Published online Dec 21, 2009. doi: 10.3748/wjg.15.5946
Revised: September 12, 2009
Accepted: September 19, 2009
Published online: December 21, 2009
AIM: To evaluate the safety of adding ketoprofen to pegylated-interferon (PEG-IFN) with or without ribavirin and the effect on viral kinetics, STAT1 activity and expression of 2’-5’-oligoadenylate synthetase (2’-5’OAS) in genotype 1 chronic hepatitis C in a phase II study.
METHODS: Forty-five patients were studied: fifteen were randomized to PEG-IFN plus ribavirin (PR), 16 to PEG-IFN plus ketoprofen and 14 to PR and ketoprofen. The molecular study of IFN-dependent signal transduction was conducted in 9 patients from each group.
RESULTS: The combination of ketoprofen and PEG-IFN with or without ribavirin was safe and well tolerated. An early activation of STAT1 was observed in ketoprofen-treated patients, but this activation was less sustained over time. Conversely, ketoprofen plus PEG-IFN and ribavirin induced an early and sustained increase of 2’-5’OAS transcription starting 24 h after the first dose until the 36th wk. These data are consistent with the clinical results, showing a better sustained virological response and a lower relapse rate in patients receiving ketoprofen plus PEG-IFN and ribavirin.
CONCLUSION: The addition of ketoprofen to the standard therapy of chronic hepatitis C should be explored in larger randomized clinical studies.
- Citation: Gramenzi A, Cursaro C, Margotti M, Balsano C, Spaziani A, Anticoli S, Loggi E, Salerno M, Galli S, Furlini G, Bernardi M, Andreone P. Ketoprofen, peginterferon 2a and ribavirin for genotype 1 chronic hepatitis C: A phase II study. World J Gastroenterol 2009; 15(47): 5946-5952
- URL: https://www.wjgnet.com/1007-9327/full/v15/i47/5946.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5946
The treatment of chronic hepatitis C virus (HCV) infection has dramatically improved over the last 20 years. Interferon α (IFNα) was one of the first agents used to treat this infection, but its efficacy in terms of sustained virological response (SVR, i.e. sustained absence of HCV from serum up to 6 mo after stopping therapy) was extremely poor[1]. Strategies to overcome this limitation have included increasing the duration of therapy or adding ribavirin (an antiviral drug) to the treatment regimen[2,3]. The introduction of a “pegylated” preparation substantially increased the antiviral activity of IFNα. Today, pegylated-interferon (PEG-IFN) in combination with ribavirin is the standard treatment for patients with chronic hepatitis C[4]. Large international controlled clinical trials have demonstrated that this combination therapy yields SVR rates in 54%-63% of treated HCV-infected patients[5-7]. However, patients infected with HCV genotype 1 are particularly resistant to antiviral treatment, as demonstrated by the lower SVR rates observed in this patient subset, ranging from 42% to 52%[5-7]. Thus, a substantial proportion of patients remain unresponsive to antiviral treatment.
IFN signaling pathways are activated by binding of IFN to its specific receptor, which induces autophosphorylation of protein tyrosine kinases Tyk-2 and Jak-1 on tyrosine residues, thus activating signal transducer and activator of transcription (STAT1 and STAT2) proteins. Activated STATs translocate to the nucleus where they activate the transcription of IFN-inducible genes, such as 2’-5’-oligoadenylate synthetase (2’-5’OAS)[8]. Non-steroidal antiinflammatory drugs (NSAIDs) have been demonstrated to amplify the IFN signaling pathways and to enhance the anti-viral effect of IFN[9-14]. Furthermore, it has recently been found that acetylsalicylic acid suppresses HCV expression in a hepatoma cell line containing HCV subgenomic replicon[15]. However, clinical studies evaluating the use of NSAIDs in combination with standard IFNα in patients with chronic HCV infection have given conflicting results[16-20], although results with ketoprofen have generally been encouraging[17-21].
A rational approach to combination therapies for patients with chronic HCV infection demands a detailed knowledge of how the different drugs affect viral kinetics and IFN intracellular signaling. Therefore, we conducted a pilot phase II study to evaluate the effect of ketoprofen plus PEG-IFN with or without ribavirin compared with PEG-IFN plus ribavirin (PR) on viral kinetics, STAT1 activity and expression of the IFN-dependent gene 2’-5’OAS in patients with chronic hepatitis C. We also assessed the safety and tolerability of these treatment schedules. In order to minimize the influence of HCV viral variability on our results, only patients infected with HCV genotype 1 were included.
Treatment-naive patients aged 18 to 65 years with chronic HCV infection genotype 1a or 1b were eligible for enrollment if they had: elevated alanine aminotransferase (ALT) levels within the previous 6 mo, a positive test for serum HCV-RNA and a liver biopsy specimen consistent with chronic hepatitis C obtained in the previous 12 mo. Patients were excluded if they had neutropenia (neutrophil count < 1.5 × 109 cells/L), thrombocytopenia (platelet count < 70 × 109 cells/L), anemia (hemoglobin level < 12.0 g/dL in women and < 13.0 g/dL in men) or a medical condition that would be clinically worsened by anemia, serum creatinine levels more than 1.5 times the upper limit of normal, evidence of liver disease due to causes other than chronic HCV infection, human immunodeficiency virus positivity, esophageal varices, decompensated liver disease, organ transplant, severe or poorly controlled psychiatric disease (especially depression), malignant neoplastic disease, severe cardiac or chronic pulmonary disease, history of peptic disease, autoimmune disease (except controlled thyroid disease), seizure disorder, alcohol or drug dependency within 1 year of study entry, clinically significant comorbid conditions; and, if female, pregnancy or unwilling to use contraception.
This was an open-label, randomized, phase II, pilot study. Patients eligible for the study were randomized into three groups: (1) PR group: patients received subcutaneous PEG-IFNα2a 180 μg/wk plus oral ribavirin 800 mg/d for 48 wk; (2) PEG-IFN plus ketoprofen (PK) group: patients received subcutaneous PEG-IFNα2a 180 μg/wk for 48 wk plus oral ketoprofen 200 mg twice daily for the first 4 wk and then 200 mg/d for the next 20 wk; (3) PEG-IFN plus ribavirin and ketoprofen (PRK) group: patients received subcutaneous PEG-IFNα2a 180 μg/wk plus oral ribavirin 800 mg/d for 48 wk plus oral ketoprofen 200 mg twice daily for the first 4 wk and then 200 mg/d for the next 20 wk.
After 24 wk, treatment was withdrawn in patients who did not experience a decrease from baseline in viral load of ≥ 2 log10 IU/mL or a negative qualitative serum HCV-RNA. A safety assessment was conducted in these patients between 4 and 8 wk after their last dose of study drug.
After the 48-wk study there was a 24-wk treatment-free follow-up period. Virological response was defined as a negative test for qualitative serum HCV-RNA. An end of treatment response (ETR) was defined as undetectable levels of HCV-RNA at the end of treatment (week 48) and SVR was defined as undetectable levels of HCV-RNA at the end of follow-up (24 wk after treatment cessation). Relapsers were defined as patients who obtained an ETR, but relapsed after completion of treatment and tested HCV-RNA positive at the end of follow-up.
PEG-IFNα2a and ribavirin were kindly supplied by Roche S.p.A., Monza (MI), Italy and ketoprofen by IBI (Istituto Biochimico Italiano) S.p.A., Aprilia (LT), Italy. All subjects gave written informed consent before entering the study. The study protocol and patient-informed consent forms were approved by the Institutional Ethics Committee of Azienda Ospedaliero-Universitaria di Bologna, Policlinico S.Orsola-Malpighi, Bologna, Italy (registration number: 58/2002/U) and the study was conducted according to the ethical guidelines of the Declaration of Helsinki.
All patients underwent liver biopsy within 12 mo before entry. Each liver biopsy was scored according to the histological activity index proposed by Knodell et al[22]. After the screening evaluations, laboratory parameters were monitored and recorded at regular intervals throughout the 48-wk study period and a physical examination was conducted at the end of treatment. Adverse events and serious adverse events were recorded throughout the study and up to the end of follow-up period.
Serum samples were collected from each patient before treatment (on day -1), at baseline (day 0), at 24 and 48 h after the first dose and at 1, 2, 4, 12, 24, 36, 48, 60 and 72 wk after the beginning of treatment. Quantitative HCV-RNA serum levels were assessed in all samples using a branched DNA assay (Versant® RNA 3.0 assay; Siemens, Milano, Italy). Virological response was assessed on weeks 1, 2, 4, 12, 24, 36, 48 and 72 by means of Transcription Mediated Amplification technique (Versant® HCV-RNA Qualitative Assay; Siemens, Milano, Italy; low limit of detection: 6 HCV IU/mL).
Peripheral blood mononuclear cells (PBMCs) were collected from patients at 0, 24 and 48 h and at 1, 2, 4 and 36 wk after the beginning of treatment. Total RNA was extracted from PBMCs using an “RNeasy Protect Mini Kit” (Qiagen, GmbH, Hilden, Germany). Full length cDNAs were synthesized from 1 μg total RNA using the “Thermoscript RT-PCR system” kit (Invitrogen), according to the manufacturer’s instructions.
Comparative RTQ-PCR was performed with the “Platinum SYBR Green qPCR SuperMix-UDG” kit (Invitrogen) and analyzed on Mx3000P apparatus from Stratagene.
A 0.2 μmol/L concentration of the following primers was used: for 2’-5’OAS forward 5'-ATTGACAGTGCTGTTAACATCATCC-3' and reverse 5'-GTGAGTTATGGAACACGACGAG-3'; for GAPDH forward 5'-GAAGGTGAAGGTCGGAGTC-3' and reverse 5'-GAAGATGGTGATGGGATTTC-3'. RTQ-PCR conditions used to amplify 2’-5’OAS and GAPDH cDNAs were: 95°C for 5 min, followed by 40 cycles comprising 30 s at 95°C, 30 s at 60°C and 1 min at 72°C. In order to check for DNA contamination, amplification of total RNA before cDNA synthesis was performed in parallel with amplification of the cDNA. Reactions were run in triplicate, and a mean value of the three samples was calculated. 2’-5’OAS mRNA levels were expressed as the relative amount of product adjusted for the level of GAPDH, using the Mx3000P software (Stratagene) and employing a comparative Ct (ΔΔCt) value method. Dissociation curves were generated to ensure that a single amplicon had been produced.
PBMCs were lysed with Ripa buffer containing 50 mmol/L Tris (pH 7.5), 100 mmol/L NaCl, 0.1% Nonidet P-40, 1 mmol/L EDTA, 2 mmol/L phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, 1 μg/mL of leupeptin and phosphatase inhibitors. Protein concentrations were measured using a colorimetric assay (Bio-Rad, Hercules, CA, USA). Equal amounts of proteins were electrophoresed on SDS-PAGE. Proteins were transferred to PVDF membrane (Immobilon-PVDF, Millipore) and treated with 1:300 specific primary antibody anti-STAT1 (Santa Cruz Biotechnology, Inc., CA, USA) and its activated form (Biosource). After incubation with peroxidase-coupled secondary antibodies (Santa Cruz Biotechnology), the sheets were visualized by ECL kits (Amersham, GE Healthcare Life Science, Germany). Antibody anti-actin was purchased from Santa Cruz Biotechnology.
This was a pilot study and therefore no formal sample size calculation was performed. Data were analyzed on an intention-to-treat basis. Patients who dropped out of the trial for any reason were classified as non responders. The safety population comprised all patients who received at least one dose of study drugs. χ2 test and Mann-Whitney U test were used to compare quantitative and qualitative variables between the groups, respectively. Differences in virological response rates between treatment groups were analyzed using Fisher’s exact test and/or Yates corrected χ2 test, as necessary. A P < 0.05 was considered to be significant. Data analysis was carried out using the SPSS for Windows version 11.0.1. Plots of the viral load were carried out using SigmaPlot version 9.0.
Forty-five patients were enrolled: 15 were randomized to the PR group, 16 to the PK group and 14 to the PRK group.
The baseline characteristics of the three treatment groups are reported in Table 1. Patients were comparable with respect to age, gender, serum ALT levels and histological features. Overall, 4 out 15 (27%) of PR patients, 3 out 16 of PK (19%) and 2 out of 14 (14%) of PRK patients had a liver fibrosis score equal to 3. The viral load was significantly different between the three groups, being significantly lower in the PR group (P = 0.01).
| PR group (n = 15) | PK group (n = 16) | PRK group (n = 14) | P-value | |
| Age (yr) | 45 ± 12 | 48 ± 12 | 42 ± 10 | NS |
| Sex (M/F) | 9/6 | 9/7 | 9/5 | NS |
| HCV-RNA × 103 IU/mL | 429 ± 578 | 1261 ± 1073 | 1555 ± 1322 | 0.01 |
| HCV-RNA > 700 × 103 IU/mL (%) | 4 (27) | 8 (50) | 10 (71) | 0.02 |
| ALT (U/L) | 82 ± 45 | 85 ± 33 | 87 ± 49 | NS |
| Median total HAI1 (range) | 8 (3-14) | 10 (6-13) | 8 (3-10) | NS |
| Median grading (range) | 7 (2-11) | 9 (5-10) | 7 (3-9) | NS |
| Median fibrosis (range) | 1 (1-3) | 1 (1-3) | 1 (0-3) | NS |
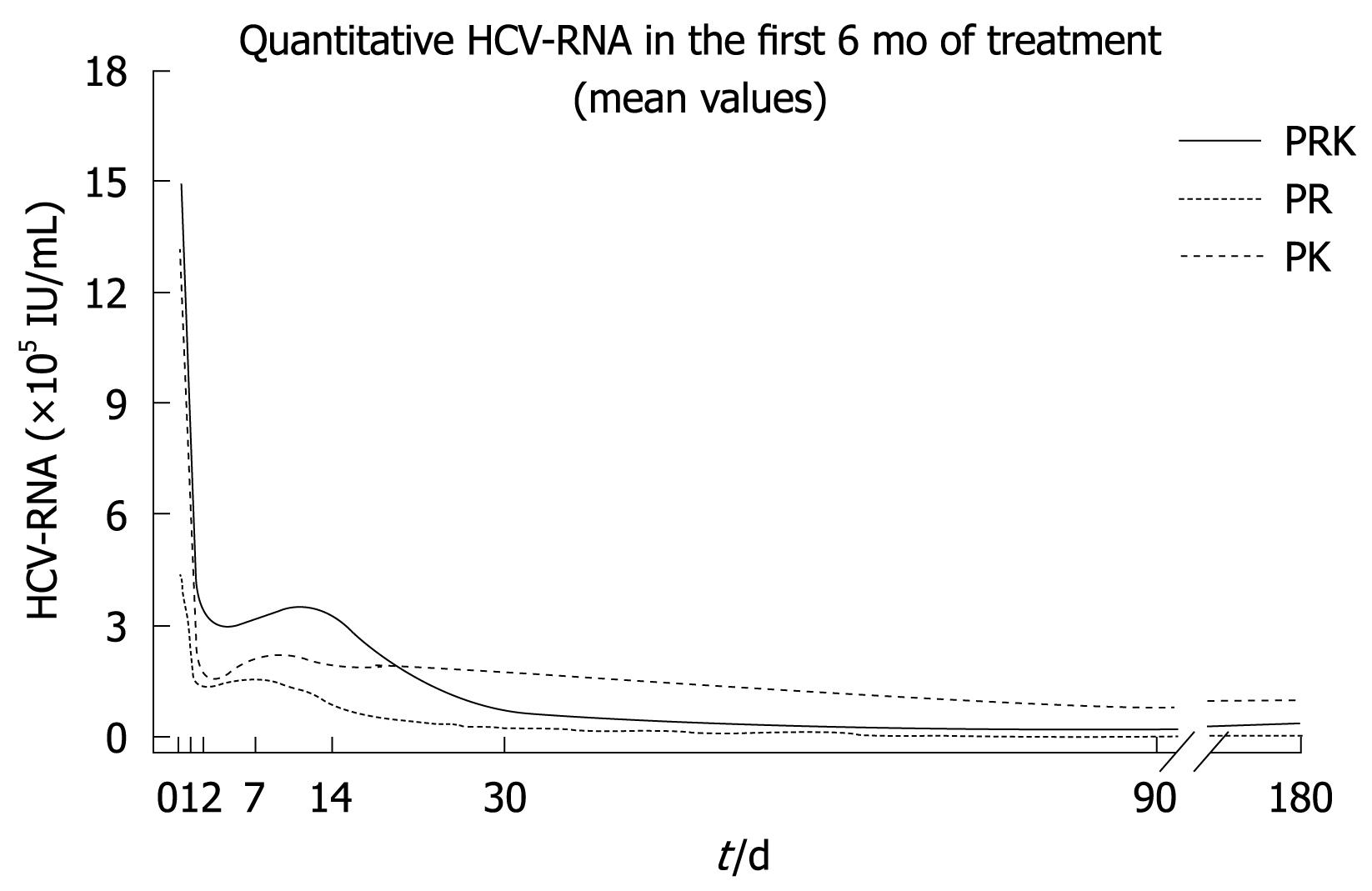
Mathematical modeling of the decline in HCV-RNA serum levels revealed a triphasic response in all treatment groups (Figure 1). The rapid first phase (1-2 d) was similar in the three groups, although HCV-RNA levels were consistently higher in the PRK group during this time. A second, or “shoulder” phase was observed between 2 and 7-14 d, where the decline in HCV-RNA levels was faster in the PR group compared with both PK and PRK groups. In particular, the PR group showed a slight and progressive reduction of viremia over time after week 1. In the third phase (week 2 onwards), the decrease in HCV-RNA levels was slowest in the PK group while it was more rapid in the PRK group. In this latter group, HCV-RNA levels declined to those observed in the PR group by study end.
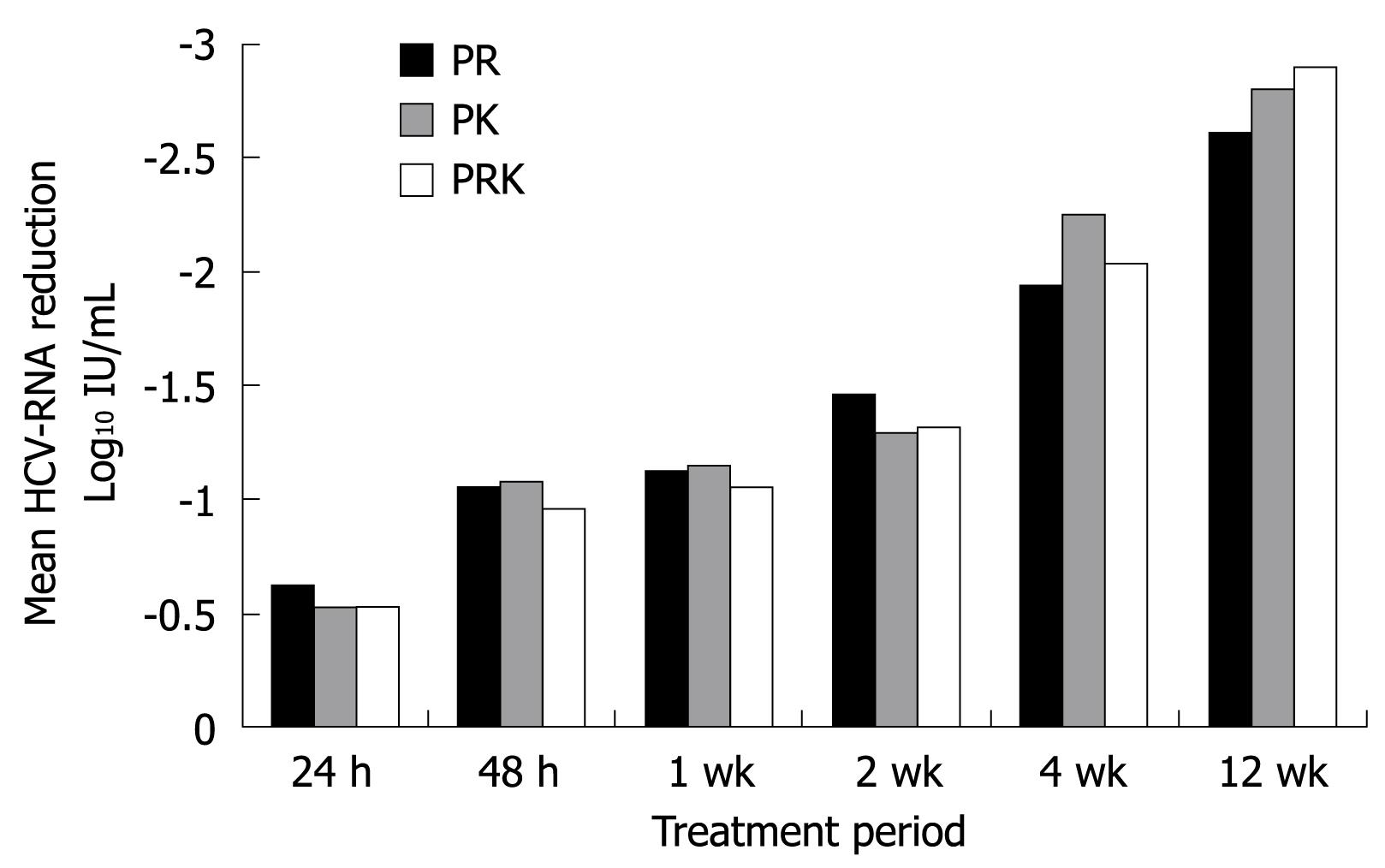
The mean log10 reduction from baseline in HCV-RNA levels over the course of the first 12 wk of treatment is shown in Figure 2. At week 4, the mean log10 decrease from baseline was comparable between the three groups (-1.95 in the PR group, -2.25 in the PK group and -2.05 in the PRK group). As shown in Table 2, some patients had undetectable HCV-RNA levels as early as 7-14 d after starting treatment. The PR treatment group displayed an earlier virological response when compared with the other two groups (Table 2).
| PR group (n = 15) | PK group (n = 16) | PRK group (n = 14) | P-value | |
| Virological response rate | ||||
| Week 1 | 0 | 0 | 1 (7.1) | NS |
| Week 2 | 1 (6.7) | 1 (6.3) | 2 (14.3) | NS |
| Week 4 | 5 (33.3) | 2 (12.5) | 3 (21.4) | NS |
| Week 12 | 10 (66.7) | 7 (43.8) | 7 (50) | NS |
| Week 24 | 11 (73.3) | 11 (68.8) | 10 (71.4) | NS |
| ETR | 11 (73.3) | 11 (68.8) | 10 (71.4) | NS |
| Relapse rate | 4/11 (36.4) | 6/11 (54.5) | 2/10 (20) | NS |
| SVR | 7 (46.7) | 5 (31.3) | 8 (57.1) | NS |
An ETR was obtained in 11/15 patients (73%) in the PR group, 11/16 (69%) in the PK group and 10/14 (71%) in the PRK group. During the treatment-free follow-up period, the relapse rate was lower in the PRK group than in both the other two groups, but the differences were not statistically significant. A SVR was obtained in 7 patients in the PR group (47%), in 5 in the PK group (31%) and in 8 in the PRK group (57%). No association was found between baseline viremia and SVR in any treatment group. However, in the subgroup of patients with high baseline viremia (> 700 × 103 IU/mL), 6/10 (60%) of the PRK group achieved a SVR compared to a quarter (25%) of the PR group and 3/8 (37.5%) of the PK group.
The first 9 randomized patients in each group were enrolled in the molecular study of IFN-dependent signal transduction. Their characteristics are described in Table 3.
| PR group (n = 9) | PK group (n = 9) | PRK group (n = 9) | P-value | |
| Age (yr) | 42 ± 13 | 54 ± 9 | 42 ± 12 | 0.04 |
| Sex (M/F) | 5/4 | 4/5 | 5/4 | NS |
| HCV-RNA × 103 IU/mL | 263 ± 232 | 1032 ± 878 | 1742 ± 1392 | 0.01 |
| HCV-RNA > 700 × 103 IU/mL | 1 (27) | 4 (44) | 7 (78) | 0.02 |
| ALT (U/L) | 70 ± 21 | 80 ± 27 | 100 ± 57 | NS |
| ETR | 7 (78) | 5 (56) | 6 (67) | NS |
| SVR | 5 (56) | 3 (33) | 5 (56) | NS |
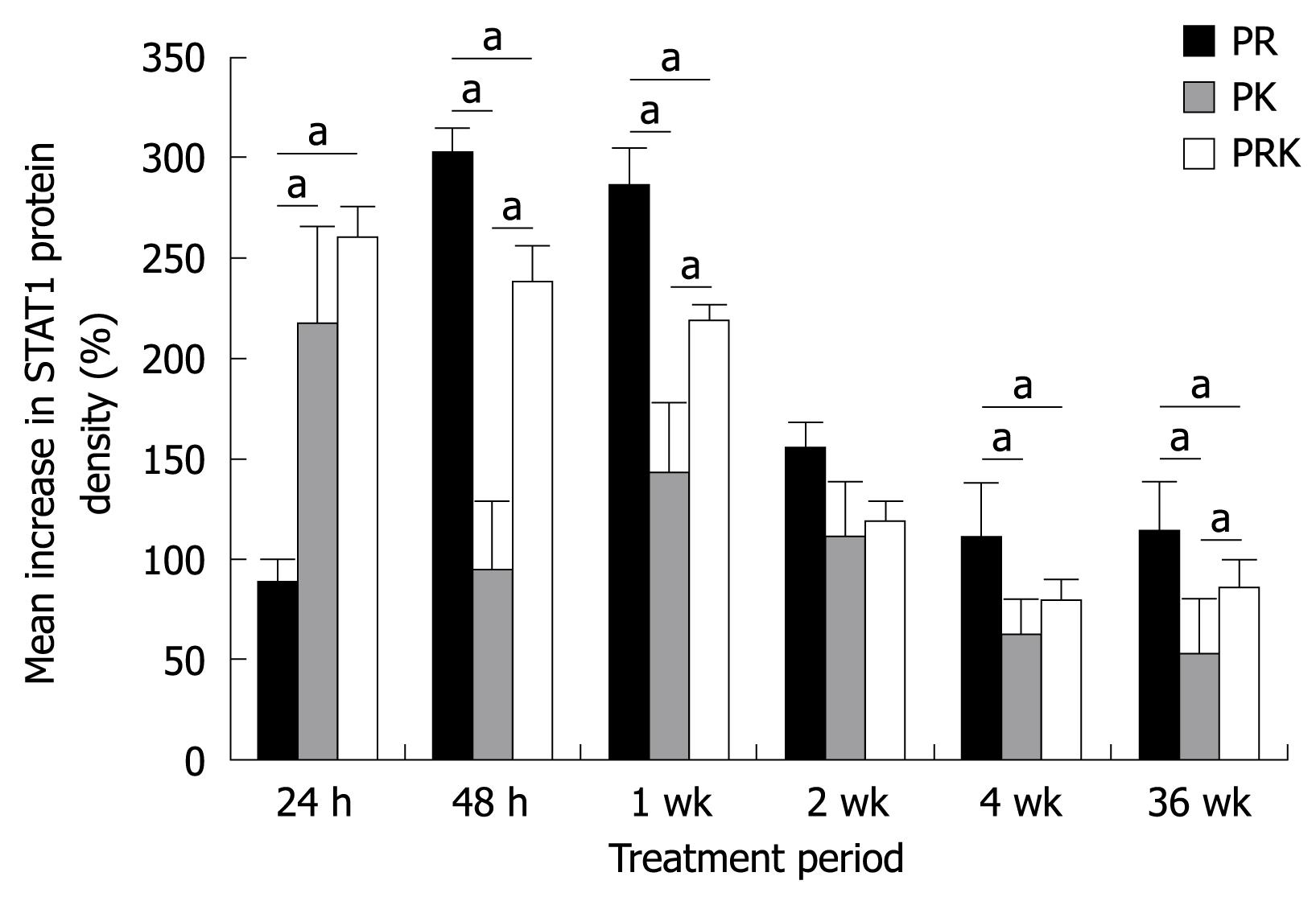
A time course analysis of STAT1 activity over 36 wk by means of densitometric analysis was performed. STAT1 activity was consistently up-regulated, as shown by an increase from baseline in mean STAT1 density as early as 24 h after the first drug administration. Nevertheless, a difference between the three treatment groups can be seen in Figure 3. At 24 h, the increase from baseline in STAT1 density was significantly higher in both ketoprofen-treated groups as compared with patients of the PR group. However, in PK patients STAT1 activation not only peaked earlier, but it also decreased more rapidly, while in the PRK group a strong STAT1 activation was still present after 1 wk. In contrast, in PR patients the activation of STAT1 peaked at 48 h, 24 h later than in both PK and PRK patients, but was still maintained at higher levels after 1 wk. A steady state was gained between 2 to 36 wk. Thus, the activation of STAT1 in the presence of ribavirin was slower and peaked later but was sustained longer as compared with that observed in the presence of ketoprofen. No relationship was found between STAT1 and viral load. There was no significant difference in STAT1 activity between patients with a SVR and non responders (data not shown).
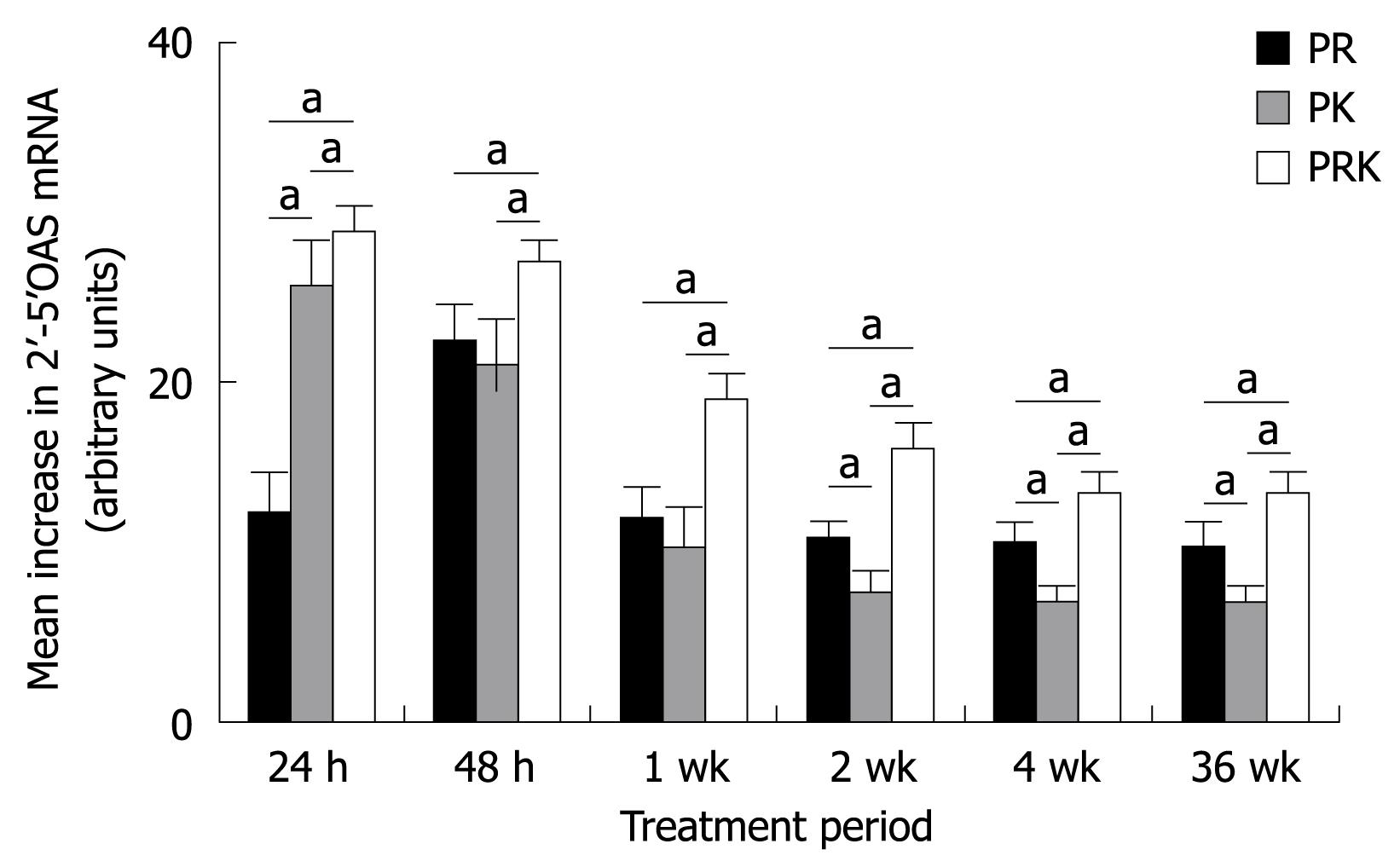
The analysis of 2’-5’OAS mRNA levels by RTQ-PCR (Figure 4) showed that the presence of ketoprofen increased the transcription rate of this gene, especially early in treatment (24 h after the first dose). The addition of ketoprofen resulted in an early upregulation of 2’-5’OAS mRNA level and permitted the maintenance of this transcript at significantly higher levels in the PRK group compared with the PR and PK groups, from the beginning of treatment until week 36. Finally, 2’-5’OAS mRNA levels were significantly higher in patients with a SVR than in non responders at each time point and in each group (data not shown).
The type and frequency of adverse events were similar in the three treatment arms and are summarized in Table 4. Anemia, defined as hemoglobin level < 12.0 g/dL in women and < 13.0 g/dL in men, and neutropenia, defined as neutrophil count < 1000/mm3 were the most common side effects. However, only one patient in the PRK group met the hemoglobin criterion for ribavirin dose reduction (hemoglobin level < 10 and ≥ 8.5 g/dL), while the dose of PEG-IFN was reduced because of neutropenia (neutrophil count of < 750 and ≥ 500/mm3) in one patient each in the PR and in PK group. The latter developed a pneumonia requiring antibiotic treatment. No patient experienced renal dysfunction. In particular, in no case did creatinine serum levels exceed the upper limit of normal range (1.2 mg/dL) during treatment. Time course evaluation of hemoglobin and creatinine serum levels during treatment in the three groups is reported in Table 5.
| PR group (n = 15) | PK group (n = 16) | PRK group (n = 14) | P-value | |
| Anemia | 8 (53) | 7 (44) | 11 (79) | NS |
| Neutropenia | 9 (60) | 7 (44) | 10 (71) | NS |
| Arthro-myalgia | 4 (27) | 4 (25) | 5 (36) | NS |
| Headache | 3 (20) | 3 (19) | 2 (14) | NS |
| Fatigue | 5 (33) | 5 (31) | 4 (29) | NS |
| Flu-like symptoms | 4 (27) | 4 (25) | 2 (14) | NS |
| Psychiatric disorders | 5 (33) | 4 (25) | 4 (29) | NS |
| Gastrointestinal disorders | 6 (40) | 3 (19) | 4 (29) | NS |
| Pruritus | 4 (27) | 1 (6) | 0 | NS |
| Insomnia | 2 (13) | 2 (12.5) | 2 (14) | NS |
| PR group (n = 15) | PK group (n = 16) | PRK group (n = 14) | |
| Hemoglobin (g/dL) | |||
| Baseline | 14.5 ± 1.3 | 14.2 ± 1.9 | 14.9 ± 1.5 |
| Week 12 | 12.2 ± 1.4 | 12.5 ± 1.7 | 12.2 ± 1.6 |
| Week 24 | 12.2 ± 1.6 | 12.6 ± 1.8 | 11.9 ± 1.7 |
| Week 48 | 11.6 ± 1.4 | 13.1 ± 1.8 | 12.0 ± 1.6 |
| Creatinine (mg/dL) | |||
| Baseline | 0.97 ± 0.13 | 0.82 ± 0.22 | 1.01 ± 0.07 |
| Week 12 | 0.87 ± 0.11 | 0.82 ± 0.16 | 0.92 ± 0.12 |
| Week 24 | 0.86 ± 0.15 | 0.79 ± 0.16 | 0.91 ± 0.15 |
| Week 48 | 0.44 ± 0.15 | 0.80 ± 0.17 | 0.92 ± 0.15 |
One patient each in the PR group and the PK group withdrew prematurely from the study. The patient in the PR group dropped out after 1 mo because of severe depression and the patient in the PK group dropped out after 6 mo because of poor compliance.
The objective of this phase II study was to assess the safety of ketoprofen in combination with PEG-IFNα2a with or without ribavirin in treatment-naive patients with HCV genotype 1 infection, and the effect of these regimens on viral kinetics and IFNα signaling modulation. Our results showed that ketoprofen was safe and well tolerated. In particular, gastrointestinal-related adverse events were mild and did not lead to dose reduction or to premature treatment discontinuation in any patient. Given the importance of managing the tolerability of HCV antiviral treatment, our observation that the addition of ketoprofen to PEG-IFN and ribavirin is as well tolerated as the standard regimen is reassuring.
The kinetics of viral decay during treatment showed a triphasic response that was more evident in patients receiving ketoprofen, independent of the use of ribavirin. Following the first phase of rapid decline in HCV-RNA levels, patients receiving ketoprofen showed a pronounced “shoulder phase” starting 2 to 14 d after initiation of therapy. It has recently been suggested that the triphasic decline in HCV-RNA levels occurs only in patients in whom a majority of hepatocytes are infected before therapy[23]. Thus, the higher baseline viremia of both ketoprofen groups could help to explain the differences in viral kinetics between the groups receiving ketoprofen and the PR group. Interestingly, in the third and final phase, the HCV-RNA levels were lower in the two groups receiving ribavirin than in the PK group. This enhanced response to treatment in ribavirin recipients supports the hypothesis that ribavirin not only improves the anti-HCV immune response, but also had a mutagenic effect against HCV[23,24]. However, it should be pointed out that in both the PK and PRK groups, ketoprofen was administered only during the first 24 wk of treatment. Thus, the similarity between the PRK and PR groups in HCV-RNA levels during the final phase could be attributable to the absence of ketoprofen in the PRK group during this time.
As far as the IFNα signaling modulation is concerned, the activation of STAT1 occurred very early after treatment initiation in the two groups receiving ketoprofen, being evident after 24 h from the start of treatment. However, there was a rapid decline in STAT1 activation thereafter, particularly in the PK group. In contrast, the PR group exhibited the greatest activation after 48 h and this activation was more sustained over time compared with the ketoprofen-containing regimens. The mechanisms responsible for these differences in the control of IFN-induced responses probably include down-regulation and degradation of receptors[13]. Moreover, it has been demonstrated that both ribavirin and NSAIDs act synergistically with IFN-α in induction of STAT1 activation[13,25], yet there was lower STAT1 activation in the PRK group compared with the PR group. Thus, an antagonistic effect between ketoprofen and ribavirin cannot be excluded.
On the other hand, our data demonstrated an early and sustained increase of 2’-5’OAS transcription in the PRK group compared with the PR group, suggesting that the addition of ketoprofen to the conventional combination therapy induces early activation of the IFNα pathway, followed by a better activation of the IFNα-dependent intracellular pathway.
Even if this study was not designed to assess antiviral efficacy, the clinical results are consistent with the molecular data. At baseline, the PR group had a significantly lower mean HCV viral load than both the PK and PRK groups. Thus, the proportion of patients with low viral load (≤ 700 000 IU/mL) was significantly higher in the PR group (73%) than in the PK (50%) or in the PRK group (29%). It is well known that in genotype 1 patients, baseline viral load is the best prognostic factor for response to antiviral treatment[5-7,26]. Thus, it was not surprising that patients in the PR group obtained a better virological response at week 4. Nevertheless, the SVR rate observed in the PRK group (57%) was better than that observed in PR (47%) and in PK (31%). Furthermore, among patients with high viral load (> 700 000 IU/mL), those of the PRK group obtained the better SVR (60% vs 25% and 38% in the PR and PK groups, respectively). It should be pointed out that our study was initiated before the optimal dose of ribavirin for patients with HCV genotype 1 (i.e. 1000 mg/d or 1200 mg/d according to body weight) was determined[7]. However, even if the dose of ribavirin utilized in this study was suboptimal and might have influenced the SVR, both groups receiving ribavirin had the same dose regimen.
In conclusion, considering that the use of ketoprofen does not add further side effects, is associated with better viral kinetics and early activation of the IFN signaling pathway, and in combination with PR improves virological response rates, this pilot study suggests the exploration of the clinical efficacy of this three-drug combination in well-designed randomized clinical trials. We conclude that such studies are warranted since, in this era of development of new drugs for HCV, the clinical use of novel compounds up till now has been hampered by toxicity issues and rapid promotion of drug-resistant HCV viruses[27].
The current standard treatment for chronic hepatitis C with pegylated-interferon (PEG-IFN) and ribavirin is effective in approximately 50%-60% of patients, so that a substantial proportion of patients remain unresponsive. A rational approach to develop alternative therapeutic strategies for patients with chronic hepatitis C virus (HCV) infection demands a detailed knowledge of how the different drugs affect viral kinetics and IFN intracellular signaling. Non-steroidal antiinflammatory drugs (NSAIDs) have been demonstrated to amplify the IFN signaling pathways and to enhance the anti-viral effect of IFN. This phase II study evaluated the effect of ketoprofen (a NSAID) plus PEG-IFN with or without ribavirin compared with PEG-IFN plus ribavirin (PR) on viral kinetics, STAT1 activity and expression of the IFN-dependent gene, 2’-5’-oligoadenylate synthetase (2’-5’OAS), in patients with genotype 1 chronic hepatitis C.
The results of this pilot study support the proposal of an evaluation of the clinical efficacy of the addition of ketoprofen to the standard PR treatment for chronic hepatitis C in well-designed randomized clinical trials.
This is the first study to report both molecular and clinical data about the use of ketoprofen in association with PEG-IFNα and ribavirin in chronic hepatitis C. The authors found that the addition of ketoprofen to the conventional combination therapy is associated with better viral kinetics and early activation of the IFNα signaling pathway, thus improving virological response rates.
The results may stimulate further experimental and clinical investigations regarding the role of NSAIDs in association with IFN-based therapy in the context of HCV-related liver diseases.
IFN signaling pathways are activated by binding of IFN to its specific receptor, which induces autophosphorylation of protein tyrosine kinases Tyk-2 and Jak-1 on tyrosine residues, thus activating signal transducer and activator of transcription (STAT1 and STAT2) proteins. Activated STATs translocate to the nucleus where they activate the transcription of IFN-inducible genes, such as 2’-5’OAS.
The authors postulate that a larger trial should be done with this 3 drug combination, compared to standard of care. While a small study, there is useful data.
| 1. | Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, Zarski JP. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24:778-789. |
| 2. | Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426-1432. |
| 3. | Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493-1499. |
| 4. | National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3-S20. |
| 5. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. |
| 6. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. |
| 7. | Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. |
| 8. | Hannigan GE, Williams BR. Signal transduction by interferon-alpha through arachidonic acid metabolism. Science. 1991;251:204-207. |
| 9. | Tsuboi I, Tanaka H, Nakao M, Shichijo S, Itoh K. Nonsteroidal anti-inflammatory drugs differentially regulate cytokine production in human lymphocytes: up-regulation of TNF, IFN-gamma and IL-2, in contrast to down-regulation of IL-6 production. Cytokine. 1995;7:372-379. |
| 10. | Baskin G. Interferon signalling through arachidonic acid-dependent pathways: a clue to adjuvant therapy for chronic viral hepatitis? Hepatology. 1991;14:392-394. |
| 11. | Andreone P, Cursaro C, Gasbarrini G. Interferon-alpha increases prostaglandin E2 production by cultured liver biopsy in patients with chronic viral hepatitis: can non-steroidal anti-inflammatory drugs improve the therapeutic response to interferon? J Hepatol. 1993;19:228-231. |
| 12. | Andreone P, Cursaro C, Gramenzi A, Buzzi A, Miniero R, Sprovieri G, Gasbarrini G. Indomethacin enhances serum 2'5'-oligoadenylate synthetase in patients with hepatitis B and C virus chronic active hepatitis. J Hepatol. 1994;21:984-988. |
| 13. | Giambartolomei S, Artini M, Almerighi C, Moavero SM, Levrero M, Balsano C. Nonsteroidal anti-inflammatory drug metabolism potentiates interferon alfa signaling by increasing STAT1 phosphorylation. Hepatology. 1999;30:510-516. |
| 14. | Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439-454. |
| 15. | Trujillo-Murillo K, Rincón-Sánchez AR, Martínez-Rodríguez H, Bosques-Padilla F, Ramos-Jiménez J, Barrera-Saldaña HA, Rojkind M, Rivas-Estilla AM. Acetylsalicylic acid inhibits hepatitis C virus RNA and protein expression through cyclooxygenase 2 signaling pathways. Hepatology. 2008;47:1462-1472. |
| 16. | Zarski JP, Maynard-Muet M, Chousterman S, Baud M, Barnoud R, Abergel A, Bacq Y, Combis JM, Causse X, Tran A. Tenoxicam, a non-steroid anti-inflammatory drug, is unable to increase the response rate in patients with chronic hepatitis C treated by alpha interferon. Hepatology. 1998;27:862-867. |
| 17. | Andreone P, Cursaro C, Gramenzi A, Fiorino S, Di Giammarino L, Miniero R, D'Errico A, Grigioni WF, Gasbarrini G, Bernardi M. Interferon alpha plus ketoprofen or interferon alpha plus ribavirin in chronic hepatitis C non-responder to interferon alpha alone: results of a pilot study. Ital J Gastroenterol Hepatol. 1999;31:688-694. |
| 18. | Fabris P, Tositti G, Negro F, Marranconi F, Infantolino D, Rassu M, De Lalla F. Interferon alfa-2b alone or in combination with ketoprofen as treatment for interferon-naive chronic hepatitis C patients. Aliment Pharmacol Ther. 1999;13:1329-1334. |
| 19. | Muñoz AE, Levi D, Podestá A, Gorín JM, González J, Bartellini MA, Munne MS, Cabanne A, Flichman D, Terg R. Interferon-alpha 2b combined with daily ketoprofen administration improves virological response in chronic hepatitis C: a prospective and randomised trial. Gut. 2000;46:427-431. |
| 20. | Andreone P, Gramenzi A, Cursaro C, Biselli M, Lorenzini S, Loggi E, Felline F, Fiorino S, Di Giammarino L, Porzio F. Interferon-alpha combined with ketoprofen as treatment of naïve patients with chronic hepatitis C: a randomized controlled trial. J Viral Hepat. 2003;10:306-309. |
| 21. | Hadziyannis SJ. Combination of interferon alpha therapy with non-steroidal anti-inflammatory drugs in chronic hepatitis C. Gut. 2000;46:306-307. |
| 22. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. |
| 23. | Dahari H, Ribeiro RM, Perelson AS. Triphasic decline of hepatitis C virus RNA during antiviral therapy. Hepatology. 2007;46:16-21. |
| 24. | Herrmann E, Lee JH, Marinos G, Modi M, Zeuzem S. Effect of ribavirin on hepatitis C viral kinetics in patients treated with pegylated interferon. Hepatology. 2003;37:1351-1358. |
| 25. | Zhang Y, Jamaluddin M, Wang S, Tian B, Garofalo RP, Casola A, Brasier AR. Ribavirin treatment up-regulates antiviral gene expression via the interferon-stimulated response element in respiratory syncytial virus-infected epithelial cells. J Virol. 2003;77:5933-5947. |
| 26. | Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46:37-47. |
| 27. | Deutsch M, Hadziyannis SJ. Old and emerging therapies in chronic hepatitis C: an update. J Viral Hepat. 2008;15:2-11. |
Peer reviewer: Paul Y Kwo, Professor, Gastroenterology and Hepatology Division, Indiana University School of Medicine, 975 West Walnut, IB 327, Indianapolis, IN 46202-5121, United States
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM