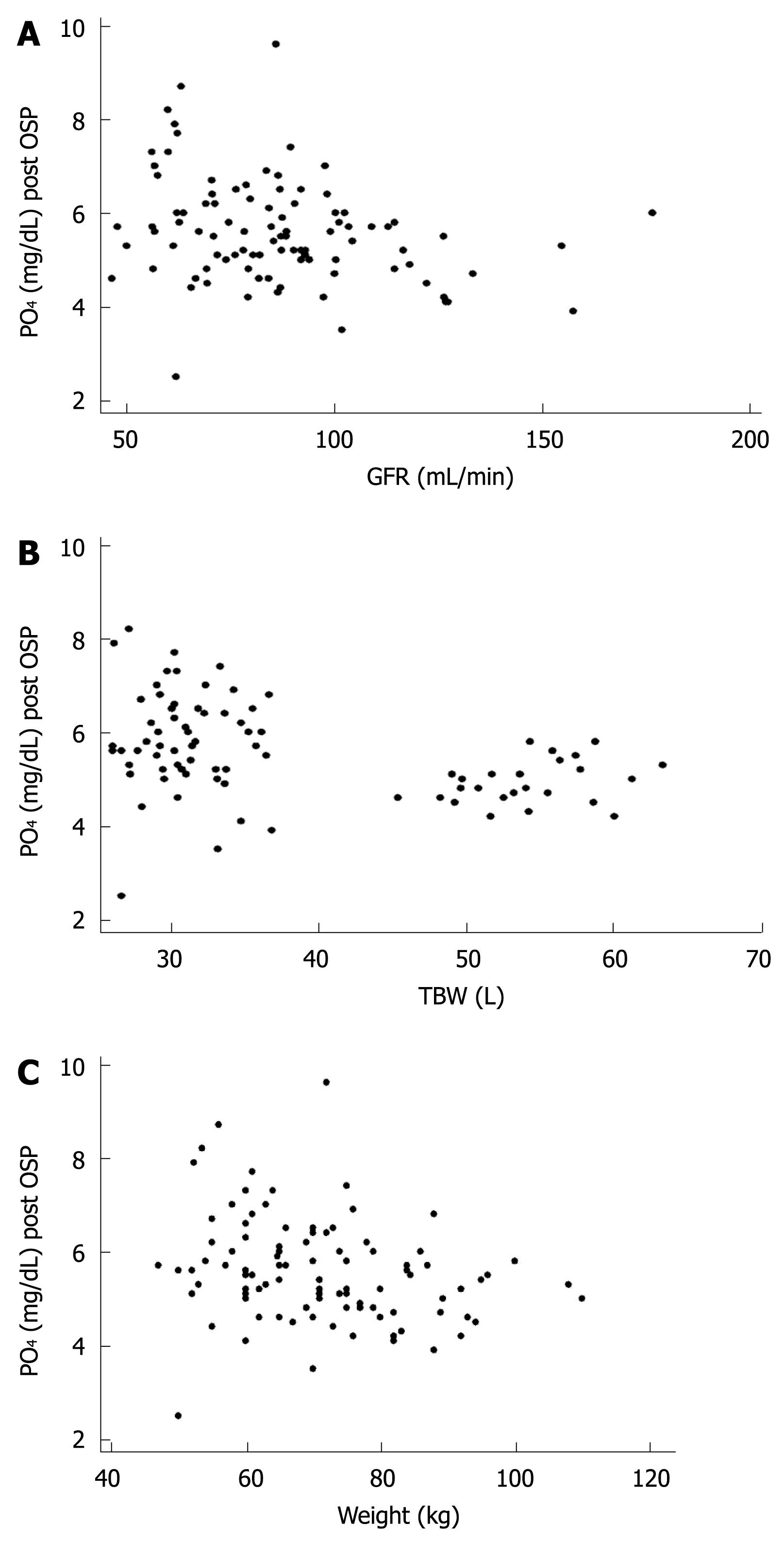
RESULTS: The main demographic data (mean ± SD) were: age, 58.9 ± 8.4 years; height, 163.8 ± 8.6 cm; weight, 71 ± 13 kg; body mass index, 26 ± 4; women, 66%. Serum phosphate increased from 3.74 ± 0.56 to 5.58 ± 1.1 mg/dL, which surpassed the normal value (2.5-4.5 mg/dL) in 87% of the patients. The highest serum phosphate was 9.6 mg/dL. Urea and creatinine remained within normal limits. Post-treatment OSP serum phosphate concentration correlated inversely with glomerular filtration rate (P < 0.007, R2 = 0.0755), total body water (P < 0.001, R2 = 0.156) and weight (P < 0.013, R2 = 0.0635).
CONCLUSION: In low-risk, well-hydrated patients, the standard dose of OSP-laxative-induced hyperphosphatemia is related to body weight.
- Citation: Casais MN, Rosa-Diez G, Pérez S, Mansilla EN, Bravo S, Bonofiglio FC. Hyperphosphatemia after sodium phosphate laxatives in low risk patients: Prospective study. World J Gastroenterol 2009; 15(47): 5960-5965
- URL: https://www.wjgnet.com/1007-9327/full/v15/i47/5960.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5960
The widespread use of colonoscopy for early detection of colorectal pathology has increased the use of osmotic laxatives for colonic cleansing. Among these, oral sodium phosphate (OSP) is the preparation of choice because is the best tolerated given the small volume in which it is administered, and it results in better colonic cleansing[1]. Under normal conditions, phosphate is absorbed in the small intestine and eliminated by the kidney as calcium phosphate[2]. Several complications associated with the use of OSP have been reported in recent years, especially hyperphosphatemia and acute and chronic renal failure. There is evidence linking these complications to conditions that increase the absorption of phosphate or render its renal elimination difficult. Although many of these complications are facilitated by dehydration and inadequate selection of patients when indicating the laxative[3-5], some patients without such conditions also have been reported[6]. Most of the information comes from retrospective studies or case reports.
In this prospective clinical trial, we investigated the frequency of hyperphosphatemia in low-risk ASA I-II patients[7], who were chosen to avoid high-risk patients. To avoid dehydration, we administered 4 L of clear liquids. The main aim was to identify the percentage of patients with hyperphosphatemia following the administration of OSP for video colonoscopy, and before anesthesia induction. A secondary objective was to establish the frequency of dehydration and hypocalcemia.
This study was approved by the Institutional Review Board. From May to December 2007, 100 consecutive patients who underwent elective colonoscopy were enrolled. Inclusion criteria were: 18-75 years of age, ASA I and II physical status, written informed consent, and colon cleansing with OSP.
Individuals with congestive heart failure, chronic kidney disease, diabetes, liver cirrhosis, intestinal obstruction, decreased bowel motility, increased bowel permeability (Crohn’s disease, ulcerative or ischemic colitis) and hyperparathyroidism were prevented from entering this trial. These conditions were ruled out in the pre-anesthetic evaluation by medical history and anamnesis. Those patients who refused to participate were also excluded. Patients who had not undergone colonic cleansing were also excluded.
All the participants received full information regarding the study protocol and procedures in the pre-anesthetic interview and signed the informed consent to participate. Vital parameters were measured and laboratory tests, including hematocrit, hemoglobin concentration, and serum osmolarity, phosphate, Ca2+, electrolytes, creatinine and urea were carried out.
Forty-eight hours before the test, a fiber- and dairy-free diet (without fruit and vegetable products) was prescribed, and from 20 to 26 h before the study, 4 L of clear liquids (tea, coffee, infusions, jelly, broth and drained juices, or isotonic drinks[8]) with sugar or honey (on demand) were administered up to 2 h before the test.
The day before colonoscopy, all the participants were given 90 mL (60 g) of OSP (fosfo-dom®; Laboratorio Dominguez S.A, Buenos Aires, Argentina) diluted in 400 mL of water in two divided doses, administered 5 h apart (17:00 pm and 22:00 pm) on the day before colonoscopy. Ten micrograms metoclopramide were also administered 1 h before the laxative.
The day after colonic cleansing and immediately before starting anesthesia with propofol and sevofluorane, blood pressure and heart rate were measured and a second venous sample was drawn and sent to the laboratory to assess hematocrit, hemoglobin, and serum osmolality, phosphate, Ca2+, electrolytes, creatinine and urea. The results obtained were compared with those obtained at baseline.
The following formulas were used to calculate plasma volume, total body water and glomerular filtration rate: Plasma volume (PV) (Beaumont formula)[9] %PV: 100/(100 - HCT1) × 100 (HCT1 - HCT2)/HCT2. HCT = hematocrit.
Total body water (TBW; L) (Watson formula)[10]: Male: TBW - W = 2.447 - (0.09156 × age) + (0.1074 × height) + (0.3362 × weight); Female: TBW - W = -2.097 + (0.1069 × height) + (0.2466 × weight).
Glomerular filtration rate (GFR; mL/min) (Cockroft-Gault equation)[11]: (140 - age) × weight kg (× 0.85 if female)/creatinine × 72.
All data are expressed as mean ± SD. The Student t test was used to analyze normally distributed variables. A univariate linear correlation model that considered post-treatment OSP serum phosphate as a dependent variable was also performed. STATA® version 8.0 (StataCorp LP, http://www.stata.com) statistical software was used to carry out the statistical analysis. P < 0.05 was considered as statistically significant.
The main demographic data (mean ± SD) were: age, 58.9 ± 8.4 years; height, 163.8 ± 8.6 cm; weight, 71 ± 13 kg; body mass index (BMI), 26 ± 4; women, 66% (Table 1). The main laboratory data (mean ± SD) are shown in Table 2. Serum phosphate (mg/dL; mean ± SD) increased from a basal value of 3.74 ± 0.56 to 5.58 ± 1.1 after OSP (P = 0.001). Hyperphosphatemia appeared in 87% of the patients. The highest serum phosphate was 9.6 mg/dL. Post-OSP serum phosphate had a significant inverse correlation with GFR (P < 0.007, R2 = 0.0755, Figure 1A), TBW (P < 0.001, R2 = 0.156, Figure 1B), and weight (P < 0.013, R2 = 0.0635, Figure 1C). No correlation was observed between post-OSP serum phosphate and creatinine, height or BMI. The prevalence of hyperphosphatemia increased in parallel and steadily with stage of chronic renal disease according to the National Kidney Foundation classification[12], which approached 80% for stage 1, 88% for stage 2, and 100% for stage 3.
| Demographic data | |
| Age (yr) | 58.9 ± 8.4 |
| Height (cm) | 163.8 ± 8.6 |
| Weight (kg) | 71 ± 13 |
| BMI | 26 ± 4 |
| Sex (%) | 66% women, 34% men |
| TBW | 36.8 ± 8.63 |
| GFR | 95.25 ± 21.27 |
| Mean | SD | Min | Max | P | ||
| Na+ (mmol/L) | Pre | 139.26 | 2.05 | 135.00 | 146.00 | NS |
| Post | 139.72 | 2.95 | 133.00 | 146.00 | ||
| CI- (mmol/L) | Pre | 104.88 | 2.68 | 98.00 | 111.00 | NS |
| Post | 104.46 | 3.64 | 95.00 | 120.00 | ||
| K+ (mmol/L) | Pre | 4.46 | 0.39 | 3.50 | 5.80 | 0.001 |
| Post | 3.62 | 0.46 | 2.40 | 5.10 | ||
| PO4 (mg/dL) | Pre | 3.74 | 0.56 | 2.60 | 5.70 | 0.001 |
| Post | 5.58 | 1.10 | 2.50 | 9.60 | ||
| Ca2+ (mmol/L) | Pre | 1.14 | 0.10 | 0.76 | 1.37 | 0.001 |
| Post | 1.04 | 0.12 | 0.50 | 1.28 | ||
| Hto (%) | Pre | 40.28 | 3.13 | 31.70 | 48.30 | 0.070 |
| Post | 41.18 | 4.00 | 27.10 | 50.90 | ||
| Urea (mg/dL) | Pre | 32.57 | 9.93 | 15.00 | 68.00 | 0.001 |
| Post | 21.36 | 7.53 | 6.00 | 43.00 | ||
| Osm (mosm/kg) | Pre | 291.03 | 5.35 | 277.00 | 304.00 | 0.002 |
| Post | 288.56 | 6.01 | 274.00 | 307.00 | ||
| Creatinine (mg/dL) | Pre | 0.87 | 0.193 | 0.40 | 1.50 | NS |
| Post | 0.87 | 0.190 | 0.50 | 1.40 | ||
| AP S/D (mmHg) | Pre | 125/78 | 14/10 | 90/60 | 170/100 | NS |
| Post | 128/74 | 29/12 | 80/40 | 185/100 |
After OSP, Ca2+ decreased significantly (P = 0.001), although the difference was not clinically relevant. Pre- and post-OSP urea and creatinine levels remained within normal limits.
Plasma volume decreased by 3.65% after OSP. This represents a dehydration of < 1.46%, which was not significant[13,14]. There was a decrease in serum osmolality (Tables 1 and 2). There was a low incidence (4%) of hypotension (arterial pressure reduction ≥ 30%) after colonic cleansing (Table 2).
The osmotic effect of OSP causes dehydration[15]; an average loss of 3-4 L of fluids is estimated during colonic cleansing with 60 g OSP[16,17]. In support of these data, increases in the concentration of hemoglobin[18], hematocrit and serum osmolality[15] have been reported. Several authors have stated that maintaining appropriate hydration is possible to dilute the urine, and reduce its calcium and phosphate concentration[19,20]. In consequence, the risk of calcium phosphate crystalluria and precipitation in the renal tubules is diminished[16,21]. Sanders et al[18] have corroborated the efficiency of intravenous hydration (average 2 L) during colonic cleansing for surgery, but this requires a hospital stay and makes ambulatory procedures difficult. Markowitz et al[21] has suggested that patients must be encouraged to drink eight cups of fluids (1920 mL) and Rex et al[19] have promoted taking 3.6 L of clear fluids.
Following the 1999 American Society of Anesthesiologists recommendations for all interventions that require general anesthesia or sedation, oral fluid intake is allowed up to 2 h before colonoscopic evaluation[22]. The rationale for the preoperative fasting is to reduce the content and acidity of the stomach, thus avoiding the risk of aspiration pneumonitis at induction of anesthesia[23,24].
Since the seminal studies of Beaumont in 1833[25], it is widely known that emptying of clear liquids is passive, without the need for gastric motility, and is completed in < 60 min[26]. Clear fluids have a washing and dragging effect that allows the gastric content to move easily to the duodenum[27]. Patients with 2 h fasting with clear liquids (i.e. no liquid intake for 2 h before colonoscopy) had less volume and gastric acidity than those with complete 8 h fasting[28-32]. These results also have been reported in children[33-36].
The absence of fluid intake before surgery favors the development of hypotensive reactions during anesthesia induction, as well as dehydration, hypoglycemia and a strong sensation of thirst and hunger that leads to irritability, especially in older patients and infants[8,37]. Clear liquid intake not only diminishes the risk of aspiration pneumonia and notably improves patient wellbeing, but it also facilitates adequate hydration.
To evaluate the changes produced by the administration of OSP, it was vital to avoid dehydration. We encouraged patients to freely take 4 L of clear liquids during colonic preparation, up to 2 h before the test. This did not lead to a significant incidence of dehydration and hypotension, which was reinforced by no significant modifications in haemoglobin and hematocrit. The average reduction in PV was 3.65%, which represented dehydration of < 1.46%, which was not significant[13,14]. Besides, in contrast to Gutierrez Santiago’s study[15], we observed a decrease in the average osmolality. Only 4% of the patients developed hypotension, a degree of blood pressure reduction of 20%-30%. These results support the efficiency of this oral hydration regime for avoiding dehydration.
At the onset of our study, the suggested interval between doses was 5-10 h, and we used a 5-h interval. As 28% of the phosphate taken is retained by the body for up to 18 h[16,38], recent studies have recommended longer intervals between doses[4].
The maximum safe dose of sodium phosphate is 90 mL[39]. Several studies on the adverse effects of high doses of OSP have suggested that these should be avoided[2,3], as is the case with their association with phosphate enemas[40-42]. If the recommended dose of 60 g (90 mL) is surpassed, or if the interval between doses is < 5 h, severe hyperphosphatemia could develop[2,19,39,43-46].
Many authors make reference to the fact that administering laxatives and sodium phosphate enemas[40,41] leads to a slight though statistically significant increase in phosphorus and a decrease in calcium concentration[2,19,21,47-49], due to intestinal absorption[2]. However, they have also suggested that well-hydrated adults who have normal renal function tolerate the amount of phosphate loading without showing significant adverse effects[6,4,49-52]. This trend was confirmed in our study, with a maximum registered plasma phosphate level of 9.6 mmol/L and a minimum calcium level of 0.5 mmol/L.
The serious electrolyte disturbances reported have appeared in patients in whom sodium phosphate was contraindicated: inflammatory colonic diseases (Crohn’s disease, ulcerative colitis)[53,54], delayed intestinal transit (megacolon, obstruction), and in conditions with intestinal vascular alteration (congestive heart failure, ischemic colitis)[49]. It also has been reported in patients with impaired renal function[55-57], or who receive drugs that affect kidney perfusion (diuretics, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers). To avoid the administration of OSP in the sub-clinical kidney disease, measurement of urea and creatinine is recommended[43]. Hyperphosphatemia has been observed in patients with dehydration, ascites[4] or vomiting[45].
Fine et al[57] have found that the mortality rate was 33%, and that the risk of death was high if serum phosphate increased beyond 32.69 mg/dL (10.56 mmol/L). Most of the deaths reported in the literature have been caused by arrhythmia or heart attack associated with electrolyte changes and dehydration[4]. Fatal cases have been observed among patients with a history of renal damage[41,43,50], ischemic colitis[50], cirrhosis[43], and in elderly patients with normal renal function[42,58]. Azzam et al[43]and Wexner et al[5] have described high levels of phosphate and kidney damage in patients without previous kidney pathology.
Gutierrez-Santiago et al[15] have found an increase in phosphatemia in 57% of patients, while Lieberman et al[51] have found it in 25%. Both studies were retrospective and they did not specify the patient’s clinical condition. In our study in low-risk patients, we found an increase in phosphate in a significant percentage (87%). The average increase of serum phosphate was 1.84 mg/dL, which was less than that reported by Tan et al[59] (3.09-3.18 mg/dL). The maximum plasma phosphate value registered was 9.6 mg/dL (3.1 mmol/L), which was twice the normal concentration. This result shows that OSP used as laxative is not free of complications, even in low-risk patients. These values do catch our attention because the careful selection of patients anticipated a much lower incidence. It is possible to assume that the wide hydration plan and careful selection of participants avoided reaching the values described by Fine et al[57].
All of the patients had normal urea and creatinine values before and after colonic cleansing. We linked the phosphate values with the TBW and GFR, and both showed a negative linear correlation with the increase in phosphate. We observed that the lower the GFR and TBW, the higher the chance of developing hyperphosphatemia. These parameters describe the relationship of weight with a specific function, which shows that the increase in phosphatemia has a negative linear correlation with weight. We avoided dehydration and there was no renal impairment, therefore, these findings contribute towards the concept that hyperphosphatemia is the result of an excessive dose of laxative, as suggested by Rex et al[4].
Tan et al[2] have stated that the decrease in plasma calcium associated with OSP-induced hyperphosphatemia is the result of the binding of calcium to the high phosphate level, and thus, the tubular deposition that induces kidney damage. Gutierrez-Santiago et al[15] have observed hypocalcemia in 36% of patients. In our study, the decrease in calcium concentration developed in 29% of the patients, but none had symptoms related to hypocalcemia.
The reported OPS-induced hypernatremia is the result of intestinal sodium absorption and can worsen due to dehydration[2,15]. We did not observe an increase of plasma sodium in our patients, which suggests that the hydration level achieved with this diet was appropriate.
The sodium and potassium exchange across the colonic epithelium can generate hypokalemia, which is accentuated by renal potassium loss induced as a consequence of the volume contraction-associated secondary aldosteronism[2,15]. The decrease in potassium in our sample coincided with that observed by Rex et al[4]. It appeared in 4% of the patients and reached 2.4 mmol/L in one case.
Unlike previous studies by other investigators, we did not observe changes in plasma chloride values in our patients[4].
The results in this study show that, in low-risk, well-hydrated patients, hyperphosphatemia following standard OSP doses is related to weight. This is the reason why we believe that, in low-weight patients, lower doses of the laxative should be administered. We consider that further studies are necessary to establish the adequate dose according to weight.
Oral hydration with 4 L of clear liquids during colonic preparation has proven its efficacy in avoiding dehydration.
The possibility of achieving high phosphate levels in low-risk, well-hydrated patients is certainly alarming, especially given the fact that few medical professionals currently take this possibility into account. These discoveries emphasize the need to carry out an adequate hydration and selection of patients to avoid administration of OSP to those individuals at risk of developing hyperphosphatemia or renal failure.
Colon cleansing is used widely for colonoscopic exploration and colonic and gynecological surgery. Oral sodium phosphate (OSP) solution is the osmotic laxative most commonly used for this purpose. It is known that OSP can induce severe hyperphosphatemia and hypocalcemia due to excessive absorption of phosphates, and there have been reports of deaths and irreversible dialysis-requiring renal insufficiency.
Hyperphosphatemia after OSP develops in patients with conditions that increase its intestinal absorption (ulcerative colitis, Crohn’s disease, ischemic colitis), in conditions in which its elimination is difficult (kidney disease, dehydration, aging), or after OSP overdose (> 60 g). These findings have come from case reports and some rare retrospective studies. No prospective studies have investigated the prevalence of hyperphosphatemia in low-risk patients.
This was a prospective study that was carried out in low-risk patients. Even though, the authors avoided the conditions that are known to facilitate hyperphosphatemia such as dehydration (inducing oral intake of 4 L of clear liquids) and the diseases described above, 87% of the patients had high serum phosphate levels. None of them developed symptoms of hypocalcemia, and there was no evidence of renal impairment. Hyperphosphatemia was related inversely to body weight. These results highlight the importance of being cautious with the administration of OSP in patients with contraindications and promoting aggressive oral hydration.
Taking into account the results of this study, the authors recommend: performing preoperative evaluation aimed at avoiding administration of OSP laxatives to patients at risk; reducing the dose of OSP in patients with low weight; and avoiding dehydration with an adequate oral intake of clear liquids. Additional studies are necessary to establish the appropriate dose adjusted to body weight.
Hyperphosphatemia: serum phosphate levels above normal (2.5-4.5 mg/dL). Hypocalcemia: ionized calcium levels below normal values (1.0-1.35 mmol/L).
This paper presented provides reliable information on the side effects of OSP in low-risk patients. The conclusions addressed are useful for managing patients’ prescribed OSP for colon cleansing.
| 1. | Hwang KL, Chen WT, Hsiao KH, Chen HC, Huang TM, Chiu CM, Hsu GH. Prospective randomized comparison of oral sodium phosphate and polyethylene glycol lavage for colonoscopy preparation. World J Gastroenterol. 2005;11:7486-7493. |
| 2. | Tan HL, Liew QY, Loo S, Hawkins R. Severe hyperphosphataemia and associated electrolyte and metabolic derangement following the administration of sodium phosphate for bowel preparation. Anaesthesia. 2002;57:478-483. |
| 3. | Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointest Endosc. 2002;56:895-902. |
| 4. | Rex DK. Dosing considerations in the use of sodium phosphate bowel preparations for colonoscopy. Ann Pharmacother. 2007;41:1466-1475. |
| 5. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. |
| 6. | Beyea A, Block C, Schned A. Acute phosphate nephropathy following oral sodium phosphate solution to cleanse the bowel for colonoscopy. Am J Kidney Dis. 2007;50:151-154. |
| 7. | Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49:233-236. |
| 8. | Ferrari LR, Rooney FM, Rockoff MA. Preoperative fasting practices in pediatrics. Anesthesiology. 1999;90:978-980. |
| 9. | Van Beaumont W. Evaluation of hemoconcentration from hematocrit measurements. J Appl Physiol. 1972;32:712-713. |
| 10. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. |
| 11. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. |
| 12. | K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. |
| 13. | Da Silva AI, Fernandez R. Dehydration of football referees during a match. Br J Sports Med. 2003;37:502-506. |
| 14. | Costill DL, Cote R, Fink W. Muscle water and electrolytes following varied levels of dehydration in man. J Appl Physiol. 1976;40:6-11. |
| 15. | Gutierrez-Santiago M, Garcia-Unzueta M, Amado JA, Gonzalez-Macias J, Riancho JA. [Electrolyte disorders following colonic cleansing for imaging studies]. Med Clin (Barc). 2006;126:161-164. |
| 16. | Patel V, Emmett M, Santa Ana CA, Fordtran JS. Pathogenesis of nephrocalcinosis after sodium phosphate catharsis to prepare for colonoscopy: Intestinal phosphate absorption and its effect on urine mineral and electrolyte excretion. Hum Pathol. 2007;38:193-194; author reply 194-195. |
| 17. | Schiller LR. Clinical pharmacology and use of laxatives and lavage solutions. J Clin Gastroenterol. 1999;28:11-18. |
| 18. | Sanders G, Mercer SJ, Saeb-Parsey K, Akhavani MA, Hosie KB, Lambert AW. Randomized clinical trial of intravenous fluid replacement during bowel preparation for surgery. Br J Surg. 2001;88:1363-1365. |
| 19. | Rex DK. Phosphate nephropathy. Am J Gastroenterol. 2008;103:807. |
| 20. | Curran MP, Plosker GL. Oral sodium phosphate solution: a review of its use as a colorectal cleanser. Drugs. 2004;64:1697-1714. |
| 21. | Markowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389-3396. |
| 22. | Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology. 1999;90:896-905. |
| 23. | Ng A, Smith G. Gastroesophageal reflux and aspiration of gastric contents in anesthetic practice. Anesth Analg. 2001;93:494-513. |
| 24. | Smith G, Ng A. Gastric reflux and pulmonary aspiration in anaesthesia. Minerva Anestesiol. 2003;69:402-406. |
| 25. | Beaumont W. Gastric juice and physiology of digestion. Pittsburg. Allen. 1833;159-160 ISBN: 0486692132. |
| 26. | Moore JG, Christian PE, Coleman RE. Gastric emptying of varying meal weight and composition in man. Evaluation by dual liquid- and solid-phase isotopic method. Dig Dis Sci. 1981;26:16-22. |
| 27. | Meakin G. Preoperative fasting of children. Anesthesiology. 1990;72:579-580. |
| 28. | Soreide E, Stromskag KE, Steen PA. Statistical aspects in studies of preoperative fluid intake and gastric content. Acta Anaesthesiol Scand. 1995;39:738-743. |
| 29. | Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003;CD004423. |
| 30. | Fasting S, Soreide E, Raeder JC. Changing preoperative fasting policies. Impact of a national consensus. Acta Anaesthesiol Scand. 1998;42:1188-1191. |
| 31. | Pandit SK, Loberg KW, Pandit UA. Toast and tea before elective surgery? A national survey on current practice. Anesth Analg. 2000;90:1348-1351. |
| 32. | Martay K, Vater Y, Hunter C, Ross B. Preoperative fasting after soft drink intake: 2 hours may be enough. J Anesth. 2002;16:179-180. |
| 33. | Brady M, Kinn S, O'Rourke K, Randhawa N, Stuart P. Preoperative fasting for preventing perioperative complications in children. Cochrane Database Syst Rev. 2005;CD005285. |
| 34. | Splinter WM, Schreiner MS. Preoperative fasting in children. Anesth Analg. 1999;89:80-89. |
| 35. | Guideline Development Group. Perioperative fasting in adults and children. Royal College of Anaesthetists. Royal College of Nursing, London. 2005; ISBN: 1-904114-20-2. |
| 36. | Tolia V, Peters JM, Gilger MA. Sedation for pediatric endoscopic procedures. J Pediatr Gastroenterol Nutr. 2000;30:477-485. |
| 37. | Nygren J, Thorell A, Ljungqvist O. Are there any benefits from minimizing fasting and optimization of nutrition and fluid management for patients undergoing day surgery? Curr Opin Anaesthesiol. 2007;20:540-544. |
| 38. | Barkun A, Chiba N, Enns R, Marcon M, Natsheh S, Pham C, Sadowski D, Vanner S. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety--a Canadian Association of Gastroenterology position paper. Can J Gastroenterol. 2006;20:699-710. |
| 39. | Food and Drug Administration. Science backgrounder: Safety of sodium phosphates oral solution. Available from: http://www.fda.gov/cder/drug/safety/sodiumphospate.htm 2001. |
| 40. | Everman DB, Nitu ME, Jacobs BR. Respiratory failure requiring extracorporeal membrane oxygenation after sodium phosphate enema intoxication. Eur J Pediatr. 2003;162:517-519. |
| 41. | Martin RR, Lisehora GR, Braxton M Jr, Barcia PJ. Fatal poisoning from sodium phosphate enema. Case report and experimental study. JAMA. 1987;257:2190-2192. |
| 42. | Farah R. Fatal acute sodium phosphate enemas intoxication. Acta Gastroenterol Belg. 2005;68:392-393. |
| 43. | Azzam I, Kovalev Y, Storch S, Elias N. Life threatening hyperphosphataemia after administration of sodium phosphate in preparation for colonoscopy. Postgrad Med J. 2004;80:487-488. |
| 44. | Gonlusen G, Akgun H, Ertan A, Olivero J, Truong LD. Renal failure and nephrocalcinosis associated with oral sodium phosphate bowel cleansing: clinical patterns and renal biopsy findings. Arch Pathol Lab Med. 2006;130:101-106. |
| 45. | Available from: http://www.fda.gov/cder/drug/infopage/OSP_solution/backgrounder.htm. |
| 46. | Huynh T, Vanner S, Paterson W. Safety profile of 5-h oral sodium phosphate regimen for colonoscopy cleansing: lack of clinically significant hypocalcemia or hypovolemia. Am J Gastroenterol. 1995;90:104-107. |
| 47. | Gumurdulu Y, Serin E, Ozer B, Gokcel A, Boyacioglu S. Age as a predictor of hyperphosphatemia after oral phosphosoda administration for colon preparation. J Gastroenterol Hepatol. 2004;19:68-72. |
| 48. | Lien YH. Is bowel preparation before colonoscopy a risky business for the kidney? Nat Clin Pract Nephrol. 2008;4:606-614. |
| 49. | Markowitz GS, Nasr SH, Klein P, Anderson H, Stack JI, Alterman L, Price B, Radhakrishnan J, D'Agati VD. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol. 2004;35:675-684. |
| 50. | Ullah N, Yeh R, Ehrinpreis M. Fatal hyperphosphatemia from a phosphosoda bowel preparation. J Clin Gastroenterol. 2002;34:457-458. |
| 51. | Lieberman DA, Ghormley J, Flora K. Effect of oral sodium phosphate colon preparation on serum electrolytes in patients with normal serum creatinine. Gastrointest Endosc. 1996;43:467-469. |
| 52. | Desmeules S, Bergeron MJ, Isenring P. Acute phosphate nephropathy and renal failure. N Engl J Med. 2003;349:1006-1007. |
| 53. | Campisi P, Badhwar V, Morin S, Trudel JL. Postoperative hypocalcemic tetany caused by fleet phospho-soda preparation in a patient taking alendronate sodium: report of a case. Dis Colon Rectum. 1999;42:1499-1501. |
| 54. | Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, Grosso B, Jimenez A, Ortega J, Quintero E. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006;12:6161-6166. |
| 55. | Hookey LC, Vanner S. Recognizing the clinical contraindications to the use of oral sodium phosphate for colon cleansing: a case study. Can J Gastroenterol. 2004;18:455-458. |
| 56. | Chan A, Depew W, Vanner S. Use of oral sodium phosphate colonic lavage solution by Canadian colonoscopists: pitfalls and complications. Can J Gastroenterol. 1997;11:334-338. |
| 57. | Fine A, Patterson J. Severe hyperphosphatemia following phosphate administration for bowel preparation in patients with renal failure: two cases and a review of the literature. Am J Kidney Dis. 1997;29:103-105. |
| 58. | Aydogan T, Kanbay M, Uz B, Kaya A, Isik A, Bozalan R, Erkman M, Akcay A. Fatal hyperphosphatemia secondary to a phosphosoda bowel preparation in a geriatric patient with normal renal function. J Clin Gastroenterol. 2006;40:177. |
| 59. | Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy - a meta-analysis. Colorectal Dis. 2006;8:247-258. |
Peer reviewer: Dr. Josep M Bordas, Department of Gastroenterology, Hospital Clinic, Barcelona 08022, Spain
S- Editor Cheng JX L- Editor Kerr C E- Editor Ma WH